Abstract
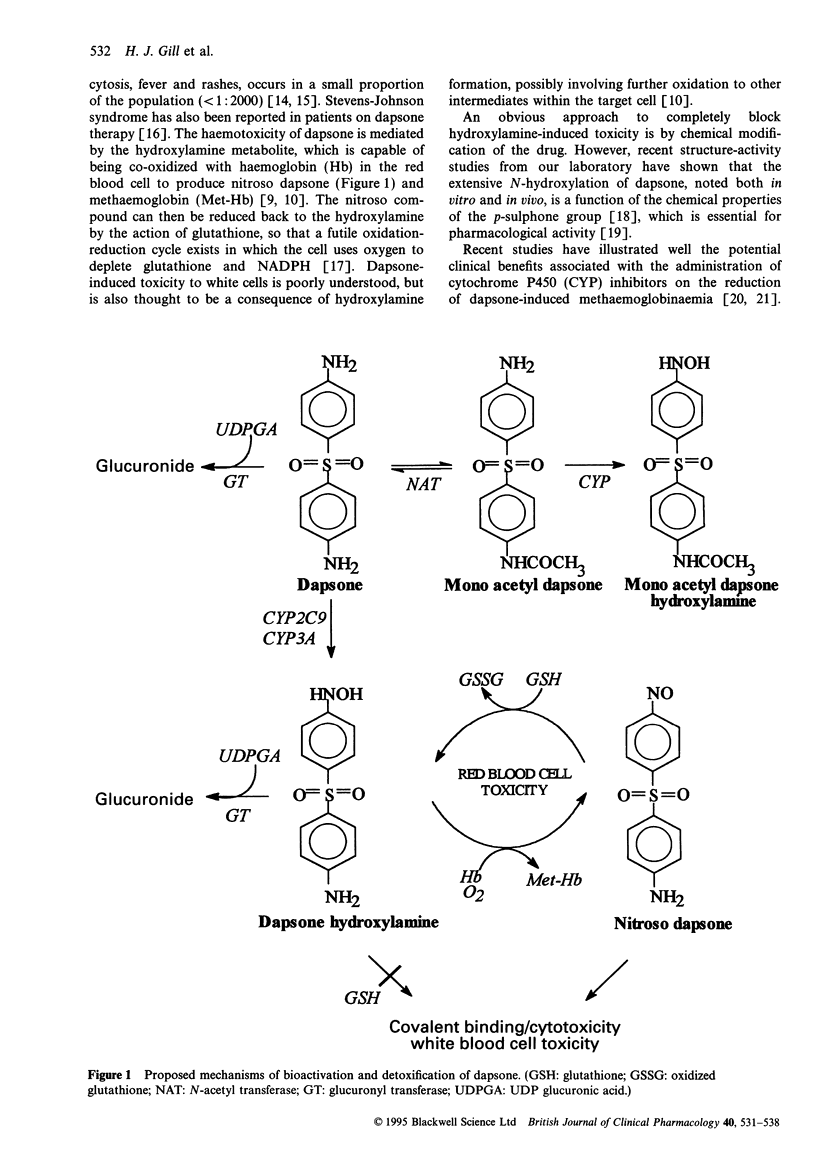
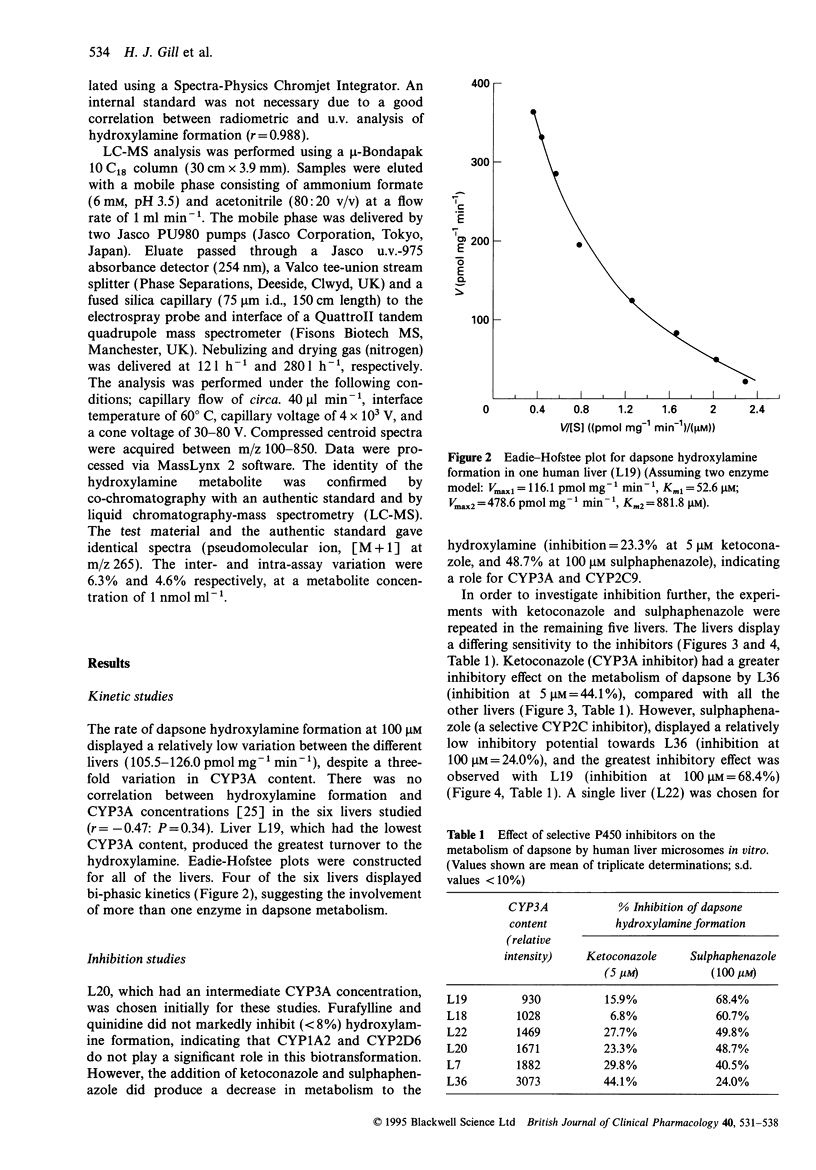
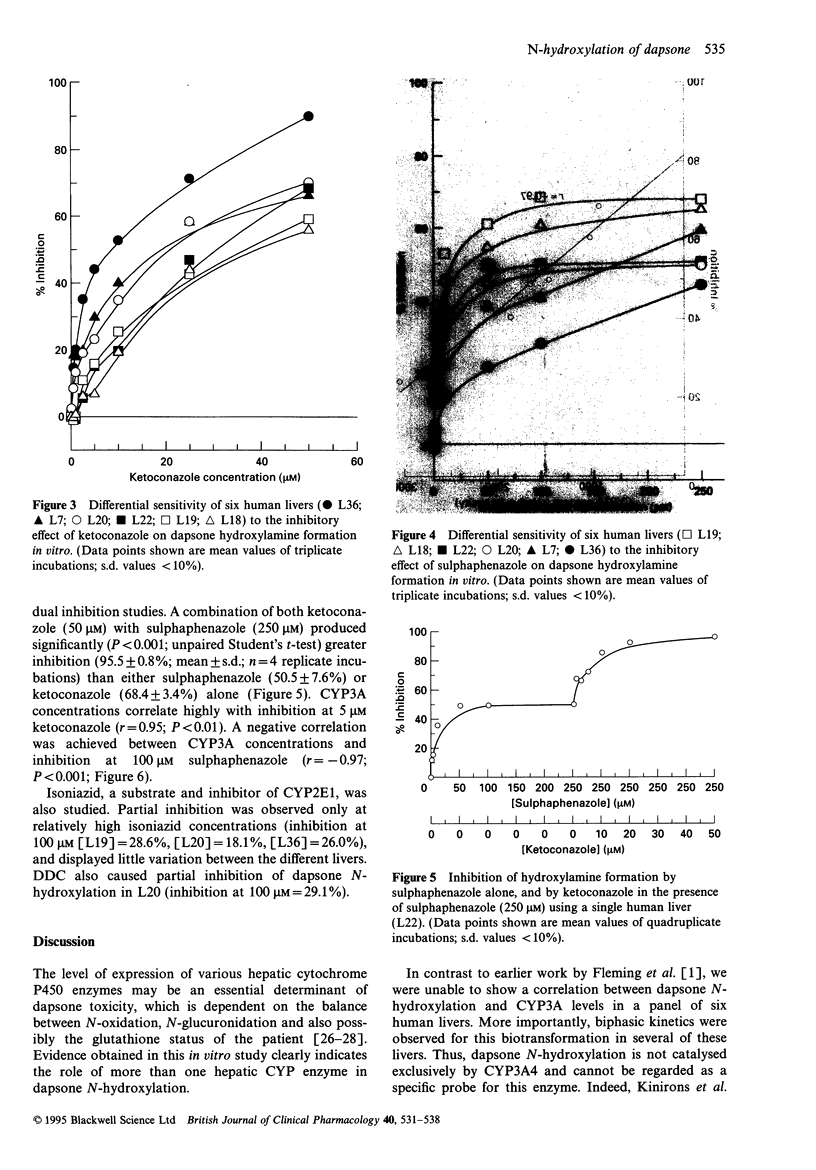
1. The adverse reactions associated with the administration of dapsone are believed to be caused by metabolism to its hydroxylamine. Previous reports suggest that CYP3A4 is responsible for this biotransformation [1]. 2. Data presented in this paper illustrate the involvement of more than one cytochrome P450 enzyme in dapsone hydroxylamine formation using human liver microsomes. Eadie-Hofstee plots demonstrated bi-phasic kinetics in several livers. No correlation could be established between hydroxylamine formation and CYP3A concentrations in six human livers (r = -0.47; P = 0.34). 3. Studies with low molecular weight inhibitors illustrate the importance of CYP2C9 and CYP3A in dapsone N-hydroxylation. 4. Differential sensitivity of dapsone N-hydroxylation to selective CYP inhibitors indicated that the contribution of individual CYP enzymes varies between livers. Selective inhibition ranged from 6.8 to 44.1% by 5 microM ketoconazole, and from 24.0 to 68.4% by 100 microM sulphaphenazole. The extent of inhibition, by either ketoconazole or sulphaphenazole was dependent on the CYP3A content of the liver. 5. The levels of expression of these cytochrome P450 enzymes may be an important determinant of individual susceptibility to the toxic effects of dapsone, and may influence the ability of an enzyme inhibitor to block dapsone toxicity in vivo. Because of the inability to produce complete inhibition, selective CYP inhibitors are unlikely to offer any clinical advantage over cimetidine in decreasing dapsone hydroxylamine formation in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back D. J., Tjia J., Mönig H., Ohnhaus E. E., Park B. K. Selective inhibition of drug oxidation after simultaneous administration of two probe drugs, antipyrine and tolbutamide. Eur J Clin Pharmacol. 1988;34(2):157–163. doi: 10.1007/BF00614553. [DOI] [PubMed] [Google Scholar]
- Baldwin S. J., Bloomer J. C., Smith G. J., Ayrton A. D., Clarke S. E., Chenery R. J. Ketoconazole and sulphaphenazole as the respective selective inhibitors of P4503A and 2C9. Xenobiotica. 1995 Mar;25(3):261–270. doi: 10.3109/00498259509061850. [DOI] [PubMed] [Google Scholar]
- Bayard P. J., Berger T. G., Jacobson M. A. Drug hypersensitivity reactions and human immunodeficiency virus disease. J Acquir Immune Defic Syndr. 1992 Dec;5(12):1237–1257. [PubMed] [Google Scholar]
- Buhl R., Jaffe H. A., Holroyd K. J., Wells F. B., Mastrangeli A., Saltini C., Cantin A. M., Crystal R. G. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet. 1989 Dec 2;2(8675):1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- Chang T. K., Gonzalez F. J., Waxman D. J. Evaluation of triacetyloleandomycin, alpha-naphthoflavone and diethyldithiocarbamate as selective chemical probes for inhibition of human cytochromes P450. Arch Biochem Biophys. 1994 Jun;311(2):437–442. doi: 10.1006/abbi.1994.1259. [DOI] [PubMed] [Google Scholar]
- Coleman M. D., Breckenridge A. M., Park B. K. Bioactivation of dapsone to a cytotoxic metabolite by human hepatic microsomal enzymes. Br J Clin Pharmacol. 1989 Oct;28(4):389–395. doi: 10.1111/j.1365-2125.1989.tb03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. D., Scott A. K., Breckenridge A. M., Park B. K. The use of cimetidine as a selective inhibitor of dapsone N-hydroxylation in man. Br J Clin Pharmacol. 1990 Nov;30(5):761–767. doi: 10.1111/j.1365-2125.1990.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming C. M., Branch R. A., Wilkinson G. R., Guengerich F. P. Human liver microsomal N-hydroxylation of dapsone by cytochrome P-4503A4. Mol Pharmacol. 1992 May;41(5):975–980. [PubMed] [Google Scholar]
- Friman G., Nyström-Rosander C., Jonsell G., Björkman A., Lekås G., Svendsrup B. Agranulocytosis associated with malaria prophylaxis with Maloprim. Br Med J (Clin Res Ed) 1983 Apr 16;286(6373):1244–1245. doi: 10.1136/bmj.286.6373.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya H., Meyer U. A., Gelboin H. V., Gonzalez F. J. Polymerase chain reaction-directed identification, cloning, and quantification of human CYP2C18 mRNA. Mol Pharmacol. 1991 Sep;40(3):375–382. [PubMed] [Google Scholar]
- Gonzalez F. J. Human cytochromes P450: problems and prospects. Trends Pharmacol Sci. 1992 Sep;13(9):346–352. doi: 10.1016/0165-6147(92)90107-h. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Idle J. R. Pharmacogenetic phenotyping and genotyping. Present status and future potential. Clin Pharmacokinet. 1994 Jan;26(1):59–70. doi: 10.2165/00003088-199426010-00005. [DOI] [PubMed] [Google Scholar]
- Gordin F. M., Simon G. L., Wofsy C. B., Mills J. Adverse reactions to trimethoprim-sulfamethoxazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1984 Apr;100(4):495–499. doi: 10.7326/0003-4819-100-4-495. [DOI] [PubMed] [Google Scholar]
- Green S. T., Goldberg D. J., Leach J., Christie P. R., Kennedy D. H. AIDS-related Pneumocystis carinii pneumonia successfully treated with dapsone-trimethoprim. Br J Clin Pharmacol. 1988 Oct;26(4):487–488. doi: 10.1111/j.1365-2125.1988.tb03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman S. J., Jollow D. J. Role of dapsone hydroxylamine in dapsone-induced hemolytic anemia. J Pharmacol Exp Ther. 1988 Jan;244(1):118–125. [PubMed] [Google Scholar]
- Halpert J. R., Guengerich F. P., Bend J. R., Correia M. A. Selective inhibitors of cytochromes P450. Toxicol Appl Pharmacol. 1994 Apr;125(2):163–175. doi: 10.1006/taap.1994.1061. [DOI] [PubMed] [Google Scholar]
- Israili Z. H., Cucinell S. A., Vaught J., Davis E., Lesser J. M., Dayton P. G. Studies of the metabolism of dapsone in man and experimental animals: formation of N-hydroxy metabolites. J Pharmacol Exp Ther. 1973 Oct;187(1):138–151. [PubMed] [Google Scholar]
- Jick H. Adverse reactions to trimethoprim-sulfamethoxazole in hospitalized patients. Rev Infect Dis. 1982 Mar-Apr;4(2):426–428. doi: 10.1093/clinids/4.2.426. [DOI] [PubMed] [Google Scholar]
- Jorde U. P., Horowitz H. W., Wormser G. P. Utility of dapsone for prophylaxis of Pneumocystis carinii pneumonia in trimethoprim-sulfamethoxazole-intolerant, HIV-infected individuals. AIDS. 1993 Mar;7(3):355–359. doi: 10.1097/00002030-199303000-00008. [DOI] [PubMed] [Google Scholar]
- Kinirons M. T., O'Shea D., Downing T. E., Fitzwilliam A. T., Joellenbeck L., Groopman J. D., Wilkinson G. R., Wood A. J. Absence of correlations among three putative in vivo probes of human cytochrome P4503A activity in young healthy men. Clin Pharmacol Ther. 1993 Dec;54(6):621–629. doi: 10.1038/clpt.1993.199. [DOI] [PubMed] [Google Scholar]
- Knodell R. G., Browne D. G., Gwozdz G. P., Brian W. R., Guengerich F. P. Differential inhibition of individual human liver cytochromes P-450 by cimetidine. Gastroenterology. 1991 Dec;101(6):1680–1691. doi: 10.1016/0016-5085(91)90408-d. [DOI] [PubMed] [Google Scholar]
- Kramer P. A., Glader B. E., Li T. K. Mechanism of methemoglobin formation by diphenylsulfones. Effect of 4-amino-4'-hydroxyaminodiphenylsulfone and other p-substituted derivatives. Biochem Pharmacol. 1972 May 1;21(9):1265–1274. doi: 10.1016/0006-2952(72)90288-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manfredi G., De Panfilis G., Zampetti M., Allegra F. Studies on dapsone induced haemolytic anaemia. I. Methaemoglobin production and G-6-PD activity in correlation with dapsone dosage. Br J Dermatol. 1979 Apr;100(4):427–432. doi: 10.1111/j.1365-2133.1979.tb01644.x. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Cox P. H., Beck K., Styer C. M., Beall G. N. A comparison of the effectiveness of three regimens in the prevention of Pneumocystis carinii pneumonia in human immunodeficiency virus-infected patients. Arch Intern Med. 1992 Mar;152(3):523–528. [PubMed] [Google Scholar]
- Maurice M., Pichard L., Daujat M., Fabre I., Joyeux H., Domergue J., Maurel P. Effects of imidazole derivatives on cytochromes P450 from human hepatocytes in primary culture. FASEB J. 1992 Jan 6;6(2):752–758. doi: 10.1096/fasebj.6.2.1371482. [DOI] [PubMed] [Google Scholar]
- Newton D. J., Wang R. W., Lu A. Y. Cytochrome P450 inhibitors. Evaluation of specificities in the in vitrometabolism of therapeutic agents by human liver microsomes. Drug Metab Dispos. 1995 Jan;23(1):154–158. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Park B. K., Kitteringham N. R. Drug-protein conjugation and its immunological consequences. Drug Metab Rev. 1990;22(1):87–144. doi: 10.3109/03602539008991445. [DOI] [PubMed] [Google Scholar]
- Persson J. O., Terelius Y., Ingelman-Sundberg M. Cytochrome P-450-dependent formation of reactive oxygen radicals: isozyme-specific inhibition of P-450-mediated reduction of oxygen and carbon tetrachloride. Xenobiotica. 1990 Sep;20(9):887–900. doi: 10.3109/00498259009046904. [DOI] [PubMed] [Google Scholar]
- Pertel P., Hirschtick R. Adverse reactions to dapsone in persons infected with human immunodeficiency virus. Clin Infect Dis. 1994 Apr;18(4):630–632. doi: 10.1093/clinids/18.4.630. [DOI] [PubMed] [Google Scholar]
- Rhodes L. E., Tingle M. D., Park B. K., Chu P., Verbov J. L., Friedmann P. S. Cimetidine improves the therapeutic/toxic ratio of dapsone in patients on chronic dapsone therapy. Br J Dermatol. 1995 Feb;132(2):257–262. doi: 10.1111/j.1365-2133.1995.tb05022.x. [DOI] [PubMed] [Google Scholar]
- Somogyi A., Gugler R. Clinical pharmacokinetics of cimetidine. Clin Pharmacokinet. 1983 Nov-Dec;8(6):463–495. doi: 10.2165/00003088-198308060-00001. [DOI] [PubMed] [Google Scholar]
- Tingle M. D., Coleman M. D., Park B. K. The effect of preincubation with cimetidine on the N-hydroxylation of dapsone by human liver microsomes. Br J Clin Pharmacol. 1991 Jul;32(1):120–123. doi: 10.1111/j.1365-2125.1991.tb05623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetrecht J. P., Sweetman B. J., Woosley R. L., Oates J. A. Metabolism of procainamide to a hydroxylamine by rat and human hepatic microsomes. Drug Metab Dispos. 1984 Jan-Feb;12(1):77–81. [PubMed] [Google Scholar]
- Uetrecht J., Zahid N., Shear N. H., Biggar W. D. Metabolism of dapsone to a hydroxylamine by human neutrophils and mononuclear cells. J Pharmacol Exp Ther. 1988 Apr;245(1):274–279. [PubMed] [Google Scholar]
- Vadher A., Lalljee M. Patient treatment compliance in leprosy; a critical review. Int J Lepr Other Mycobact Dis. 1992 Dec;60(4):587–607. [PubMed] [Google Scholar]
- de Quay B., Malinverni R., Lauterburg B. H. Glutathione depletion in HIV-infected patients: role of cysteine deficiency and effect of oral N-acetylcysteine. AIDS. 1992 Aug;6(8):815–819. [PubMed] [Google Scholar]


