Abstract
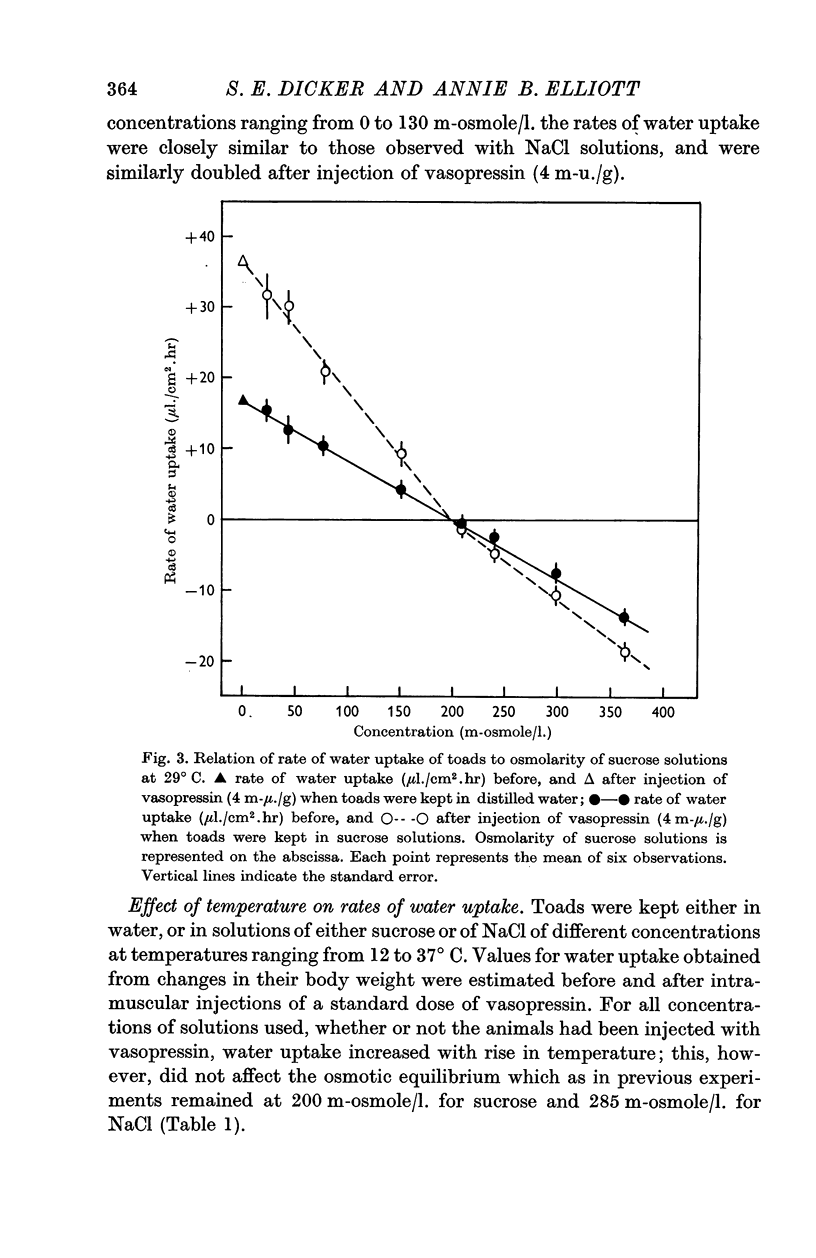
1. The rate of water uptake across the skin was studied in the live toad, Bufo melanostictus. When toads were kept in distilled water at 29° C the uptake of water amounted to 16·9 ± 1·3 μl./cm2/hr; when bathed in sucrose or urea solutions, the water uptake diminished with increasing osmotic pressure. There was no water uptake observed when toads were kept in 200 m-osmolar sucrose or urea.
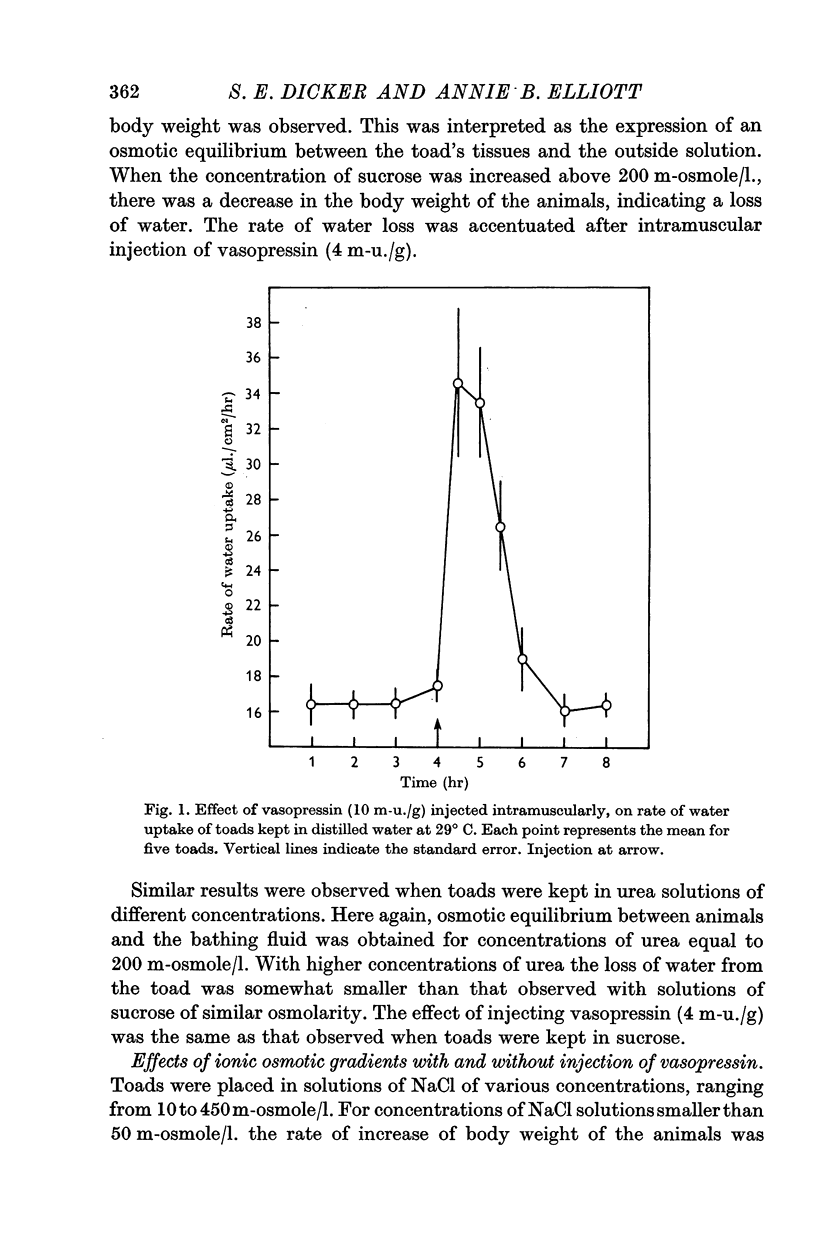
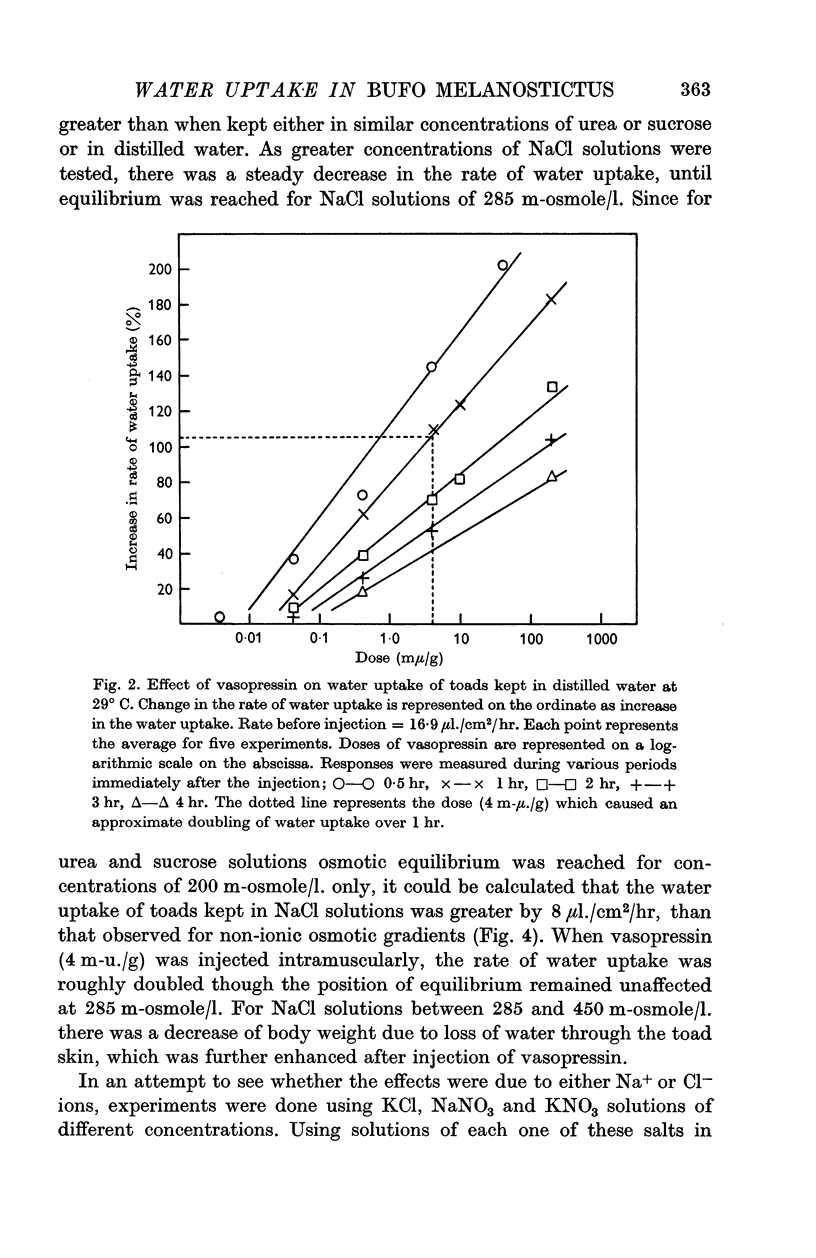
2. Intramuscular injections of vasopressin increased the rate of water uptake from distilled water. There was a good relation between doses and responses over various time intervals. A dose of 4 m-u. vasopressin/g body wt. doubled the rate of water uptake over a period of 1 hr. The same dose of vasopressin doubled the rate of water uptake when the toads were kept in solutions of sucrose or urea of different osmolarity.
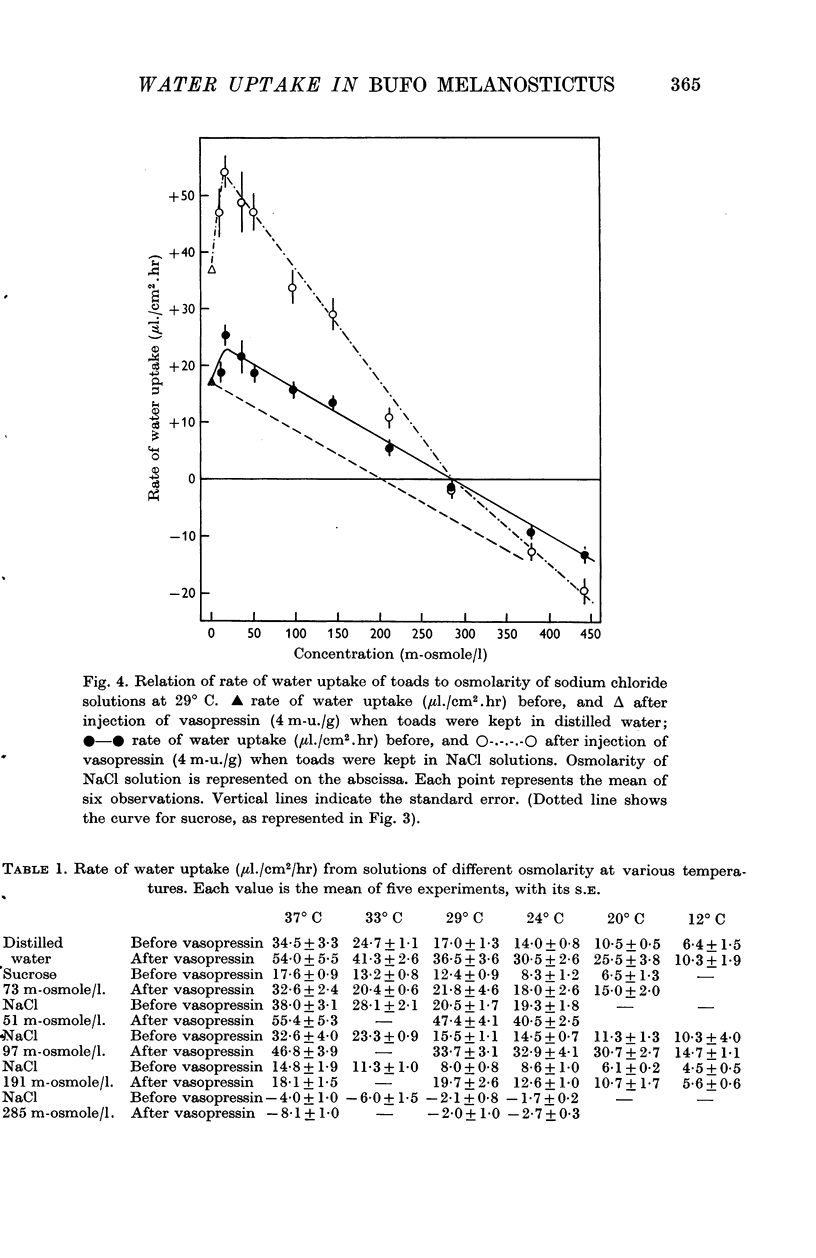
3. The rate of water uptake when the toads were bathed in sodium chloride solutions was consistently 8 μl./cm2/hr greater than when bathed in sucrose or urea solutions of equal osmolarity. There was no water uptake when the sodium chloride solution was 285 m-osmolar.
4. Vasopressin (4 m-u./g) injected intramuscularly doubled the rate of water uptake from sodium chloride solutions of different osmolarity.
5. With solutions of potassium chloride, sodium nitrate, and potassium nitrate, in concentrations up to 150 m-osmoles/l., the rate of water uptake was found to be the same as with solutions of sodium chloride of the same osmolarity. Similarly, it was doubled by injection of vasopressin (4m-u./g).
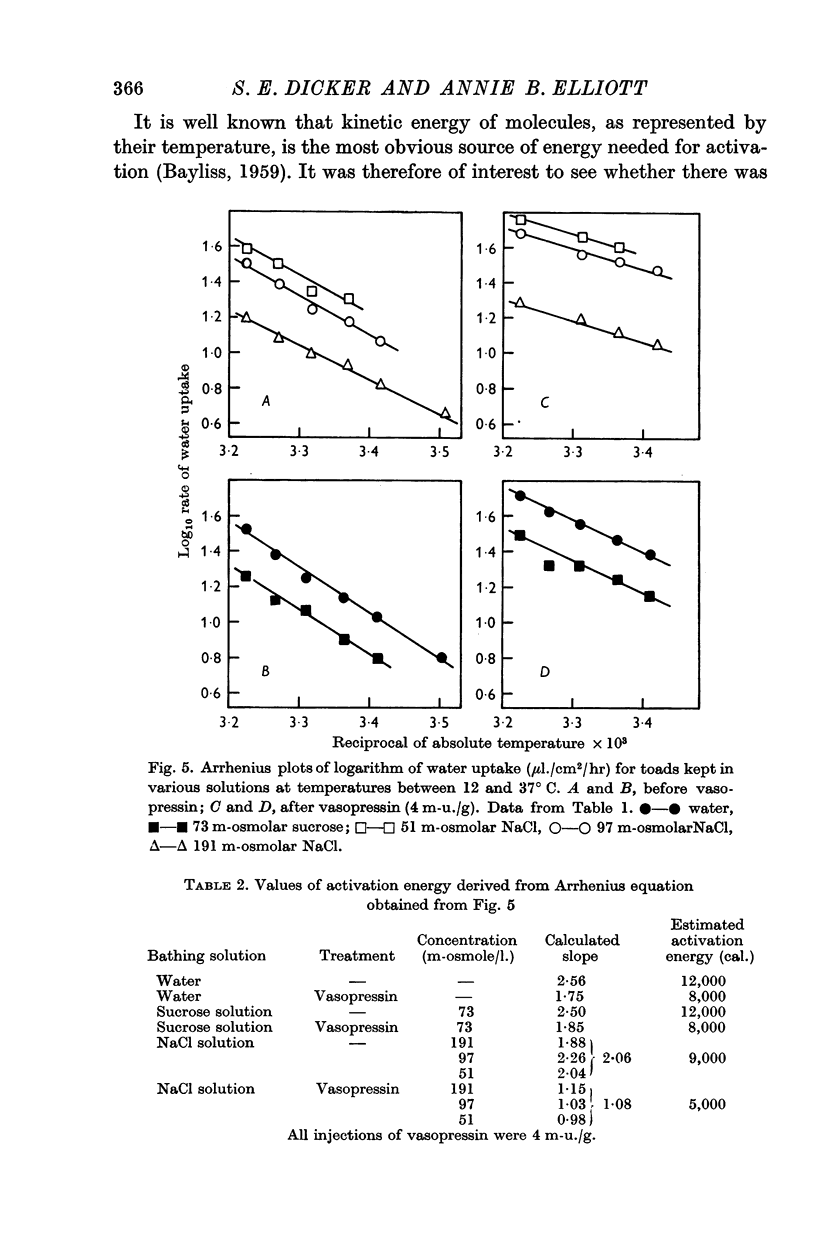
6. The effect of temperature on the rate of water uptake before and after injection of vasopressin was investigated in toads kept in distilled water, sucrose, or sodium chloride solutions. For temperatures between 20 and 37° C, vasopressin (4 m-u./g) reduced the activation energy involved in the process of water uptake by 4000 cal.
7. The results agree with the view that water uptake follows a diffusion process which is facilitated by vasopressin, possibly as a result of increasing the size or number of available pores.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENTLEY P. J. The effects of vasopressin on water uptake of the toad, Bufo marinus, while bathed in different hypotonic solutions. J Endocrinol. 1957 Dec;16(2):126–134. doi: 10.1677/joe.0.0160126. [DOI] [PubMed] [Google Scholar]
- BOURGUET J., MAETZ J. [Arguments in favor of the independence of the mechanisms of action of various neurohypophyseal hormones on the osmotic flux of water and on the active transport of sodium in the same receptor; studies on the bladder and the skin of Rana esculenta L]. Biochim Biophys Acta. 1961 Sep 30;52:552–565. doi: 10.1016/0006-3002(61)90414-0. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. The mechanism of water transport by the gall-bladder. J Physiol. 1962 May;161:503–527. doi: 10.1113/jphysiol.1962.sp006900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYS R. M., LEAF A. Studies on the movement of water through the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 May;45:905–919. doi: 10.1085/jgp.45.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The contributions of diffusion and flow to the passage of D2O through living membranes; effect of neurohypophyseal hormone on isolated anuran skin. Acta Physiol Scand. 1953 Mar 31;28(1):60–76. doi: 10.1111/j.1748-1716.1953.tb00959.x. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S. The similarity of effects of vasopressin, adenosine-3',5'-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest. 1962 Apr;41:702–709. doi: 10.1172/JCI104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYER W. H. Effect of posterior pituitary extract on permeability of frog skin to water. Am J Physiol. 1951 Jan;164(1):44–48. doi: 10.1152/ajplegacy.1950.164.1.44. [DOI] [PubMed] [Google Scholar]
- WHITTEMBURY G., WINDHAGER E. E. Electrical potential difference measurements in perfused single proximal tubules of Necturus kidney. J Gen Physiol. 1961 Mar;44:679–687. doi: 10.1085/jgp.44.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZERAHN K. Oxygen consumption and active sodium transport in the isolated and short-circuited frog skin. Acta Physiol Scand. 1956 May 31;36(4):300–318. doi: 10.1111/j.1748-1716.1956.tb01327.x. [DOI] [PubMed] [Google Scholar]



