Abstract
Studies were carried out to examine the mechanism of action of WAY-150138, a member of a novel group of thiourea compounds recently shown to inhibit replication of herpes simplex virus type 1 (HSV-1). Previous studies have shown that the drug acts by preventing DNA encapsidation and that resistant mutants map to UL6, the gene encoding the protein subunit of the portal complex through which DNA enters the capsid. We tested the idea that WAY-150138 acts by preventing the incorporation of DNA-packaging proteins into capsids as they are assembled. Capsids were isolated from HSV-1-infected, drug-treated cells and examined by Western immunoblotting for the presence of two packaging proteins, the portal subunit (UL6) and a candidate terminase subunit (UL15). The results showed that both proteins were depleted in the capsids, suggesting that WAY-150138 antagonizes DNA encapsidation by depriving capsids of packaging proteins during the assembly process.
WAY-150138 (Fig. 1) and a related thiourea compound have recently been shown to be potent inhibitors of herpes simplex virus type 1 (HSV-1) replication in cell culture (17). Modest inhibition of HSV-2 growth was also observed, but little or no inhibition was detected with other herpesviruses tested, including human cytomegalovirus and varicella-zoster virus.
FIG. 1.
Structure of WAY-150138.
Studies designed to identify the mechanism of WAY-150138 inhibition suggest that it acts by preventing DNA encapsidation. Electron micrographs of HSV-1-infected, drug-treated cells demonstrated an abundance of DNA-free B capsids but few DNA-containing capsids or progeny virions despite normal DNA replication (17). In further support of an effect on DNA packaging, it was observed that WAY-150138-resistant HSV-1 mutants map to UL6, the gene encoding the protein subunit of the portal complex through which DNA enters the capsid as it is packaged (5, 17).
With the goal of further clarifying the mechanism of WAY-150138 action, we tested the idea that the drug may antagonize DNA encapsidation by inhibiting the incorporation of packaging proteins into capsids as they are assembled. Packaging proteins should be depleted in such capsids. To determine whether this is the case, capsids were isolated from infected, WAY-150138-treated cells and examined by Western immunoblotting for the presence of UL6, the portal protein described above, and UL15, a candidate terminase subunit considered to provide the energy required for packaging (1, 10, 12, 19).
Experiments were performed with the KOS strain of HSV-1, which was grown on Vero cells propagated in 150-cm2 flasks at 34°C in minimal essential medium as described previously (5). WAY-150138 (molecular weight, 492) was a gift from Marja van Zeijl (Wyeth Research, Pearl River, N.Y.). Stock solutions (10 mg/ml) were prepared in dimethyl sulfoxide and were added to cell cultures at the same time as infecting virus. Control cells received only dimethyl sulfoxide. The titer of cell-associated progeny virus (the major progeny virus population) was determined 24 h postinfection. Infected cells were suspended in minimal essential medium containing 1% calf serum at a concentration of 2 × 107 cells/ml and lysed by freezing and thawing followed by a brief (15-s) sonication at 4°C in a bath sonicator (model HD-50; Heat Systems). The lysate was clarified by centrifugation for 2 min at 2,000 × g, diluted, and used to infect Vero cell monolayers contained in 24-well microtiter plates. Foci of virus infection were counted by light microscopy 24 h after infection.
In agreement with the results of van Zeijl et al. (17), WAY-150138 was found to inhibit HSV-1 replication in cultured Vero cells. In each of two representative experiments, 10-μg/ml (20 μM) WAY-150138 was found to reduce the titer of progeny virus tenfold (Table 1). The observed reduction in virus titer was more modest than the 300- to 800-fold reduction seen by van Zeijl et al. (17), even when the drug dose was increased and the host cell line was changed. We suggest that the difference in drug effects may be due to the different HSV-1 strains employed (KOS and Patton in the present and earlier studies, respectively).
TABLE 1.
Effect of WAY-150138 on replication of HSV-1 in cell culturea
| Treatment | HSV-1 titer (PFU/ml of cell extract)
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| Control | 109 | 108 |
| 10-μg/ml (20 μM) WAY-150138 | 108 | 107 |
The methods used for growth of HSV-1 and for determination of the titer of progeny virus on Vero cell monolayers are described in the text.
Capsid isolation.
Capsids were isolated from control and WAY-150138-treated cells by cell lysis followed by sucrose density gradient ultracentrifugation. Previously described procedures were employed for capsid isolation, beginning with the infection of Vero cells (multiplicity of infection = 5) for 24 h (6). A, B, and C capsids were separated by sucrose gradient ultracentrifugation on 5-ml gradients of 25 to 50% sucrose in TNE (0.01 M Tris-HCl [pH 7.5], 0.5 M NaCl, 1 mM EDTA). Gradients were centrifuged for 40 min at 24,000 rpm in a Beckman SW50.1 rotor and photographed (with top illumination) to record the positions of capsid bands. Capsids from individual bands were removed from the gradient with a Pasteur pipette, concentrated by centrifugation into a pellet (23,000 rpm for 1 h in a Beckman SW50.1 rotor), and resuspended in phosphate-buffered saline for further analysis by electron microscopy or gel electrophoresis. Previously described procedures were employed for electron microscopy of negatively stained capsids (16) and for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (7).
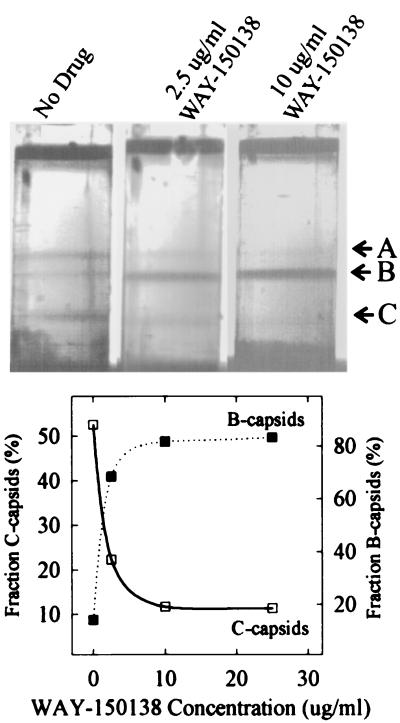
Sucrose gradient analysis of capsid populations revealed that A (empty), B (containing the scaffolding protein), and C (containing DNA) capsids were all present in control cells, with C capsids being the predominant species, accounting for more than 50% of the capsids present (Fig. 2). In contrast, when infected cells were treated with WAY-150138, the proportion of A and C capsids decreased while the number of B capsids increased with increasing drug concentration. At the highest WAY-150138 concentrations tested, B capsids accounted for more than 80% of the total capsid population (Fig. 2). The high proportion of B capsids observed in drug-treated cells is consistent with the results of thin-section electron microscopic analyses, which also show a preponderance of B capsids (17). The decrease seen in the number of C capsids supports the view that WAY-150138 functions by inhibiting DNA encapsidation.
FIG. 2.
Sucrose density gradient analysis of capsids isolated from HSV-1-infected control and WAY-150138-treated cells. The methods employed for capsid isolation and centrifugation are described in the text. Photographs of the gradients after centrifugation are shown in the top panel, with the positions of the A, B, and C capsids indicated on the right. The lower panel shows a quantitative measure of the proportions of B and C capsids as determined by densitometric scanning of the photographs in the top panel. Note that B capsids predominate and C capsids are depleted in infected cells treated with WAY-150138.
WAY-150138 was found to alter the relative proportions of A, B, and C capsids but did not appear to have a dramatic effect on the total number of capsids present in infected cells (Fig. 2, top panel). A quantitative comparison of the total numbers of capsids in infected control and drug-treated cells was performed by densitometric scanning of gradient photographs such as those shown in the top panel of Fig. 2. This analysis showed that the ratios of the total number of capsids present in drug-treated cells to that in control cells were 1.04 (2.5 μg of WAY-150138/ml), 1.00 (10 μg of WAY-150138/ml), and 0.81 (10 μg of WAY-150138/ml) in three experiments.
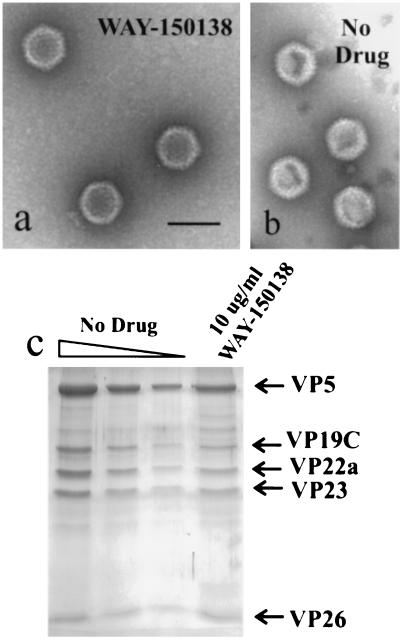
Capsids prepared from infected control and WAY-150138-treated cells were found to be closely similar in structure and in their content of the predominant capsid protein species. For example, in electron micrographs of negatively stained preparations, B capsids isolated from the two types of cells yielded images that were indistinguishable (Fig. 3, compare panels a and b). Similarly, analysis by SDS-polyacrylamide gel electrophoresis followed by Coomassie staining showed that the major capsid protein species present in B capsids from control cells, including VP5, VP19C, VP23, pre-VP22a, and VP26 (2, 11, 14), were also present in B capsids isolated from WAY-150138-treated cells (Fig. 3c).
FIG. 3.
Structure and protein composition of B capsids isolated from control and WAY-150138-treated cells. Shown are electron micrographs of negatively stained capsids prepared from WAY-150138-treated cells (a) and untreated control cells (b). Note that capsids from WAY-150138-treated and control cells are indistinguishable in morphology. Also shown are the results of SDS-polyacrylamide gel electrophoresis (c), with the three leftmost lanes containing decreasing amounts (twofold dilutions) of capsids isolated from control cells. Note that the predominant capsid protein species found in capsids from control cells (i.e., VP5, VP19C, VP22a, VP23, and VP26) are also present in capsids isolated from WAY-150138-treated cells (rightmost lane).
Western immunoblotting.
Western immunoblot experiments were carried out with B capsids that were isolated as described above and purified by a second step of sucrose density gradient ultracentrifugation. Twenty-five microliters of capsids in phosphate-buffered saline (at a concentration of ∼1 mg/ml) was centrifuged on 0.7-ml gradients of 20 to 50% sucrose for 30 min at 23,000 rpm in an SW50.1 rotor. Gradient fractions were then used for SDS-polyacrylamide gel electrophoresis followed by Coomassie staining or Western immunoblotting. Fractions from control and WAY-150138-treated specimens were analyzed on the same gel and the same blot by previously described procedures for SDS-polyacrylamide gel electrophoresis and Western immunoblotting (13). The primary antisera employed in the immunoblots were anti-MBP-UL6 (a gift of Joel Baines, Cornell University) (15), which was used at a dilution of 1:1,000, and AS9 (specific for UL15 [13]), which was used at a dilution of 1:500.
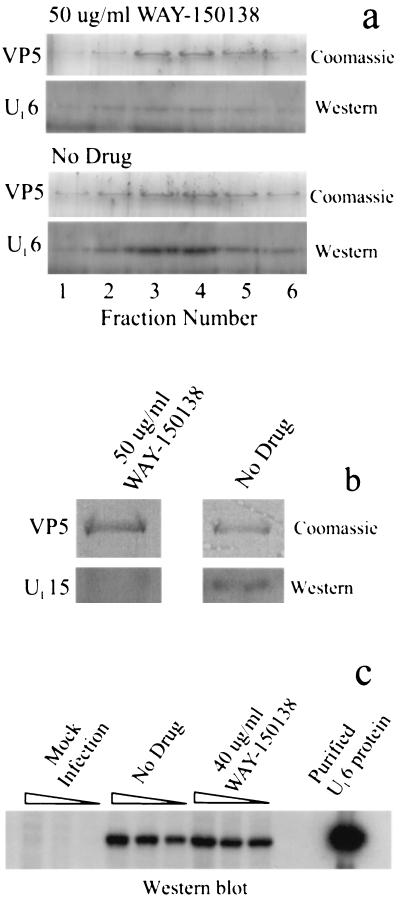
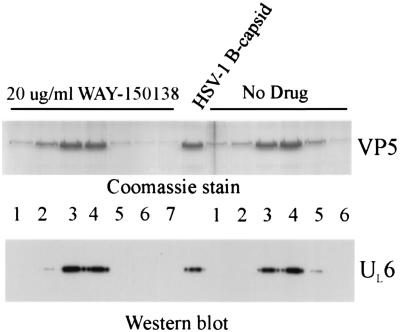
Western immunoblot analysis of capsids isolated from control cells showed that they contain UL6, as expected (Fig. 4a) (5, 8); UL6 staining above the background level was observed only in capsid-containing gradient fractions, which were identified by the presence of VP5, the major capsid protein. In contrast, the level of UL6 was reduced in capsids isolated from WAY-150138-treated cells (Fig. 4a), with reductions of 24.5- and 3.6-fold (average, 14.1-fold) observed in two experiments. A decrease in UL15 content was also observed in capsids from WAY-150138-treated cells compared to that in capsids from control cells; reduction was found to be 16-fold in one experiment (Fig. 4b).
FIG. 4.
Western immunoblot analysis of capsids and cells. (a and b) Analysis of capsids isolated from infected control and WAY-150138-treated cells. UL6 staining (a) was performed with sucrose density gradient fractions bracketing the B capsid band, as identified visually on the gradient and by Coomassie staining of fractions for VP5 (lanes labeled Coomassie). The peak B capsid fraction was stained for UL15 (b). Note that staining of both UL6 and UL15 is decreased in capsids from WAY-150138-treated cells compared to those from control cells (No Drug), while the amounts of capsids present (as measured by VP5 staining with Coomassie) are almost the same. (c) Western blot staining for UL6 in uninfected Vero cells (three leftmost lanes) and cells infected with HSV-1 in the absence (middle group of three lanes) and presence (rightmost group of three lanes) of 40 μg of WAY-150138/ml. Samples in each group of three lanes contained 4 × 105, 2 × 105, and 0.8 × 105 cells, respectively. The lane at far right shows the staining of authentic, purified UL6 protein (5). Note that UL6 protein is absent in uninfected cells but present in both control and drug-treated infected cells.
Control immunoblot experiments were performed to determine whether WAY-150138 affects the synthesis of UL6 protein. Control and drug-treated (40 μg of WAY-150138/ml) cells were infected for 24 h at 37°C and solubilized by boiling in 2% SDS. The proteins were then subjected to SDS-polyacrylamide gel electrophoresis, Western immunoblotting, and staining for UL6 as described above. The results (Fig. 4c) showed that UL6 protein was synthesized in both control and drug-treated cells, with little quantitative difference observed between the two groups of samples in three matched-sample concentrations. A control experiment demonstrated that, as expected, UL6 was not detected in uninfected Vero cells (Fig. 4c, three leftmost lanes).
Control studies were also carried out to determine whether WAY-150138 acts by dissociating UL6 protein from capsids that already contain it. HSV-1 B capsids containing UL6 were incubated in vitro with the drug, purified by sucrose density gradient centrifugation, and tested by Western immunoblotting for the presence of UL6. Experiments were performed with B capsids isolated as described above from Vero cells infected with wild-type HSV-1 KOS. Samples containing 125 μg of capsids in 50 μl of TNE were treated with 20 μg of WAY-150138/ml or solvent (dimethyl sulfoxide) for 30 min at room temperature. Capsids were then purified by banding on 0.7-ml gradients of 20 to 50% sucrose in TNE; gradients were centrifuged for 40 min at 23,000 rpm in a Beckman SW 50.1 rotor and fractionated. Capsid-containing and flanking fractions were then analyzed by SDS-polyacrylamide gel electrophoresis followed by Coomassie staining and by Western immunoblotting for UL6. Fractions containing capsids were identified by the presence of VP5, the major capsid protein, as shown in Fig. 5. The same fractions were also found to contain UL6, as shown in the lower panel of Fig. 5, with UL6 observed in both the drug-treated and control capsids. The presence of UL6 in WAY-150138-treated capsids indicates that the capsids are resistant to the extraction of UL6 by the drug.
FIG. 5.
Analysis of UL6 in control HSV-1 B capsids and B capsids treated in vitro with WAY-150138. The methods employed for preparation of B capsids, treatment of capsids with 20 μg of WAY-150138/ml in vitro, capsid purification by sucrose gradient centrifugation, and analysis of gradient fractions by SDS-polyacrylamide gel electrophoresis and Western immunoblot staining for UL6 are described in the text. Gradient fraction numbers are indicated between the panels. The center lane shows the results of the same analysis performed with untreated HSV-1 B capsids. Note that UL6 protein is present in capsids from both control and WAY-150138-treated cells.
As described above, the studies reported here were undertaken to test the idea that WAY-150138 suppresses HSV-1 replication by preventing the incorporation of packaging proteins such as UL6 into capsids as they are assembled. Such a mechanism of action would be consistent with the previously reported effect of the drug in inhibiting DNA encapsidation and with the mapping of WAY-150138 resistance mutations to the UL6 gene. Other possible mechanisms of WAY-150138 action considered at the outset of this study included the following: (i) selective inhibition of UL6 protein synthesis, (ii) degradation of UL6 after it is synthesized, (iii) an effect on UL6 function, and (iv) extraction of UL6 from capsids already containing it.
The observed depletion of UL6 and UL15 in capsids obtained from WAY-150138-treated cells supports the view that the drug acts to prevent incorporation of packaging proteins into capsids. Capsids with UL6 and UL15 depleted would produce the observed WAY-150138-induced defect in DNA encapsidation. In contrast, we do not favor the view that the drug could cause the loss of packaging proteins from capsids once they are formed. WAY-150138 was found to have no effect on HSV-1 replication in cell culture when added 6 h or more postinfection (17). Since most capsids are successfully packaged with DNA after 6 h postinfection, these capsids must retain the UL6 portal. Also, UL6 was found to be retained in B capsids incubated in vitro in the presence of WAY-150138 (Fig. 5). A direct effect of WAY-150138 on the incorporation of packaging proteins into capsids rather than on protein synthesis is suggested by the observation that the amounts of intracellular UL6 were found to be similar in control and WAY-150138-treated cells (Fig. 4c).
The finding that WAY-150138 is able to antagonize the incorporation of packaging proteins into capsids while still allowing the formation of intact, structurally normal capsids is consistent with previous conclusions regarding the roles of UL6 and UL15 in capsid assembly. Morphologically normal HSV-1 capsids are formed in vivo and in vitro in the absence of UL6 or UL15 (3, 4, 5, 9, 18, 19). Capsids with UL6 depleted or that lack UL6 are expected to be defective in the UL6-containing portal complex found at a unique capsid vertex. Apart from this difference, however, capsids with UL6 depleted are expected to be structurally the same as wild-type capsids (Fig. 3a and b).
Two previous observations indicate that WAY-150138 may have a direct effect on incorporation of UL6 into capsids, with its effect on UL15 as a consequence. First, as mentioned above, mutations causing resistance to WAY-150138 in HSV-1 map to the UL6 gene (17). Resistance mapping to UL15 would also be expected if WAY-150138 had a direct effect on the UL15 polypeptide. Second, Yu and Weller (19) have demonstrated that UL15 is associated with capsids only if UL6 is also present. Capsids isolated from cells infected with a UL6 null mutant lack both UL6 and UL15 proteins, suggesting that UL15 may bind to capsids by way of the UL6-containing portal complex. Therefore, we propose that WAY-150138 acts specifically to deprive capsids of UL6 as they are assembled. The effects of the drug on DNA packaging and on UL15 incorporation into capsids are suggested to be consequences of its primary effect on UL6. Further studies are under way to identify the individual capsid assembly steps that may be affected by WAY-150138.
Acknowledgments
We thank Marja van Zeijl and Fred Homa for thoughtful comments on the manuscript and Jonathan Bloom (Wyeth Research) for preparation of WAY-150138.
This work was supported by NIH grant AI41644 and by NSF award MCB-9904879.
REFERENCES
- 1.Brown, J. C., M. A. McVoy, and F. L. Homa. 2002. Packaging DNA into herpesvirus capsids, p. 111-153. In A. Holzenburg and E. Bogner (ed.), Structure-function relationships of human pathogenic viruses. Kluwer Academic/Plenum Publishers, London, United Kingdom.
- 2.Homa, F. L., and J. C. Brown. 1997. Capsid assembly and DNA packaging in herpes simplex virus. Rev. Med. Virol. 7:107-122. [DOI] [PubMed] [Google Scholar]
- 3.Newcomb, W. W., F. L. Homa, D. R. Thomsen, F. P. Booy, B. L. Trus, A. C. Steven, J. V. Spencer, and J. C. Brown. 1996. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid assembly. J. Mol. Biol. 263:432-446. [DOI] [PubMed] [Google Scholar]
- 4.Newcomb, W. W., F. L. Homa, D. R. Thomsen, Z. Ye, and J. C. Brown. 1994. Cell-free assembly of the herpes simplex virus capsid. J. Virol. 68:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newcomb, W. W., R. M. Juhas, D. R. Thomsen, F. L. Homa, A. D. Burch, S. K. Weller, and J. C. Brown. 2001. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 75:10923-10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newcomb, W. W., B. L. Trus, F. P. Booy, A. C. Steven, J. S. Wall, and J. C. Brown. 1993. Structure of the herpes simplex virus capsid: molecular composition of the pentons and the triplexes. J. Mol. Biol. 232:499-511. [DOI] [PubMed] [Google Scholar]
- 7.Newcomb, W. W., B. L. Trus, N. Cheng, A. C. Steven, A. K. Sheaffer, D. J. Tenney, S. K. Weller, and J. C. Brown. 2000. Isolation of herpes simplex virus procapsids from cells infected with a protease-deficient mutant virus. J. Virol. 74:1663-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel, A. H., and J. B. MacLean. 1995. The product of the UL6 gene of herpes simplex virus type 1 is associated with virus capsids. Virology 206:465-478. [DOI] [PubMed] [Google Scholar]
- 9.Patel, A. H., F. J. Rixon, C. Cunningham, and A. J. Davison. 1996. Isolation and characterization of a herpes simplex virus type-1 mutant defective in the UL6 gene. Virology 217:111-123. [DOI] [PubMed] [Google Scholar]
- 10.Poon, A. P. W., and B. Roizman. 1993. Characterization of a temperature-sensitive mutant of the UL15 open reading frame of herpes simplex virus 1. J. Virol. 67:4497-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rixon, F. J. 1993. Structure and assembly of herpesviruses. Semin. Virol. 4:135-144. [Google Scholar]
- 12.Salmon, B., D. Nalwanga, Y. Fan, and J. D. Baines. 1999. Proteolytic cleavage of the amino terminus of the UL15 gene product of herpes simplex virus type 1 is coupled with maturation of viral DNA into unit-length genomes. J. Virol. 73:8338-8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheaffer, A. K., W. W. Newcomb, M. Gao, D. Yu, S. K. Weller, J. C. Brown, and D. J. Tenney. 2001. Herpes simplex virus DNA cleavage and packaging proteins associate with the procapsid prior to its maturation. J. Virol. 75:687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steven, A. C., and P. G. Spear. 1996. Herpesvirus capsid assembly and envelopment, p. 312-351. In R. Burnett, W. Chiu, and R. Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, N.Y.
- 15.Taus, N. S., B. Salmon, and J. D. Baines. 1998. The herpes simplex virus 1 UL17 gene is required for localization of capsids and major and minor capsid proteins to intranuclear sites where viral DNA is cleaved and packaged. Virology 252:115-125. [DOI] [PubMed] [Google Scholar]
- 16.Thomas, D., W. W. Newcomb, J. C. Brown, J. S. Wall, J. F. Hainfeld, B. L. Trus, and A. C. Steven. 1985. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J. Virol. 54:598-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Zeijl, M., J. Fairhurst, T. R. Jones, S. K. Vernon, J. Morin, J. LaRocque, B. Feld, B. O'Hara, J. D. Bloom, and S. V. Johann. 2000. Novel class of thiourea compounds that inhibit herpes simplex virus type 1 DNA cleavage and encapsidation: resistance maps to the UL6 gene. J. Virol. 74:9054-9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu, D., A. K. Sheaffer, D. J. Tenney, and S. K. Weller. 1997. Characterization of ICP6::lacZ insertion mutants of the UL15 gene of herpes simplex virus type 1 reveals the translation of two proteins. J. Virol. 71:2656-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu, D., and S. K. Weller. 1998. Genetic analysis of the UL15 gene locus for the putative terminase of herpes simplex virus type 1. Virology 243:32-44. [DOI] [PubMed] [Google Scholar]