Abstract
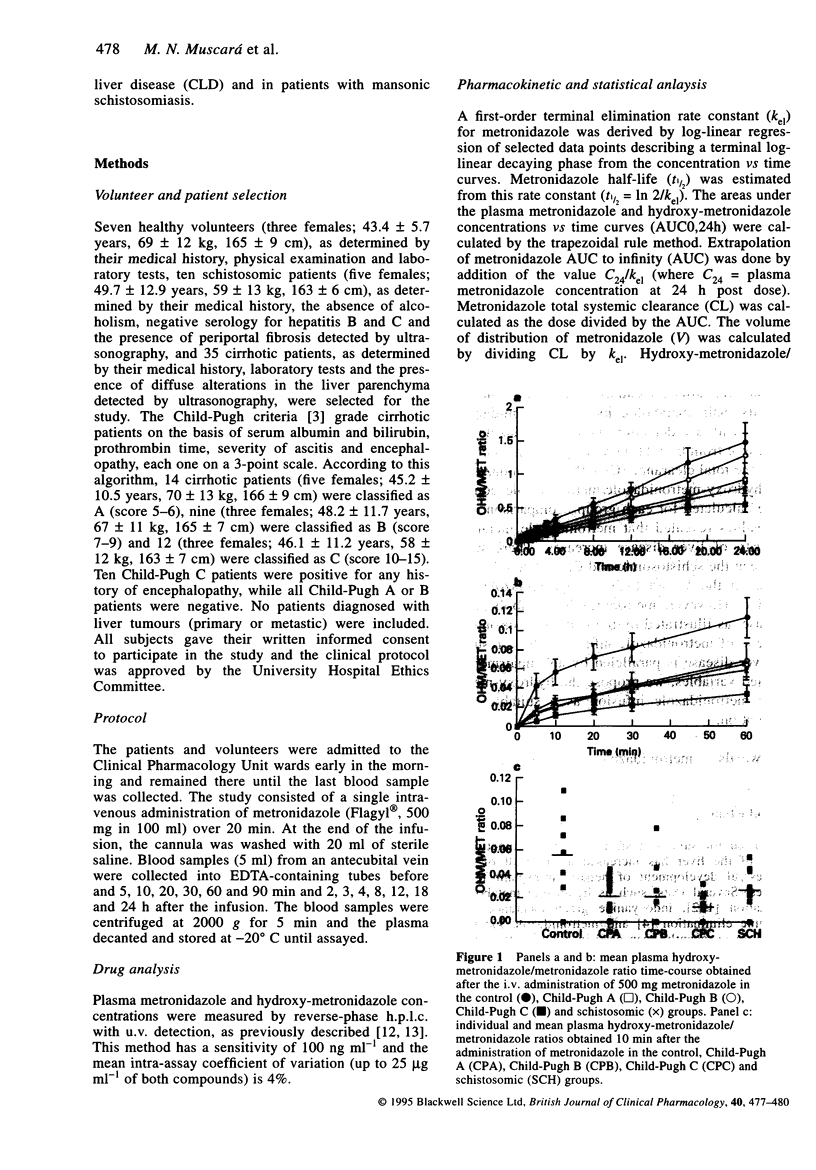
Metronidazole pharmacokinetics were studied in patients with different degrees of liver cirrhosis, classified according to the Child-Pugh algorithm (A, B or C, as liver disease severity increases) and in schistosomic patients. Metronidazole (500 mg) was administered i.v. as a slow infusion over 20 min, and blood samples were collected at set intervals after the end of the infusion. The plasma concentrations of metronidazole and its main metabolite hydroxy-metronidazole were quantified by reversed-phase h.p.l.c. with u.v. detection. The metronidazole and hydroxy-metronidazole areas under the curve from 0 to 24 h (AUC0,24h), the metronidazole terminal elimination half-life (t1/2), the total clearance (CL), the metronidazole volume of distribution (V) values and the hydroxy-metronidazole/metronidazole concentration ratios as a function of time were calculated for each group. Comparison of the metronidazole AUC0,24h, t1/2 and CL values revealed that metronidazole metabolism is progressively impaired as the severity of liver disease increases. There were no variations in these parameters between the schistosomic and Child-Pugh A groups. In addition, there were no differences in the V and hydroxy-metronidazole AUC0,24h among the various groups studied. However, metronidazole metabolism was delayed in patients with hepatic disease, as illustrated by the hydroxy-metronidazole/metronidazole ratio 10 min after the end of metronidazole infusion. These results indicate that the clinical assessment of liver disease is paralleled by an impairment of metronidazole metabolism. Of the studied variables, we propose the hydroxy-metronidazole/metronidazole ratio 10 min after metronidazole infusion as a suitable and practical index for liver function evaluation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers I., Hartmann H., Bircher J., Creutzfeldt W. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol. 1989 Apr;24(3):269–276. doi: 10.3109/00365528909093045. [DOI] [PubMed] [Google Scholar]
- Bergan T., Thorsteinsson S. B. Pharmacokinetics of metronidazole and its metabolites in reduced renal function. Chemotherapy. 1986;32(4):305–318. doi: 10.1159/000238429. [DOI] [PubMed] [Google Scholar]
- Daneshmend T. K., Homeida M., Kaye C. M., Elamin A. A., Roberts C. J. Disposition of oral metronidazole in hepatic cirrhosis and in hepatosplenic schistosomiasis. Gut. 1982 Oct;23(10):807–813. doi: 10.1136/gut.23.10.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremse D. A., A-Kader H. H., Schroeder T. J., Balistreri W. F. Assessment of lidocaine metabolite formation as a quantitative liver function test in children. Hepatology. 1990 Sep;12(3 Pt 1):565–569. doi: 10.1002/hep.1840120319. [DOI] [PubMed] [Google Scholar]
- Haller I. In vitro activity of the two principal oxidative metabolites of metronidazole against Bacteroides fragilis and related species. Antimicrob Agents Chemother. 1982 Jul;22(1):165–166. doi: 10.1128/aac.22.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S., Vickers C., Elias E. Drug metabolism in end-stage liver disease. In vitro activities of some phase I and phase II enzymes. J Hepatol. 1990 Jul;11(1):37–42. doi: 10.1016/0168-8278(90)90269-w. [DOI] [PubMed] [Google Scholar]
- Jensen J. C., Gugler R. Sensitive high-performance liquid chromatographic method for the determination of metronidazole and metabolites. J Chromatogr. 1983 Oct 14;277:381–384. doi: 10.1016/s0378-4347(00)84862-8. [DOI] [PubMed] [Google Scholar]
- Loft S., Otton S. V., Lennard M. S., Tucker G. T., Poulsen H. E. Characterization of metronidazole metabolism by human liver microsomes. Biochem Pharmacol. 1991 Apr 15;41(8):1127–1134. doi: 10.1016/0006-2952(91)90650-t. [DOI] [PubMed] [Google Scholar]
- Loft S., Sonne J., Døssing M., Andreasen P. B. Metronidazole pharmacokinetics in patients with hepatic encephalopathy. Scand J Gastroenterol. 1987 Jan;22(1):117–123. doi: 10.3109/00365528708991867. [DOI] [PubMed] [Google Scholar]
- McLean A. J., Morgan D. J. Clinical pharmacokinetics in patients with liver disease. Clin Pharmacokinet. 1991 Jul;21(1):42–69. doi: 10.2165/00003088-199121010-00004. [DOI] [PubMed] [Google Scholar]
- Muscará M. N., De Nucci G. Bioavailability of four pharmaceutical formulations of metronidazole tested on normal healthy volunteers. Braz J Med Biol Res. 1991;24(12):1251–1260. [PubMed] [Google Scholar]
- O'Keefe J. P., Troc K. A., Thompson K. D. Activity of metronidazole and its hydroxy and acid metabolites against clinical isolates of anaerobic bacteria. Antimicrob Agents Chemother. 1982 Sep;22(3):426–430. doi: 10.1128/aac.22.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oellerich M., Burdelski M., Ringe B., Lamesch P., Gubernatis G., Bunzendahl H., Pichlmayr R., Herrmann H. Lignocaine metabolite formation as a measure of pre-transplant liver function. Lancet. 1989 Mar 25;1(8639):640–642. doi: 10.1016/s0140-6736(89)92144-2. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J. E., Edson R. S. Symposium on antimicrobial agents. Metronidazole. Mayo Clin Proc. 1987 Nov;62(11):1013–1017. doi: 10.1016/s0025-6196(12)65074-5. [DOI] [PubMed] [Google Scholar]
- Sadun E. H., Williams J. S., Witherspoon C., Martin L. K. The relative role of eggs and adult worms in the development of liver damage in mice infected with Schistosoma mansoni. Ann N Y Acad Sci. 1969 Oct 6;160(2):841–862. doi: 10.1111/j.1749-6632.1969.tb15905.x. [DOI] [PubMed] [Google Scholar]
- Wang T., Kleber G., Stellaard F., Paumgartner G. Caffeine elimination: a test of liver function. Klin Wochenschr. 1985 Nov 4;63(21):1124–1128. doi: 10.1007/BF02291094. [DOI] [PubMed] [Google Scholar]


