Abstract
Genes encoding fowlpox virus (FWPV) structural proteins have been identified mainly by sequence homology with those from vaccinia virus (VACV), but little is known about the encoded proteins. Production of monoclonal antibodies (MAbs) against Poxine and HP1-440 (Munich) clone FP9 allowed the identification of three immunodominant FWPV proteins: the 39-kDa core protein (encoded by FPV168, homologous to VACV A4L), a 30- and 35-kDa protein doublet, and an abundant 63-kDa protein. The 30- and 35-kDa proteins are nonglycosylated, antigenically related proteins present in the intracellular mature virus membrane and localizing closely with the viral factories. N-terminal sequencing identified the 35-kDa protein as encoded by FPV140 (the FWPV homolog of VACV H3L). The 63-kDa protein forms covalently linked dimers and oligomers. It remained mainly insoluble upon detergent treatment of purified virus but did not localize closely with the viral factory. N-terminal sequencing was unsuccessful, suggesting N-terminal blocking. CNBr digestion generated a peptide encoded by FPV191, predicted to encode one of two FWPV A-type inclusion (ATI) proteins. The characteristics of the 63-kDa protein were inconsistent with published observations on cowpox or VACV ATI proteins (it appears to be essential). The 63-kDa protein, however, shares characteristics with both VACV p4c virus occlusion and 14-kDa fusion proteins. Gene assignment at the poxvirus ATI locus (between VACV A24R and A28L) is complicated by sequence redundancies and variations, often due to deletions and multiple frameshift mutations. The identity of FPV191 in relation to genes at this locus is discussed.
Avipoxviruses, which replicate only in avian cells, have been developed as safe recombinant vectors for human vaccination (23, 30). In mammalian cells, avipoxvirus replication is blocked but early gene expression occurs (45, 46). Late gene expression occurs in some mammalian cell types infected by fowlpox virus (FWPV), the prototypic avipoxvirus, without productive virus replication, as morphogenesis appears to be blocked (42). FWPV has been much less intensively studied than vaccinia virus (VACV). It appears to form extracellular enveloped virus (EEV) by budding rather then wrapping (6, 16). It has a larger genome (266 kbp for attenuated strain FP9 [S. M. Laidlaw and M. A. Skinner unpublished data] and 288 kbp for the United States Department of Agriculture challenge strain [1]) than does VACV (192 kbp for the Copenhagen strain [15]), with a very different genome organization (27). The complete sequence of a pathogenic strain of FWPV (1) includes genes encoding homologs of at least 21 known VACV structural proteins, but FWPV appears to lack homologs of intracellular mature virus (IMV) proteins encoded by A27L and D8L as well as an intracellular enveloped virus protein encoded by A36R and the EEV proteins encoded by B5R, A33R, and A56R (1).
Although genes encoding FWPV structural proteins have been identified by sequence homology, very little is known about the proteins they encode. We produced monoclonal antibodies (MAbs) and polyclonal antibodies against FWPV proteins as markers to study FWPV morphogenesis and identified genes encoding three FWPV immunodominant structural proteins.
MATERIALS AND METHODS
Virus and cells.
The origins, propagation, and purification of FWPV strains (FP9, HP1, and Poxine) and canarypox virus (CNPV) have been described previously (5).
MAbs.
BALB/c mice were immunized twice intraperitonally 3 weeks apart with a crude preparation of Poxine in Quil A, a saponin-derived adjuvant (Superfos, Vedbaek, Denmark). For the second fusion, mice were injected twice 4 weeks apart with 100 μg of sucrose gradient-purified FP9 virus (5). Hybridoma supernatants were screened by enzyme-linked immunosorbent assay or by indirect immunofluorescence assay, with Poxine-infected or noninfected chicken embryo fibroblasts (CEFs) as the antigen. The 3D9 MAb directed against the 39-kDa protein was kindly provided by C. P. Czerny.
Polyclonal sera.
Chicken hyperimmune sera against FP9, Poxine, or CNPV were produced as described previously (5).
Radioimmunoprecipitation.
Infected (multiplicity of infection, 10) or uninfected CEFs were incubated in methionine-free medium containing 2% dialyzed fetal calf serum. Two hours later, the medium was replaced with fresh medium containing 1.85 MBq of [35S]methionine/ml and incubated for 2 h. The medium was removed, and the cells were washed once with phosphate-buffered saline (PBS) and lysed in 2 ml of radioimmunoprecipitation assay (RIPA) buffer (2.5 mM Tris [pH 7.2], 0.15 M NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1% sodium deoxycholate). Lysates were centrifuged for 15 min (Eppendorf centrifuge), and supernatants were stored at −70°C.
Ascitic fluid (10 μl) or hybridoma supernatant (500 μl) was incubated for 1 h at 4°C with 50 μl of 10% protein A-Sepharose CL-4B (Pharmacia) in 0.1 M sodium phosphate buffer, pH 8.1. After the beads were washed twice in phosphate buffer, nonlabeled, noninfected cell lysate (100 μl), prepared as described above, was added and incubated with the beads overnight at 4°C. The cold lysate was then replaced by 20 μl of labeled lysate, preadsorbed overnight at 4°C on the same volume of protein A-Sepharose beads. After 3 h of incubation at 4°C, the beads were washed five times in RIPA buffer and twice in Tris-buffered saline (50 mM NaCl, 2.5 mM Tris [pH 7.2]), resuspended in sample buffer (50 μl), boiled, and subjected to SDS-polyacrylamide gel electrophoresis (PAGE). Labeled proteins were detected by fluorography with sodium salicylate (8).
Deglycosylation.
After radioimmunoprecipitation, the washed beads were further washed with 50 mM sodium phosphate buffer, pH 7. Digestion buffer (25 μl; 50 mM sodium phosphate buffer [pH 6.1], 25 mM EDTA, 0.4 mM phenylmethylsulfonyl fluoride), with or without a mixture consisting of 0.1% SDS, 0.5% Triton X-100, and 1% β-mercaptoethanol, was added to the beads, which were then boiled before 0.01 to 0.2 U of endoglycosidase F (endo-F; Boehringer Mannheim) was added. After 20 h of incubation at 37°C, the samples were processed for SDS-PAGE.
Far-Western blotting.
Purified virus and infected and uninfected freeze-thawed cellular lysates were loaded on an SDS-PAGE gel and transferred onto a nitrocellulose membrane. The membrane was saturated overnight at 4°C in 5% powdered milk in Tris-buffered saline and then probed overnight at 4°C with infected cellular lysate. MAbs directed against the 63-kDa protein or the 30- and 35-kDa proteins were then added as for a conventional Western blotting experiment.
Preparation of viral membranes and cores.
Virions, harvested from both supernatant and cells, labeled with [35S]methionine, and purified on sucrose gradients, were incubated for 1 h at 37°C with 1 volume of 0.2 M Tris-HCl, pH 8.6-2% Triton X-100. Cores were pelleted by centrifugation at 200,000 × g for 30 min at 4°C (Beckman TLA-100.1 rotor). Membrane fractions were collected, and the pellets were resuspended in 0.1 M Tris-HCl, pH 8.6. All samples were centrifuged and treated again in the same way before being radioimmunoprecipitated with specific MAbs.
Confocal immunofluorescence.
CEFs, seeded on coverslips in 24-well plates (0.5 × 105 per ml of M199 medium with 10% fetal calf serum), were infected with FWPV at a multiplicity of infection of 0.1 to 0.5. After incubation at 37°C, the cells were fixed in 4% paraformaldehyde in PBS for 60 min, washed with PBS, permeabilized with 0.5% Triton X-100 in PBS for 15 min, and then rewashed. The cells were blocked with 0.5% bovine serum albumin (BSA) in PBS for 30 min and then incubated with hybridoma supernatant (diluted 1/200) or preadsorbed rabbit serum in BSA-PBS for 60 min. After three 5-min washes in PBS, cells were incubated with the Alexa 488 goat anti-mouse immunoglobulin G (IgG) antibody (Molecular Probes; diluted 1/200) for 60 min. After three washes, the cells were incubated with ToPro 3 (Molecular Probes) at 1:10,000 in PBS for 5 min. After being washed, the cells were mounted in Vectashield (Vector Laboratories), and immunofluorescence was observed with a Leica TCS NT confocal microscope. Images were adjusted for levels and annotated by using Photoshop, version 5 (Adobe).
Amino acid sequence analysis.
Clarified, infected cell lysate (4 ml) was immunoprecipitated overnight at 4°C by using protein A-Sepharose (Pharmacia; 250 μl of a 10% suspension in 0.1 M sodium phosphate buffer, pH 8.1) and specific MAbs. The beads were washed three times with phosphate buffer, resuspended, and boiled in electrophoresis sample buffer, and the supernatants were subjected to SDS-PAGE. To prevent N-terminal blocking, the gel was prerun (11). Proteins were transferred onto a Fluorotrans membrane (Pall Corporation) in CAPS buffer (0.01 M CAPS [3-(cyclohexylamino)-1-propanesulfonic acid] [pH 11], 10% methanol), visualized by amido black staining, excised from the membrane, and N-terminally sequenced on an Applied Biosystems 492 automated protein sequencer, with on-line chromatography of PTH amino acids, by using the manufacturer's 140C microgradient system and 220- by 2.1-mm reverse-phase columns. For internal sequencing, the Coomassie blue-stained gel was destained in 30% methanol. Protein bands were excised, placed in clean Eppendorf tubes, and dehydrated in high-pressure liquid chromatography grade acetonitrile for 30 min. The protein was digested overnight at room temperature with CNBr (10 mg/ml in 70% trifluoroacetic acid). The slices were swollen on ice in NH4OH solution (1%), excess liquid was removed, and the slices were dried in a centrifugal evaporator. The slices were twice reswollen in distilled water on ice, and excess water was removed before the slices were dried in a centrifugal evaporator. Finally, the slices were swollen in SDS-PAGE sample buffer and then placed in the wells of a 15% gel. The electrophoresed peptides were transferred onto a Fluorotrans membrane and sequenced as described above.
Construction of deletion plasmids and transient dominant selection.
The 5′ end of the FPV191 gene of FP9 was amplified by PCR using primers M1498 (5′-CCCCAAGCTTGTATAAATCAATGGATGC; consisting of a HindIII site and sequence from 450 bp upstream of FPV191) and M1497 (5′-ATCTTCTTAGAGGTATTACTACATCATC; complementary to FPV191 sequences from nucleotide positions 34 to 51 and 1373 to 1382). The 3′ end of FPV191 was amplified with primers M1496 (5′-AGTAATACCTCTAAGAAGATAGATTACC; FPV191 sequences from nucleotide positions 42 to 51 and 1373 to 1390) and M1499 (5′-ACATGCATGCTAGATACTGTACGTCCGG; consisting of an SphI site and the complement of the sequence 450 bp downstream of FPV191). In a second-round PCR, the 5′ and 3′ end reaction products were used as sources of target DNA with primers M1498 and M1499, resulting in a product consisting of the FPV191 gene with nucleotides 52 to 1372 deleted and with 450 bp of flanking sequence on either side. The product was purified with a QIAquick PCR purification kit (Qiagen), digested with HindIII and SphI, and cloned into pGNR, which has the Escherichia coli gpt gene under the control of the VACV p7.5 early/late promoter (5), previously digested with the same enzymes. Transdominant selection (12) was performed as described previously (5). Unstable recombinants, which carried the gpt gene as well as wild-type and deleted copies of FPV191, were selected in the presence of mycophenolic acid. Stable recombinants that had lost the gpt gene in the absence of mycophenolic acid were then screened by PCR with M1498 and M1499 to see whether they carried the wild-type form of FPV191 or forms with deletions.
RESULTS
MAb isolation.
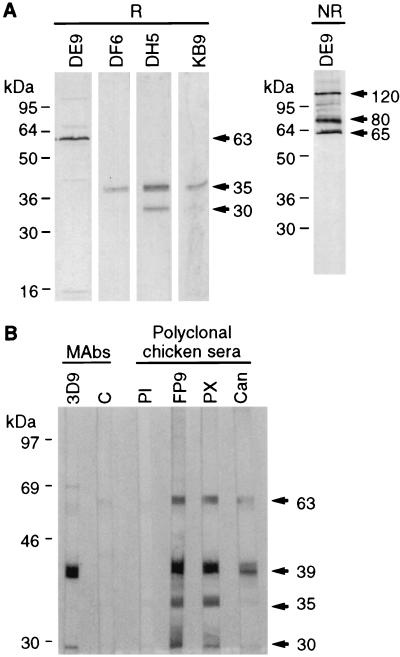
Fifteen hybridomas from two fusions, reacting strongly by indirect immunofluorescence assay or enzyme-linked immunosorbent assay, were characterized by RIPA using lysates from infected CEFs and by Western blotting using purified virus as the antigen (Table 1; see below). All six MAbs from the first fusion and three from the second reacted against a 63-kDa protein (Fig. 1A). From the second fusion, two MAbs reacted against a 39-kDa protein (data not shown) and four precipitated a doublet of 30 and 35 kDa (Fig. 1A). These proteins corresponded to the four major virus proteins detected in purified FP9 by Western blotting with chicken hyperimmune sera (Fig. 1B). The 39-kDa protein, a major FWPV core protein equivalent to the VACV A4L gene product, has already been studied in detail (5). This protein, as well as the 63-kDa protein, was also recognized by anti-CNPV polyclonal serum which, however, showed no cross-reactivity against the 30- and the 35-kDa proteins (Fig. 1B). Similarly, by Western blotting, the DE9 anti-FWPV 63-kDa protein MAb recognized the CNPV homolog, which migrated slightly faster than the FWPV protein, but none of the anti-FWPV 30- and 35-kDa protein MAbs reacted against the CNPV homologs (data not shown).
TABLE 1.
Description of the MAbs
| Antigen | MAb | Molecular mass(es) (kDa) by:
|
||
|---|---|---|---|---|
| RIPA | Western blotting
|
|||
| Reducing | Nonreducing | |||
| Crude preparation (Poxine) | AE2 | 63 | —a | — |
| BD3 | 63 | — | — | |
| DG11 | 63 | — | — | |
| DH6 | 63 | — | — | |
| HD4 | 63 | — | — | |
| IH2 | 63 | — | — | |
| Purified virus (FP9) | DE9 | 63 | 63 | 65, 80, 120 |
| FD5 | 63 | — | — | |
| HE11 | 63 | — | — | |
| GB9 | 39 | 39 | NTb | |
| GG1 | 39 | 39 | NT | |
| DF6 | 30, 35 | 35 | NT | |
| DH5 | 30, 35 | 30, 35 | NT | |
| JA7 | 30, 35 | — | NT | |
| KB9 | 30, 35 | 35 | 35 | |
—, no reaction.
NT, not tested.
FIG. 1.
(A) Western blotting performed with FWPV FP9 as the antigen under reducing (R) or nonreducing (NR) conditions revealed with MAb DE9 (anti-63-kDa protein) or with either DF6, DH5, KB9 (anti-30- and -35-kDa proteins). Molecular weight markers are shown on the left. (B) Immunodominant proteins detected by Western blotting using purified FWPV FP9 as the antigen. The antibodies were as follows: 3D9, anti-39-kDa protein MAb; C, negative control without a first antibody; PI, preimmune polyclonal chicken serum; FP9, PX, and Can, polyclonal chicken serum obtained after immunization with FWPV FP9, FWPV Poxine, and CNPV, respectively.
Structure of the 30- and 35-kDa doublet proteins.
By Western blotting analysis using purified virus as the antigen, two MAbs (DF6 and KB9) reacted only against the 35-kDa protein (although MAb DF6 also weakly immunoprecipitated the 30-kDa protein; see Fig. 3) whereas MAb DH5 revealed 30- and 35-kDa polypeptides, showing that the epitope recognized by this MAb was present on both proteins (Fig. 1A). Both proteins are, therefore, probably encoded by the same gene but processed differently. Under nonreducing conditions, no additional band was detected (data not shown), showing that there is no disulfide linkage between them. Both proteins were found in purified virus (Fig. 1A), and neither is glycosylated, as labeling in the presence of tunicamycin or monensin or treatment of radioimmunoprecipitated proteins with endo-F did not alter their apparent molecular weights (data not shown).
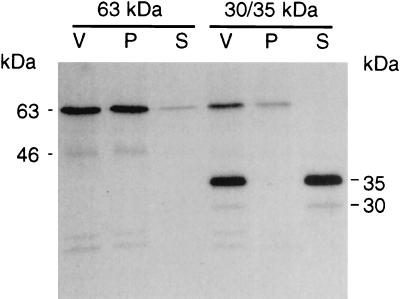
FIG. 3.
Fractionation of purified virus with detergent. Untreated [35S]methionine-labeled purified virus (V) and pelleted (P) or solubilized (S) fractions obtained after detergent treatment were radioimmunoprecipitated with either MAb DG11 (anti-63 kDa protein) or MAb DF6 (anti-30- and -35-kDa proteins).
Structure of the 63-kDa protein.
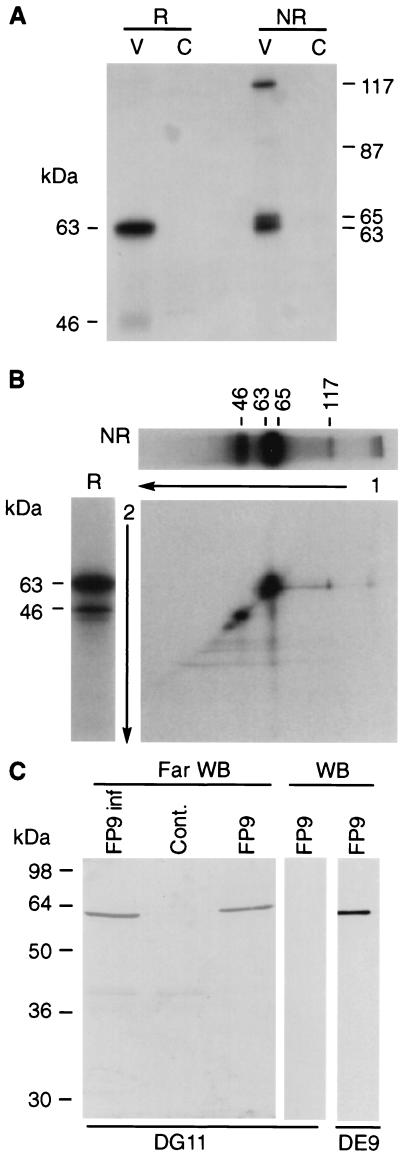
The most abundant protein of purified FWPV migrated as a 63-kDa protein (data not shown). Most MAbs obtained (9 out of 15) were directed against a similar protein. Only one such MAb (DE9) reacted by Western blotting (Table 1), suggesting that the protein has a complex secondary structure and presents mainly conformational epitopes. Under nonreducing conditions, MAb DE9 reacted with three polypeptides, of 65, 80, and 120 kDa (Fig. 1A). By radioimmunoprecipitation under reducing conditions, a major 63-kDa protein and a weak 46-kDa protein were detected (Fig. 2A). The variable relative amounts of these proteins indicates that the 46-kDa polypeptide was probably a degradation product (Fig. 2A and B) although neither phenylmethylsulfonyl fluoride nor aprotinin nor 4-(2-aminoethyl)benzenesulfonyl fluoride (Roche) prevented its formation. Under nonreducing conditions, the 63-kDa protein appeared as a doublet (63 and 65 kDa) and a 117-kDa protein was also observed (Fig. 2A). Higher-molecular-weight bands could also be detected (Fig. 1A and 2B), suggesting the formation of oligomers. Under these conditions the weak 46-kDa protein disappeared but a weak 87-kDa band, probably representing a dimer of the 46-kDa protein, was detected (Fig. 2A). Nonreducing conditions followed by reducing two-dimensional (2D) electrophoresis showed that the 117-kDa protein was a disulfide-linked dimer of the 63-kDa protein (Fig. 2B). The 63- and 65-kDa doublet formed a single band under reducing conditions, suggesting that they represent conformations with different intramolecular disulfide bonds.
FIG. 2.
(A) Radioimmunoprecipitation of the 63-kDa protein under reducing (R) and nonreducing (NR) conditions. V, FP9 infected cell lysate; C, noninfected cellular control. (B) 2D electrophoresis. The 63-kDa protein was first immunoprecipitated and run on a gel under nonreducing conditions, as in panel A, lane 3. The corresponding lane was excised (a duplicate well is shown horizontally on top of the 2D gel), treated with β-mercaptoethanol, inserted on top of a second gel devoid of wells, and run under reducing conditions (a control sample run treated under reducing conditions is shown on the left of the 2D gel). (C) Far-Western blotting. Purified virus (FP9) and clarified lysates from infected (FP9 inf) and uninfected (Cont.) cells were subjected to SDS-PAGE and then transferred onto a nitrocellulose membrane. The membrane (Far WB) was saturated overnight at 4°C in 5% powdered milk in TN buffer (10 mM Tris, pH 7.5, and 500 mM NaCl) and then probed overnight at 4°C with FP9-infected cellular lysate. MAb DG11, directed against the 63-kDa protein, was then added as for a conventional Western blotting experiment (control Western blots [WB] are shown for DG11 and DE9). The 63-kDa protein used as a probe in the cellular lysate could be distinguished from that run on the gel and transferred to the membrane by revealing the far-Western blot with MAb DG11, which does not recognize the denatured protein (Table 1; also compare lanes 3 and 4). In contrast, DE9 recognizes both forms of the protein.
Neither labeling in the presence of tunicamycin or monensin nor endo-F treatment altered the apparent molecular weight of the immunoprecipitated 63-kDa protein, suggesting that it was not glycosylated (data not shown).
Interaction of the 63-kDa protein with itself.
In a far-Western blotting experiment, performed in order to identify proteins interacting with the 63-kDa protein, the only interaction of the 63-kDa protein observed was with itself, confirming the tendency of the protein to oligomerize (Fig. 2C).
Subviral protein localization.
The 30- and 35-kDa proteins and 63-kDa protein were detected on each type of particle (IMV, cell-associated enveloped virus [CEV], and EEV) purified through a CsCl gradient as described previously (6) (data not shown). [35S]methionine-labeled, sucrose gradient-purified virus was treated with Triton X-100, and the membranes were separated from the cores by high-speed centrifugation. Immunoprecipitation showed that the 30- and 35-kDa proteins and the 63-kDa protein partitioned to different viral compartments. The 30- and 35-kDa proteins were detected exclusively in the membrane fraction (Fig. 3). The 63-kDa protein was mainly in the insoluble fraction, but a small proportion was also released into the soluble fraction.
Intracellular localization.
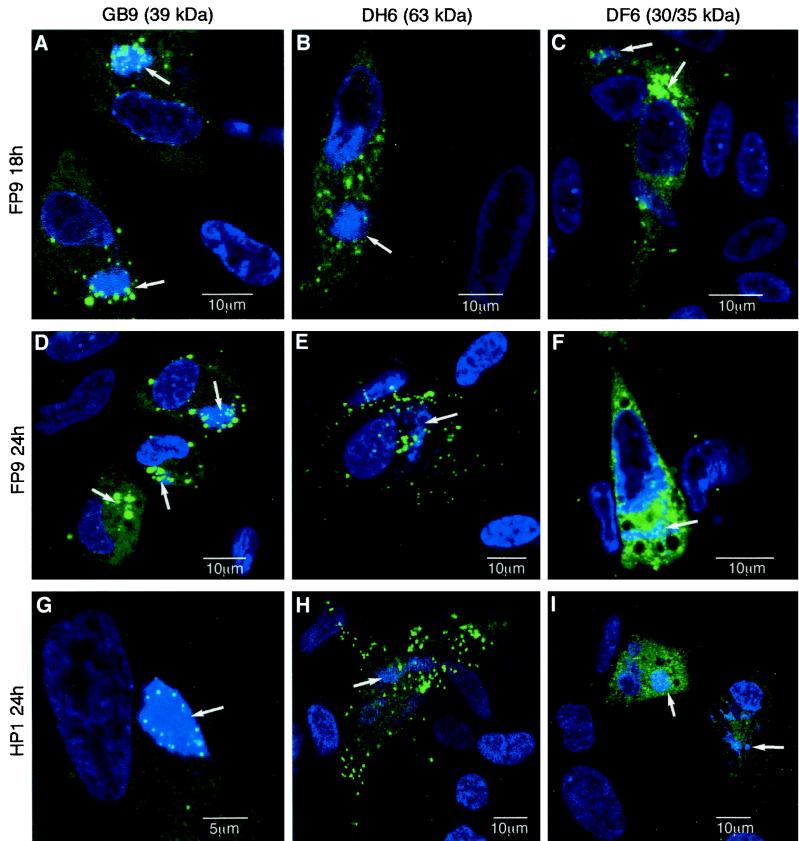
The intracellular localizations of the 63-kDa protein and 30- and 35-kDa proteins were compared, by confocal immunomicroscopy, with that of the FWPV 39-kDa core protein (encoded by the homolog of VACV A4L), which was present in the viral factories (5). Like the 39-kDa protein, the 30- and 35-kDa proteins clearly colocalized with the viral factories at 18 h postinfection (p.i.), but, unlike the 39-kDa protein (which remained closely associated with viral factories), the 30- and 35-kDa proteins became widely distributed throughout the cytoplasm at 24 h p.i. (Fig. 4). These results suggested that the 30- and 35-kDa proteins are part of the IMV membrane, acquired in the factory. Given that the 63-kDa protein seemed to be associated with virus cores, it was a surprise that it did not localize closely with viral factories (Fig. 4). Rather, it was present in diffuse cytoplasmic patches at 18 h p.i. (Fig. 4B) and became concentrated in discrete sharp points, representing virions (many of which were at the cell surface), at 24 h p.i. (Fig. 4E and H). As it was partly removed by detergent treatment (Fig. 3), it is possible that the 63-kDa protein is located on the surface of the IMV, only associating with it after it has moved away from the viral factory. This would be analogous to the addition of the 14-kDa fusion protein to the outside of VACV IMV (32, 41).
FIG. 4.
Merged double-immunofluorescence staining of paraformaldehyde-fixed, Triton X-100-permeabilized, FWPV-infected CEFs at 18 (A to C) or 24 h p.i. (D to I). Tissue culture-adapted FP9 virus (A to F) and virulent progenitor HP1 (G to I) were used. In all cases, DNA was stained with ToPro 3 (blue). Specific viral proteins were stained with MAbs and the Alexa 488 (green) goat anti-mouse immunoglobulin G second antibody. The 39-kDa core protein (encoded by the A4L homolog) was used as a control for colocalization with the viral factory and was detected with MAb GB9 (A, D, and G). The 63-kDa protein was detected with MAb DH6 (B, E, and H), and the 30- and 35-kDa proteins were detected with MAb DF6 (C, F, and I). The pictures are single optical sections recorded on a Leica TCS NT confocal microscope. Scales are as indicated. Image levels were adjusted where necessary by using Photoshop, version 5 (Adobe). Arrows, locations of viral factories, as ascertained in the blue channel (data not shown).
N-terminal sequence of the 35-kDa protein.
The N-terminal sequence APGDKKQIIFVITTI, obtained from the 35-kDa protein immunoprecipitated with MAb DH5 and screened against a partial FP9 sequence database, identified a perfect match with the N terminus of a protein, encoded by open reading frame (ORF) FPV140, which showed significant homology to the VACV p35 immunodominant membrane protein (encoded by gene H3L) and its homologs in other mammalian poxviruses.
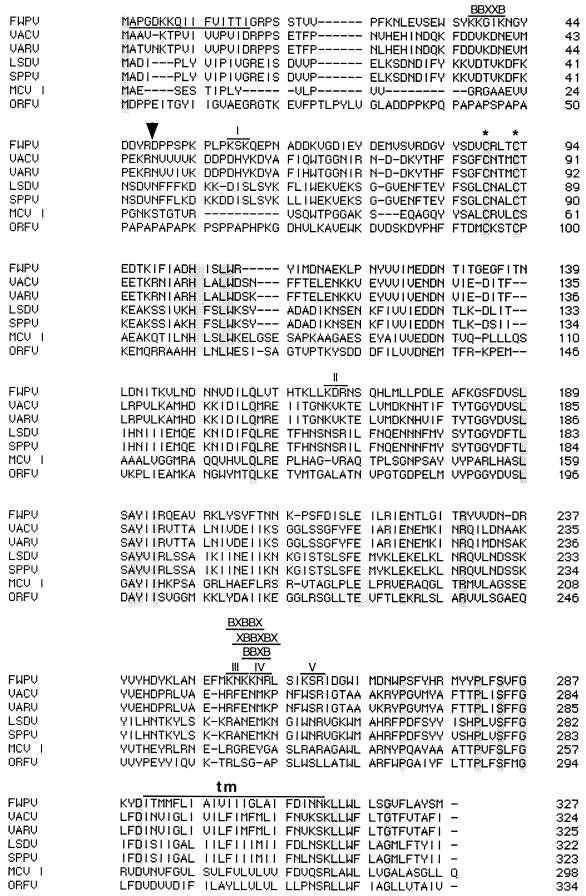
Sequence comparison of H3L homologs.
The sequence of the H3L homolog in FP9 is identical to that of FPV140 in a pathogenic strain of FWPV (1). The amino acid sequence of the H3L protein is remarkably conserved within poxvirus genera (more than 95% identity between orthopoxviruses). The percentage of identity among genera, however, drops to between 20 and 40%. All H3L protein homologs contain only two cysteine residues (equivalent to FWPV residues 89 and 93), both of which are perfectly conserved (Fig. 5), suggesting an important role. There are no conserved glycosylation sites. A short N-terminal hydrophobic sequence and a longer C-terminal hydrophobic region (Fig. 5) are conserved in all homologs. Computer analysis (SignalP, version 1.1; World Wide Web Prediction Server, Center for Biological Sequence Analysis) indicated that the N terminus is extremely unlikely to be a signal sequence, in agreement with our N-terminal sequencing results. The C-terminal hydrophobic domain was predicted to be a transmembrane region (TOPPRED2; Stockholm University Theoretical Chemistry Protein Prediction Servers). Like the VACV H3L protein (24), that of FWPV is rich in basic residues and contains potential heparan sulfate binding sites (Fig. 5).
FIG. 5.
Alignment of poxvirus proteins, encoded by H3L homologs, with the GeneWorks program. Accession numbers are as follows: FWPV, Q9J590; VACV Copenhagen, P20497; VARV Bangladesh-1975, AAA60834; lumpy skin disease virus (LSDV), AAD31773; sheeppox virus (SPPV), AAD31777; MCV I, AAC55212; orf virus strain NZ2 (ORFV), AAC69887. The FWPV peptide identified by N-terminal sequencing is underlined. Arrow, Arg residue where a cleavage might occur to generate the 30-kDa polypeptide; asterisks, conserved Cys residues. The predicted transmembrane region (tm) is indicated above the sequence. Motifs corresponding to potential sites for binding to heparan sulfate are also indicated above the FWPV protein sequence. I to V, five K/R-X-K/R motifs.
Sequence of the 63-kDa protein.
N-terminal sequencing of the immunoprecipitated 63-kDa protein was unsuccessful, suggesting that its N terminus was blocked. The 63-kDa protein, isolated by SDS-PAGE, was therefore digested with CNBr, producing two faint bands (lying between 36 and 50 kDa), from which the peptide sequence SATTASTLY(N/F)N(V/Q) was obtained. The ambiguities in the last three amino acids reflect the low level (∼1 pmol) at which sequencing was performed. A partial match was obtained from our FP9 genomic sequence database with a peptide from a predicted 473-amino acid (aa) protein (molecular mass, 55 kDa). The corresponding peptide (residues 230 to 243) follows a methionine residue (residue 229), consistent with CNBr cleavage (Fig. 6). The next methionine residue is found at position 455. Therefore, a polypeptide of 226 aa with a predicted molecular mass of 26 kDa should have been obtained after digestion. Thus the peptide, like the protein, migrated with a higher apparent mass, possibly due to the presence of 12 consecutive Asp residues at positions 369 to 380. A BLAST search with the ORF encoding this 473-aa protein identified it as equivalent to FPV191, predicted to encode a homolog of A-type inclusion (ATI) proteins in the pathogenic FWPV strain (1).
FIG. 6.
Sequence of the FWPV FP9 63-kDa protein. The peptide best match is overlined (single line). It follows a methionine residue (boldface), consistent with CNBr cleavage (arrowhead, position of the cleavage site). Compared to the homologous protein (encoded by FPV191) from a pathogenic strain of FWPV (1), three substitutions were observed (73S-Y, 220R-I, and 436V-L) (inverted triangles). The aspartate repeats, 12 residues in FP9 but 13 in the pathogenic strain sequenced by Afonso et al. (1), are doubly underlined. The RGDLXXL integrin binding motif is underlined. Leucine residues forming heptad repeats at the C terminus of the protein are in boldface. The potential transmembrane domain (residues 117 to 133) is underlined with a dashed line.
Sequence analysis of predicted FWPV ATI proteins.
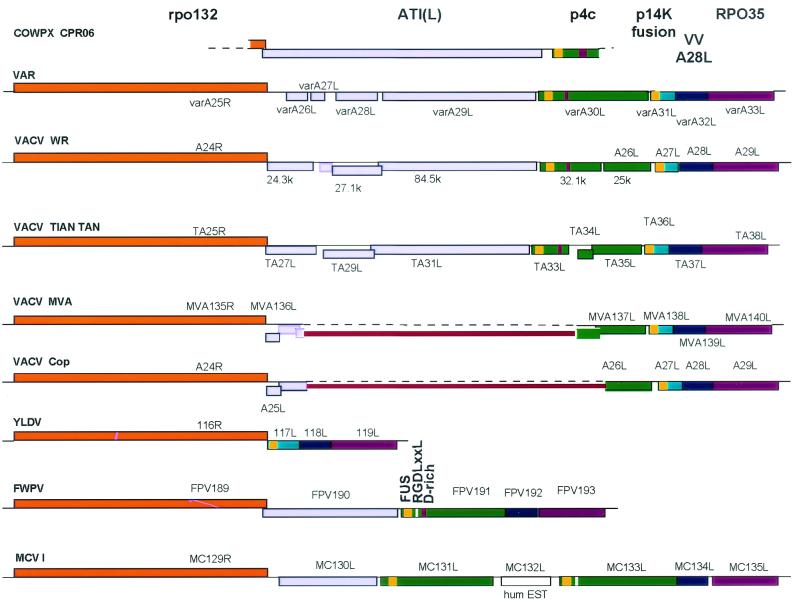
Two adjacent FWPV genes (FPV190 and -191) have been predicted to encode ATI homologs (1) (Fig. 7). They are flanked by FPV189 and FPV192, encoding homologs of VACV A24R (RNA polymerase 132-kDa subunit) and A28L, respectively.
FIG. 7.
Comparison of the organization of the region between the VACV A24R and A29L homologs in different poxvirus genomes. Maps were drawn to scale from nucleotide sequences found under the following accession numbers: COWPX CPR06, D00319 and X06343; VARV Bangladesh-1975, L22579; VACV WR, M37415 and M61187; VACV Tian Tan, AF095689; VACV MVA, U94848; VACV Copenhagen, M35027; Yaba-like disease virus (YLDV), AJ293568; FWPV, AF198100; MCV I, U60315. Leporipoxvirus (NC 001266 and NC 001132), capripoxvirus (NC 003027), and suipoxvirus (AF410153) have the same organization as YLDV, with neither A25L nor A26L homologs. For all viruses, blocks above the sequence line represent those ORFs translated left to right, blocks below the line represent ORFs translated right to left. The fill colors of the ORFs demonstrate equivalence. The disrupted ATI ORFs are represented by the resulting smaller ORFs; their overlap indicates that there are frameshifts. ORFs bound by a black line have been described in the literature or in the databases; those bound by colored lines can be extracted from the published sequence data. Open-ended blocks indicate incomplete sequence data (cowpox virus) or fused ORFs (Copenhagen and MVA). Thick red lines, ORFs fused as a result of large deletions from Copenhagen and MVA sequences; yellow blocks at the C termini of p4c-like and p14k-like proteins, locations of the Vac_fusion domain; magenta blocks near the C termini of most p4c-like proteins, locations of the aspartate-rich domains; white blocks near the C termini of proteins encoded by FPV191 and MC133L, locations of the RGDLGSL motif. VV, vaccinia virus.
FPV190 encodes a 620-aa protein (molecular mass, 75 kDa, pI 6.3); the protein's N-terminal sequence is MGXXX(S/T/A/C/N), and so it is possibly myristylated. By BLAST, the protein encoded by FPV190 was similar to three related molluscum contagiosum virus I (MCV I) proteins (encoded by MC130L, MC133L, and MC131L, in that order [36, 37]), to the FPV191 protein, and to various orthopoxvirus proteins that appear to be truncated versions of the cowpox virus ATI protein (Fig. 7). The FPV190 protein is about one-half the size of the cowpox virus ATI protein (P16602; 1,284 aa), the only known intact orthopoxvirus ATI protein. The FPV190 protein shows homology with cowpox virus ATI for the first 350 residues. There is a break in homology, and then residues 407 to 599 of the FPV190 protein show homology with cowpox virus ATI residues 936 to 1127. However, the latter region of the FPV190 protein also shows similarity to other parts of cowpox virus ATI, indicating the presence of repeats in cowpox virus ATI, which was confirmed by dot matrix analysis (data not shown). In FP9, FPV190 is heavily disrupted by three frameshift mutations (after codons for aa 409, 510, and 606; S. M. Laidlaw and M. A. Skinner, unpublished data).
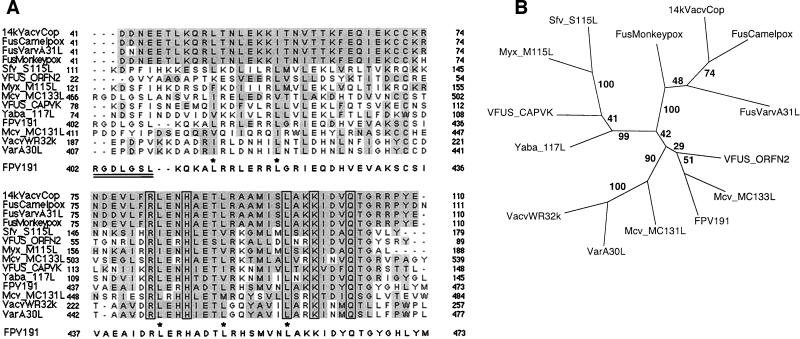
The 63-kDa protein of FP9 has 99% amino acid identity with that encoded by the published FPV191 sequence due to three substitutions and one deletion (Fig. 6). The substitutions occurred during passage of FP9 from HP1, but the deletion of 1 Asp residue from a run of 13 in the FPV191 protein differentiates the U.S. virus from the European viruses (S. M. Laidlaw and M. A. Skinner, unpublished data). A potential transmembrane helix was predicted between residues 117 and 133, but no N-terminal signal peptide sequence is predicted. The polyaspartate region (Fig. 6 and 7) confers a highly acidic character to the protein (pI 5.1). A BLAST search revealed closest similarity with proteins encoded by MC133L (28% amino acid identity over the whole length of the protein), MC131L, variola virus (VARV) A30L (variola virus major Bangladesh-1975; 24% amino acid identity over the whole protein), FPV190, and MC130L and with the VACV WR 25-kDa protein (according to the nomenclature of reference 2) (Fig. 7). The C-terminal region of the FPV191 protein (residues 429 to 467) shows a surprising similarity (53% amino acid identity over 39 residues) to the C-terminal region (residues 67 to 105) of the 14-kDa IMV fusion protein (encoded by VACV A27L; Fig. 8A).
FIG. 8.
(A) Alignment of poxvirus proteins showing a high homology to the C-terminal region of the 14-kDa fusion protein encoded by VACV A27L. Proteins containing a region with similarity to the Vac_fusion domain (at that time defined as residues 71 to 104) of the VACV p14k fusion protein (the A27L protein) were identified by BLASTP. Extended sequences were aligned with DBCLUSTAL. Two gaps were introduced in the FPV191 protein sequence after the RGDLGSL motif, so that it aligned with the same sequence in the MC133L protein (note that the RGD triplet immediately precedes the sequences aligned). The accession numbers used were as follows: 14kVacvCop, p20535 (representing all VACV fusion proteins); FusCamelpox, CAA52999; FusVarvA31L, P33816; FusMonkeypox, S37281; Sfv_S115L, AAF17998; VFUS_ORFN2, P26654; Myx_M115L, AAF15003; Mcv_MC133L, AAC55261; VFUS_CAPVK, P16717; Yaba_117L, CAC21355; FPV191, AAF44535; Mcv_MC131L, AAC55259; VacvWR32k, B40825 (the same sequence as Tian Tan TA33L); VarA30L, T28571. The RGDLXXL motif in the MC133L and FPV191 proteins is doubly underlined. Asterisks, leucine residues in FPV191 protein involved in the zipper motif. (B) Bootstrap analysis of proteins containing the Vac_fusion domain. Phylogenetic comparison was performed on the extended region (equivalent to residues 41 to 110 of p14k), as aligned in panel A, with the PHYLIP, version 3.5, programs (13) SEQBOOT (with 100 replicates), PROTDIST, and NEIGHBOR. A consensus tree was calculated with CONSENSE and drawn with TREEVIEW (28). The tree is unrooted. The numbers at the forks indicate the numbers of times the group of species beyond the fork occurred among the trees, out of 100 trees.
Knockout analysis of FPV191.
We attempted to isolate knockout viruses, by transient dominant selection, with bp 52 to 1372 (out of 1,422 bp) of the FPV191 ORF deleted, leading to truncation of the encoded protein after aa 17. Although we could isolate unstable gpt+ viruses carrying both the wild-type form of FPV191 and the deleted form, these always resolved to give only wild-type viruses (data not shown). Of the flanking genes, FPV190 is clearly nonessential but little is known about FPV192 and the other poxvirus homologs of VACV A28L. There is as yet no inducible knockout system for FWPV, so we cannot rule out inadvertently lethal effects within FPV192. However, we were also unable to isolate recombinant viruses in which bp 557 to 599 of the FPV191 ORF had been directly replaced by a lacZ/gpt selection cassette, leading to truncation of the encoded protein after amino acid 186 (data not shown). That these two different strategies both failed to produce knockout viruses indicates that FPV191 is most likely essential.
DISCUSSION
High-avidity MAbs allowed us to identify three immunodominant FP9 proteins: the 39-kDa core protein (encoded by FPV168), previously identified as the homolog of the VACV A4L protein (5), a 35-kDa protein (encoded by FPV140) that we identified as the homolog of the VACV H3L protein, and a 63-kDa protein (encoded by FPV191) homologous to proteins from two human pathogens (encoded by MCV MC133L and VARV A30L) but with no intact homolog in VACV (Fig. 7).
Like its VACV homolog (encoded by H3L), the FPV140 product is a nonglycosylated IMV membrane protein that colocalizes with viral factories. It has no signal sequence, so is not translocated into the endoplasmic reticulum, but it has a C-terminal hydrophobic sequence. In the VACV H3L protein, this hydrophobic domain is responsible for its posttranslational insertion into the IMV membrane (9). Two conserved cysteine residues (also conserved in FWPV; Fig. 5) are probably essential for the correct folding of the protein. The VACV H3L protein also has multiple forms: a major 37-kDa protein, a minor 34-kDa protein (possibly a compact form of VP37K due to intramolecular disulfide bridges), and a 30-kDa protein, which corresponds to a proteolytic product of VP37K (residues 48 to 324) (44). An Arg residue is present at position 48 of the FWPV protein, suggesting that the FWPV 30-kDa protein could be generated similarly (Fig. 5).
The two glycosaminoglycan-binding motifs (X-B-B-B-X-X-B-X and X-B-B-X-B-X, where B is a basic residue [7]) reported in the VACV H3L protein (24) are not conserved in the FPV140 protein. However, the FWPV homolog contains an X-B-B-X-B-X motif in the C-terminal part of the protein (NKKNRL, residues 252 to 257; Fig. 5). This sequence includes a B-B-X-B motif similar to the identified principal site for heparin binding in the RANTES chemokine (31). Other motifs (such as B-Xm-B-B-X and B-Xm-B-B-B-Xm, where m usually equals 1 or 2 and is never larger than 7) have been found in heparin or heparan sulfate binding proteins (14). One sequence of the first type is found in the FPV140 protein (KNKKN, residues 251 to 255) overlapping the previously mentioned X-B-B-X-B-X motif (Fig. 5). Another putative heparin binding motif is K/R-X-K/R, described in the VACV complement control protein (encoded by VACV C3L) (40). Five of these motifs (KSK, KDR, KNK, KNR, and KSR) can be found in the FPV140 protein (Fig. 5). Three of them are part of the sequence KNKKNRLSIKSR (residues 251 to 262), which accommodates all four kinds of motif (Fig. 5) and which, therefore, represents a strong candidate for a heparan sulfate binding site. This suggests that the FPV140 protein, like its VACV homolog (24), could function in IMV attachment to cells. Interestingly, FWPV has no equivalent of the other two VACV proteins involved in IMV binding (encoded by D8L and A27L).
The FP9 63-kDa protein formed dimers and oligomers, was present in IMV, CEV, and EEV, and remained partially associated with virion cores after detergent treatment. It did not colocalize with the viral factories but apparently associated with the IMVs during their maturation. It seems to be essential, as we have been unable to isolate a knockout virus either by transient dominant selection or by direct insertion mutagenesis. Finally, internal peptide sequencing identified the protein as the FPV191 gene product, predicted to be an ATI-like protein. However, the apparently essential nature of this protein represents a novel feature among ATI-like proteins.
ATI bodies are cytoplasmic eosinophilic bodies consisting of dense matrices, and they usually surround many embedded IMV particles. They can be found in cells infected by some poxviruses, such as cowpox virus (10), ectromelia virus (25), CNPV (34), raccoon poxvirus (29), and FWPV (43, 47). Other poxviruses, including VACV, rabbitpox virus, and Shope fibroma virus, do not form ATIs (19). Viruses deficient in the so-called virus occlusion protein, the p4c protein (39), encoded by intact VACV A26L (see below) (D. Pickup, personal communication), may produce ATIs that do not contain any virions.
The cowpox virus ATI protein is a 160-kDa protein (20, 29) (Fig. 7). In most strains of VACV as well as in VARV, camelpox virus, and monkeypox virus the ATI protein is truncated as a result of frameshift mutations (2, 26). This gives rise to several ORFs whose products include, in some strains, a highly immunogenic truncated product that is unable to form typical ATIs but that cross-reacts with antiserum raised against the 160-kDa protein (29).
Characteristics reported here for the FP9 63-kDa protein are inconsistent with previous observations of ATI proteins in cowpox virus or in VACV. First of all, we found that the 63-kDa protein appears to be essential for virus replication whereas the cowpox virus and VACV proteins are nonessential proteins. Moreover, the 63-kDa protein is a component of IMV, CEV, and EEV particles whereas the cowpox virus ATI protein was not found in purified virions (20, 29). The truncated IHD-J VACV ATI protein has been shown to be present on the surface of IMV particles only and to be a specific marker of a fraction of IMVs that are destined to be included in the ATIs (48). As occlusion protein p4c has also been detected only on IMVs and late intermediate immature virus particles (35, 48) and as p4c is able to coprecipitate the ATI protein (48), an interaction between the two proteins could explain the detection of the ATI protein on the IMV.
Among poxviruses, the genomic locus between homologs of VACV genes encoding 132- and 35-kDa RNA polymerase subunits (A24R and A29L in VACV Copenhagen) appears unstable and variable, with a wide spectrum of genotypes (Fig. 7). At one end of the spectrum, strains of cowpox virus that make virus-containing ATIs have, at this locus, genes encoding intact ATI and p4c (as well as VACV A27L and A28L homologs). In contrast, leporipoxviruses and yatapoxviruses have, at this locus, only A27L and A28L homologs (22, 51) (Fig. 7). Between these two extremes, the ATI genes of VACV and VARV are disrupted by numerous frameshift mutations and are substantially deleted from VACV strains Copenhagen and MVA (2, 3) (Fig. 7). The gene (VARV A30L) encoding p4c is intact in VARV (major and minor strains) but is disrupted by frameshifts and partial deletions in VACV (Fig. 7), except for the WR strain used by Sarov and Joklik (35) and for the IHD-J strain, both of which show an occlusion-positive phenotype (39, 48). As a consequence of this genome variability and also due to sequence redundancy in this region of the genome, it proved difficult to assign FPV191. Analysis and gene assignment in this region are further complicated by the fact that VACV Copenhagen gene A26L, as the result of a 4-kbp deletion relative to that of WR (2), actually encodes a protein formed by fusion between the N terminus of p4c and the C terminus of the ATI protein (Fig. 7). This leads to a confusing and complicated situation; we hope that Fig. 7 will serve as an aid in understanding the organization of this locus across the poxviruses.
The FP9 63-kDa protein (encoded by FPV191) shows 24% amino acid identity with the VARV A30L protein (varA30L) and has a similar hydrophobicity profile but is most closely related to the MCV protein encoded by MC133L. Interestingly, FWPV and MCV are the only known chordopoxviruses to lack homologs of the VACV A27L protein, the 14-kDa fusion protein which is a multifunctional IMV surface protein (1, 37, 38). Homologs of the VACV A27L protein are remarkably well conserved within the Orthopoxvirus genus (93 to 99% amino acid identity). Conservation between A27L proteins from the orthopoxviruses and related proteins from the Leporipoxvirus, Capripoxvirus, Parapoxvirus, and Yatapoxvirus genera is restricted to the C-terminal region, defined in the Conserved Domain Database as pfam02346 Vac_fusion (Fig. 8A). Such nomenclature is somewhat misleading, as this domain does not actually include that part of the A27L protein responsible for fusion (VACV Copenhagen residues 29 to 43) (49). The parts of A27L protein actually responsible for fusion, EEV formation (residues 1 to 29) (49), and the binding of IMV to the cell (residues 21 to 33) (17, 49) are not conserved in the FPV191, MC133L, and varA30L proteins. It is the C-terminal Vac_fusion conserved domain that is found at the C terminus of the FWPV FPV191 protein and that is also present in the MCV MC133L protein, as well as the MC131L, varA30L, and p4c proteins (see below). This domain in the VACV A27L protein overlaps a short leucine zipper motif (leucines 47 and 54), which resides in the triple-coiled-coil helical region (residues 44 to 72) described by Vazquez et al. (50). It also overlaps another leucine zipper (leucines 82, 89, and 96) within the region (residues 75 to 110) responsible for the interaction between the A27L protein and the 21-kDa protein (the A17L protein), anchoring the A27L protein on the surface of the IMV (33, 50). Although these two leucine zippers are conserved in the FPV191, MC133L, and varA30L proteins, the propensity of the first region to form coiled coils (as determined with MacStripe, version 2.0b1; http://www.york.ac.uk/depts/biol/units/coils/coilcoil.html) is only about 0.1 in the FWPV and MCV proteins and zero in varA30L protein. The significance of the second leucine zipper domain in the FPV191 protein is not clear. The leucine residue at position 89 in the VACV A27L protein, which plays a critical role in both the association of homotrimers to form hexamers at high concentrations and the interaction of the A27L protein with the A17L protein (50), is conserved in the FPV191 protein and varA30L (Fig. 8A). An A17L homolog exists in FWPV (FPV182, encoding a predicted 22-kDa product), but the only interaction that we detected by far-Western blotting with the 63-kDa protein was with itself (Fig. 2C). The 63-kDa protein forms covalently linked dimers (Fig. 2) but also higher-molecular-weight complexes (Fig. 2B) to which leucine zipper interactions could contribute.
We initially surmised that the presence of the Vac_fusion domain in proteins encoded by FPV191 and MC133L indicated that these genes had arisen by recombination of sequence encoding ATI-like protein into an ancestral homolog encoding a fusion protein. While this is a plausible explanation, the situation became complicated when we discovered a Vac_fusion domain at the C terminus of the p4c-like predicted protein, encoded by an ORF in WR (2). Further analysis revealed that the domain was encoded by all the ORFs encoding p4c-like proteins (Fig. 7). Phylogenetic analysis using either the isolated fusion domain or especially the intact proteins shows that they cluster into either A27L protein-like sequences (with strong groups for orthopoxvirus fusion proteins and for the variant fusion proteins found in Yaba-like disease virus, capripoxvirus, and leporipoxvirus) or into p4c-like sequences (Fig. 8B). The FWPV FPV191 protein, the MCV MC133L protein, and the orf virus fusion protein belong to a branch of the p4c arm (Fig. 8B).
Another feature, apart from the Vac_fusion domain, shared by the FPV191 protein and p4c (but not the MC133L protein) is the C terminal Asp-rich region, also present in the cowpox virus protein encoded by the ORF transcriptionally upstream of the ATI gene. Although the number of Asp residues varies, the domain is also present in varA30L and in proteins derived from the C terminus of p4c in two strains of VACV, WR (32.1 kDa) (2) and Tian Tan (encoded by TA33L; accession no. AAF34028). Interestingly, the FPV191 and MC133L proteins but not varA30L share an RGD integrin binding motif (residues 402 to 404 in the FPV191 protein and residues 466 to 468 in the MC133L protein) N-terminal of the first conserved leucine zipper domain and C-terminal of the Asp-rich domain in the FPV191 protein (Fig. 6 and 7). The context of the RGD motif, RGDLGSL, is conserved in the FPV191 and MC133L proteins, suggesting that it may be of some importance. The context is identical to that found in foot-and-mouth disease virus, where it constitutes a ligand for the αvβ6 integrin (18, 21).
In conclusion, the 63-kDa protein encoded by FPV191 (like that encoded by MC133L) appears to have features in common with both VACV p4c and the A27L protein. Although all these proteins have a Vac_fusion domain, they are clearly distinct, as shown by the bootstrapped tree analysis (Fig. 8B). The FPV191 and MC133L proteins lie somewhere between the p4c cluster and the A27L protein cluster, possibly indicating that they have inherited functions of each. This is particularly interesting in view of the fact that FWPV and MCV are the only known chordopoxviruses to lack obvious homologs of the VACV A27L protein. Absence of this protein may account for the production of FWPV EEV by budding (6, 16), but it is conceivable that the FPV191 protein undertakes some of the functions of the A27L protein. This possibility becomes more likely with our demonstration that the FPV191 protein appears to be essential.
It is not clear how the FPV191 protein associates with the virion or where, but in some ways the situation resembles that for p4c and the A27L protein. They do not colocalize with factories but bind only after formation of immature virus (35, 39, 41). The FPV191 protein is only partly solubilized by Triton X-100 alone, a situation similar to that for the VACV A27L protein, which can be solubilized completely by NP-40 only in the presence of reducing agents (36). As the leucine zipper regions are conserved, it is likely that the FPV191 protein, like the A27L protein (33, 50), binds the equivalent of the A17L protein, though we have not been able to demonstrate such an interaction. As the A17L protein is detachable from the IMV only in the presence of reducing agents (4, 36), any protein associated with it would show the same pattern. The predicted potential transmembrane domain of the FPV191 protein might also play a role in its association with the IMV membrane. This needs to be investigated.
The evolution of this part of the genome is fascinating, given the presence in the orthopoxviruses of conserved Vac_fusion domains in two adjacent genes and the presence of two genes encoding ATI-like proteins in FWPV (or more in the case of MCV). It will be interesting to see if the gene order in FWPV is conserved in other avipoxviruses. We had surmised that the FPV191 protein might be a virion occlusion protein, and this remains to be tested, but its presence on all types of virions, the fact that it appears to be essential, and the lack of an A27L homolog in FWPV raise the possibility that it has other functions. The similarity between FPV191 and MC133L, together with the absence of A27L from these viruses, is also intriguing.
Acknowledgments
This work was supported initially by an E.U. Biotech I fellowship and subsequently by the BBSRC.
We are grateful to David Pickup for helpful comments, support, and access to unpublished data. We thank C. P. Czerny for providing the 3D9 MAb.
REFERENCES
- 1.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, G. F. Kutish, and D. L. Rock. 2000. The genome of fowlpox virus. J. Virol. 74:3815-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amegadzie, B. Y., J. R. Sisler, and B. Moss. 1992. Frame-shift mutations within the vaccinia virus A-type inclusion protein gene. Virology 186:777-782. [DOI] [PubMed] [Google Scholar]
- 3.Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. [DOI] [PubMed] [Google Scholar]
- 4.Betakova, T., and B. Moss. 2000. Disulfide bonds and membrane topology of the vaccinia virus A17L envelope protein. J. Virol. 74:2438-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulanger, D., P. Green, T. Smith, C. P. Czerny, and M. A. Skinner. 1998. The 131-amino-acid repeat region of the essential 39-kilodalton core protein of fowlpox virus FP9, equivalent to vaccinia virus A4L protein, is nonessential and highly immunogenic. J. Virol. 72:170-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulanger, D., T. Smith, and M. A. Skinner. 2000. Morphogenesis and release of fowlpox virus. J. Gen. Virol. 81:675-687. [DOI] [PubMed] [Google Scholar]
- 7.Cardin, A. D., and H. J. Weintraub. 1989. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9:21-32. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain, J. P. 1979. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal. Biochem. 98:132-135. [DOI] [PubMed] [Google Scholar]
- 9.da Fonseca, F. G., E. J. Wolffe, A. Weisberg, and B. Moss. 2000. Characterization of the vaccinia virus H3L envelope protein: topology and posttranslational membrane insertion via the C-terminal hydrophobic tail. J. Virol. 74:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downie, A. W. 1939. A study of the lesions produced experimentally by cowpox virus. J. Pathol. Bacteriol. 48:361-379. [Google Scholar]
- 11.Dunbar, B., and S. B. Wilson. 1994. A buffer exchange procedure giving enhanced resolution to polyacrylamide gels prerun for protein sequencing. Anal. Biochem. 216:227-228. [DOI] [PubMed] [Google Scholar]
- 12.Falkner, F. G., and B. Moss. 1990. Transient dominant selection of recombinant vaccinia viruses. J. Virol. 64:3108-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felsenstein, J. 1989. PHYLIP—phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 14.Fromm, J. R., R. E. Hileman, E. E. Caldwell, J. M. Weiler, and R. J. Linhardt. 1997. Pattern and spacing of basic amino acids in heparin binding sites. Arch. Biochem. Biophys. 343:92-100. [DOI] [PubMed] [Google Scholar]
- 15.Goebel, S. J., G. P. Johnson, M. E. Perkus, S. W. Davis, J. P. Winslow, and E. Paoletti. 1990. The complete DNA-sequence of vaccinia virus. Virology 179:247-266 and 517-563. [DOI] [PubMed] [Google Scholar]
- 16.Hatano, Y., M. Yoshida, F. Uno, S. Yoshida, N. Osafune, K. Ono, M. Yamada, and S. Nii. 2001. Budding of fowlpox and pigeonpox viruses at the surface of infected cells. J. Electron Microsc. 50:113-124. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao, J. C., C. S. Chung, and W. Chang. 1998. Cell surface proteoglycans are necessary for A27L protein-mediated cell fusion: identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding domain. J. Virol. 72:8374-8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. Q. King. 2000. The epithelial integrin αvβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 74:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato, S., M. Takahashi, S. Kameyama, and J. Kamahora. 1959. A study on the morphological and cyto-immunological relationship between the inclusions of variola, cowpox, rabbitpox, vaccinia (variola origin) and vaccinia IHD and a consideration of the term “Guarnieri body.” Biken J. 2:353-363. [Google Scholar]
- 20.Kitamoto, N., S. Tanimoto, K. Hiroi, T. Tanaka, H. Miyamoto, N. Wakamiya, K. Ikuta, S. Ueda, and S. Kato. 1986. Polypeptide analysis with monoclonal antibodies of A type inclusion bodies induced by cowpox virus. Arch. Virol. 89:15-28. [DOI] [PubMed] [Google Scholar]
- 21.Kraft, S., B. Diefenbach, R. Mehta, A. Jonczyk, G. A. Luckenbach, and S. L. Goodman. 1999. Definition of an unexpected ligand recognition motif for αvβ6 integrin. J. Biol. Chem. 274:1979-1985. [DOI] [PubMed] [Google Scholar]
- 22.Lee, H. J., K. Essani, and G. L. Smith. 2001. The genome sequence of Yaba-like disease virus, a yatapoxvirus. Virology 281:170-192. [DOI] [PubMed] [Google Scholar]
- 23.Limbach, K. J., and E. Paoletti. 1996. Non-replicating expression vectors: applications in vaccine development and gene therapy. Epidemiol. Infect. 116:241-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, C. L., C. S. Chung, H. G. Heine, and W. Chang. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74:3353-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchal, J. 1930. Infectious ectromelia. A hitherto undescribed virus disease in mice. J. Pathol. Bacteriol. 33:713-728. [Google Scholar]
- 26.Meyer, H., and H. J. Rziha. 1993. Characterization of the gene encoding the A-type inclusion protein of camelpox virus and sequence comparison with other orthopoxviruses. J. Gen. Virol. 74:1679-1684. [DOI] [PubMed] [Google Scholar]
- 27.Mockett, B., M. M. Binns, M. E. G. Boursnell, and M. A. Skinner. 1992. Comparison of the locations of homologous fowlpox and vaccinia virus genes reveals major genome reorganization. J. Gen. Virol. 73:2661-2668. [DOI] [PubMed] [Google Scholar]
- 28.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 29.Patel, D. D., D. J. Pickup, and W. K. Joklik. 1986. Isolation of cowpox virus A-type inclusions and characterization of their major protein component. Virology 149:174-189. [DOI] [PubMed] [Google Scholar]
- 30.Perkus, M. E., J. Tartaglia, and E. Paoletti. 1995. Poxvirus-based vaccine candidates for cancer, AIDS, and other infectious-diseases. J. Leukoc. Biol. 58:1-13. [DOI] [PubMed] [Google Scholar]
- 31.Proudfoot, A. E., S. Fritchley, F. Borlat, J. P. Shaw, F. Vilbois, C. Zwahlen, A. Trkola, D. Marchant, P. R. Clapham, and T. N. Wells. 2001. The BBXB motif of RANTES is the principal site for heparin binding and controls receptor selectivity. J. Biol. Chem. 276:10620-10626. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez, D., C. Risco, J. R. Rodriguez, J. L. Carrascosa, and M. Esteban. 1996. Inducible expression of the vaccinia virus A17L gene provides a synchronized system to monitor sorting of viral proteins during morphogenesis. J. Virol. 70:7641-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez, D., J. R. Rodriguez, and M. Esteban. 1993. The vaccinia virus 14-kilodalton fusion protein forms a stable complex with the processed protein encoded by the vaccinia virus A17L gene. J. Virol. 67:3435-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadasiv, E. C., P. W. Chang, and G. Gulka. 1985. Morphogenesis of canary poxvirus and its entrance into inclusion bodies. Am. J. Vet. Res. 46:529-535. [PubMed] [Google Scholar]
- 35.Sarov, I., and W. K. Joklik. 1973. Isolation and characterization of intermediates in vaccinia virus morphogenesis. Virology 52:223-233. [DOI] [PubMed] [Google Scholar]
- 36.Sarov, I., and W. K. Joklik. 1972. Studies on the nature and location of the capsid polypeptides of vaccinia virions. Virology 50:579-592. [DOI] [PubMed] [Google Scholar]
- 37.Senkevich, T. G., J. J. Bugert, J. R. Sisler, E. V. Koonin, G. Darai, and B. Moss. 1996. Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes. Science 273:813-816. [DOI] [PubMed] [Google Scholar]
- 38.Senkevich, T. G., E. V. Koonin, J. J. Bugert, G. Darai, and B. Moss. 1997. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology 233:19-42. [DOI] [PubMed] [Google Scholar]
- 39.Shida, H., K. Tanabe, and S. Matsumoto. 1977. Mechanism of virus occlusion into A-type inclusion during poxvirus infection. Virology 76:217-233. [DOI] [PubMed] [Google Scholar]
- 40.Smith, S. A., N. P. Mullin, J. Parkinson, S. N. Shchelkunov, A. V. Totmenin, V. N. Loparev, R. Srisatjaluk, D. N. Reynolds, K. L. Keeling, D. E. Justus, P. N. Barlow, and G. J. Kotwal. 2000. Conserved surface-exposed K/R-X-K/R motifs and net positive charge on poxvirus complement control proteins serve as putative heparin binding sites and contribute to inhibition of molecular interactions with human endothelial cells: a novel mechanism for evasion of host defense. J. Virol. 74:5659-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sodeik, B., S. Cudmore, M. Ericsson, M. Esteban, E. G. Niles, and G. Griffiths. 1995. Assembly of vaccinia virus: incorporation of p14 and p32 into the membrane of the intracellular mature virus. J. Virol. 69:3560-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somogyi, P., J. Frazier, and M. A. Skinner. 1993. Fowlpox virus host range restriction: gene expression, DNA replication, and morphogenesis in nonpermissive mammalian cells. Virology 197:439-444. [DOI] [PubMed] [Google Scholar]
- 43.Tajima, M., and T. Ushijima. 1966. Electron microscopy of avian pox viruses with special reference to the significance of inclusion bodies in viral replication. Nippon Juigaku Zasshi 28:107-118. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi, T., M. Oie, and Y. Ichihashi. 1994. N-terminal amino-acid-sequences of vaccinia virus structural proteins. Virology 202:844-852. [DOI] [PubMed] [Google Scholar]
- 45.Taylor, J., and E. Paoletti. 1988. Fowlpox virus as a vector in non-avian species. Vaccine 6:466-468. [DOI] [PubMed] [Google Scholar]
- 46.Taylor, J., R. Weinberg, B. Languet, P. Desmettre, and E. Paoletti. 1988. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine 6:497-503. [DOI] [PubMed] [Google Scholar]
- 47.Thiele, J., H. Kiel, and H. D. Adolphs. 1979. Avian pox virus. An ultrastructural study on a cherrug falcon. Brief report. Arch. Virol. 62:77-82. [DOI] [PubMed] [Google Scholar]
- 48.Ulaeto, D., D. Grosenbach, and D. E. Hruby. 1996. The vaccinia virus 4c and A-type inclusion proteins are specific markers for the intracellular mature virus particle. J. Virol. 70:3372-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vazquez, M. I., and M. Esteban. 1999. Identification of functional domains in the 14-kilodalton envelope protein (A27L) of vaccinia virus. J. Virol. 73:9098-9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vazquez, M. I., G. Rivas, D. Cregut, L. Serrano, and M. Esteban. 1998. The vaccinia virus 14-kilodalton (A27L) fusion protein forms a triple coiled-coil structure and interacts with the 21-kilodalton (A17L) virus membrane protein through a C-terminal alpha-helix. J. Virol. 72:10126-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willer, D. O., G. McFadden, and D. H. Evans. 1999. The complete genome sequence of Shope (rabbit) fibroma virus. Virology 264:319-343. [DOI] [PubMed] [Google Scholar]