Abstract
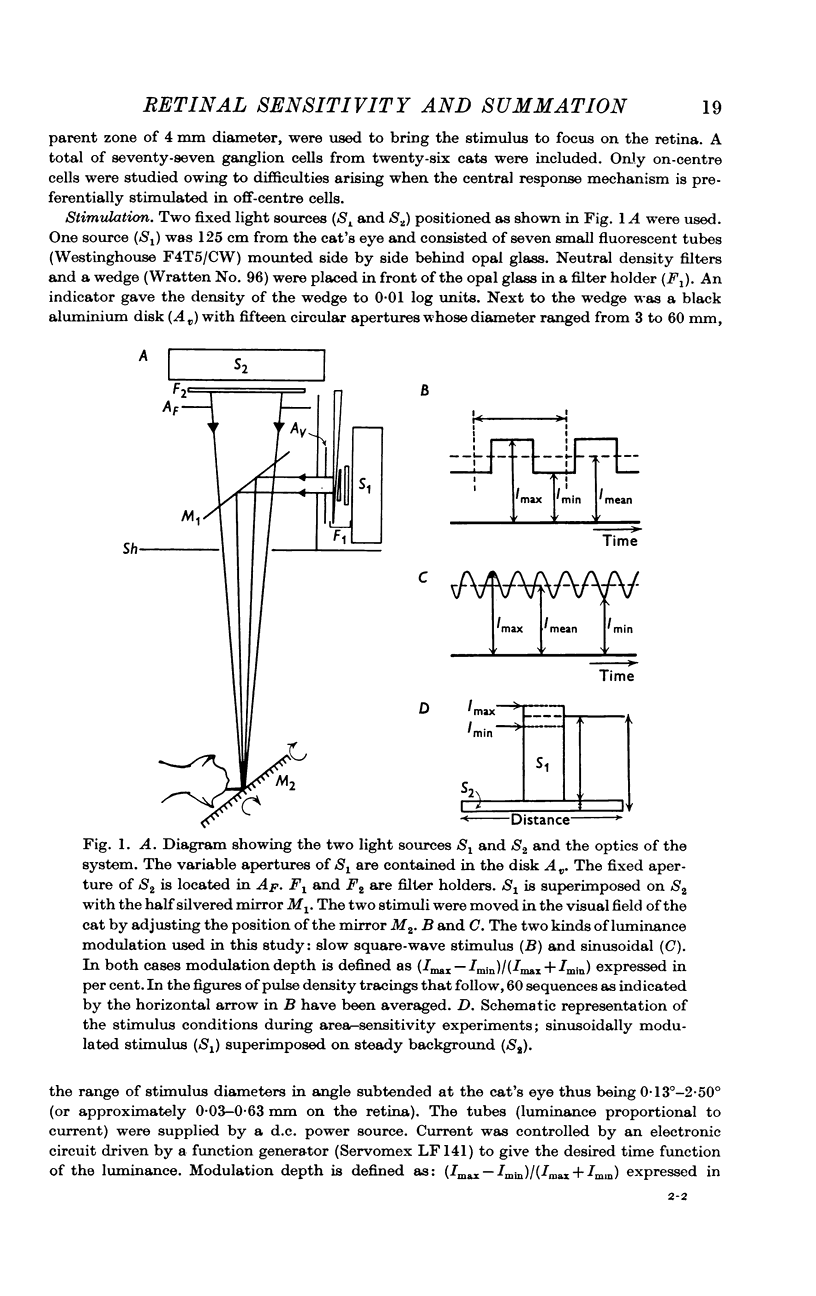
1. Properties of the central response mechanism of on-centre ganglion cells in the cat retina were studied by recording, from optic tract fibres, responses evoked by stimuli modulated with time in a sinusoidal or square-wave fashion.
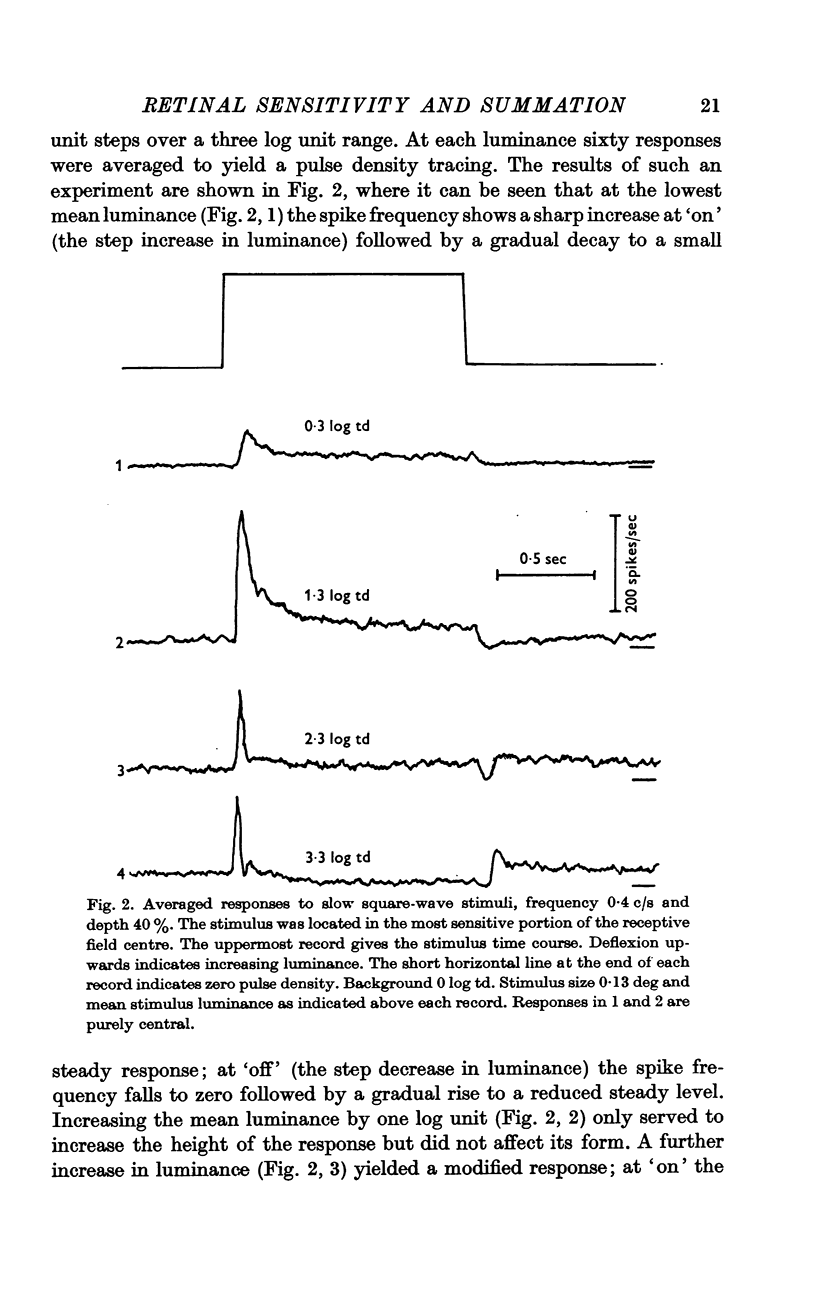
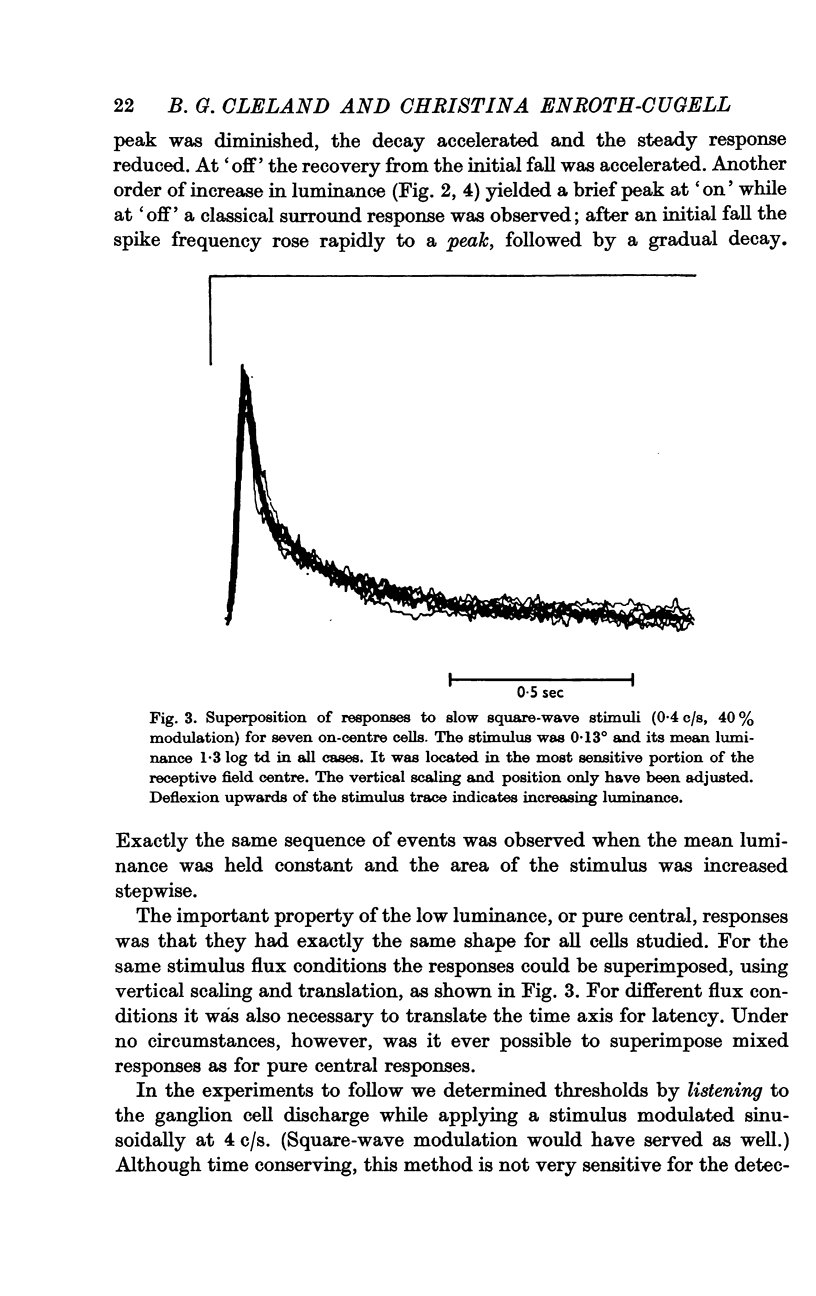
2. The shape of averaged square-wave responses resulting from the central mechanism alone was identified. This shape was identical from one cell to another. Such an identification permits the early recognition of peripheral antagonism.
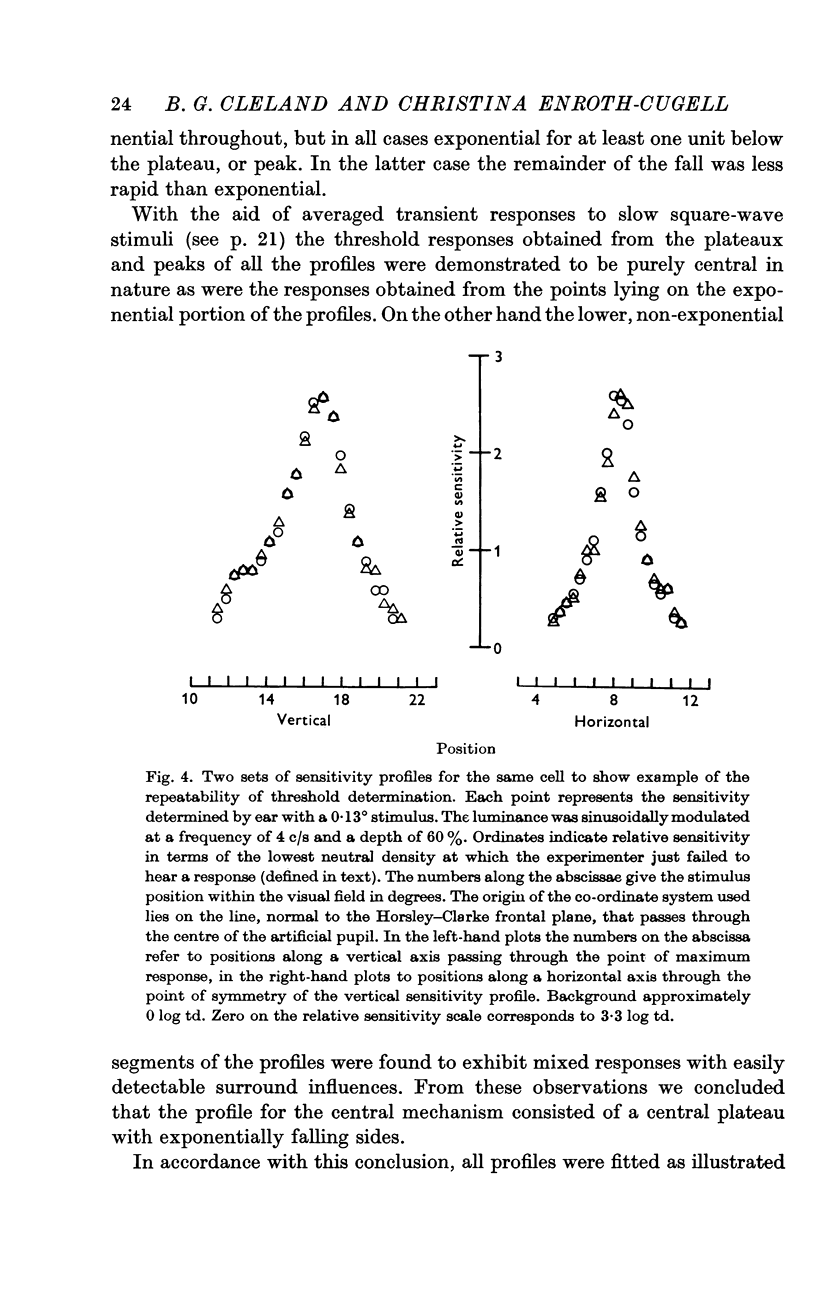
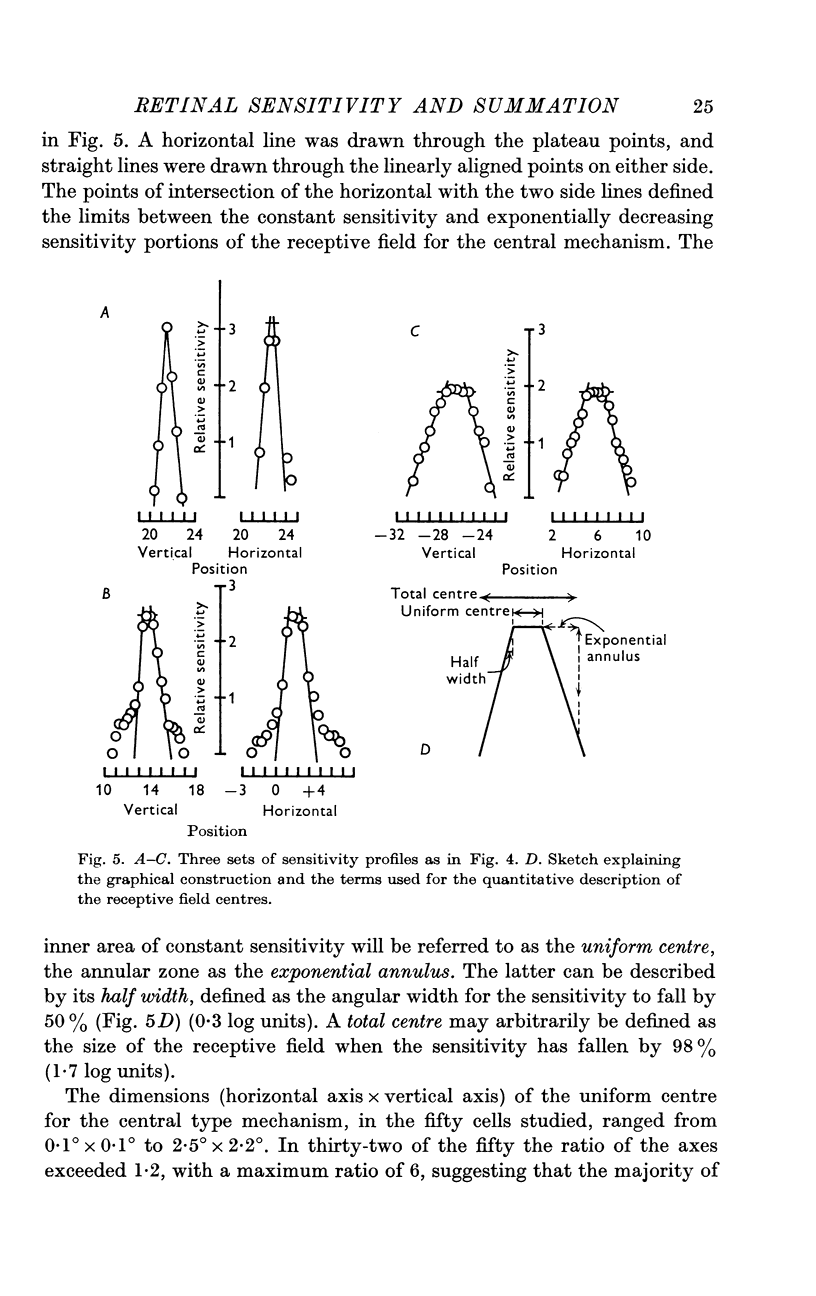
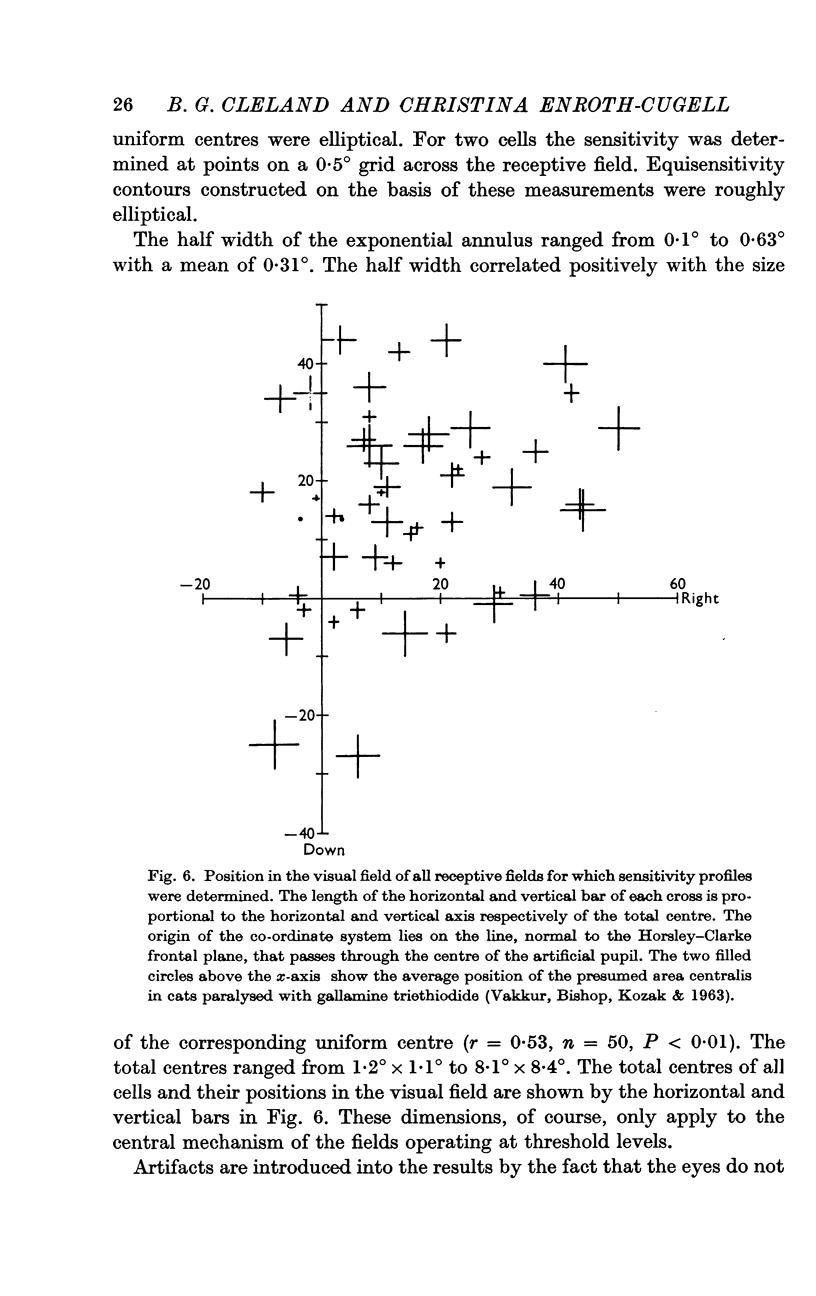
3. Threshold sensitivity for a sinusoidal stimulus was determined for fifty cells along one horizontal and vertical axis, passing through the most sensitive portion of the receptive field. These sensitivity profiles were described in terms of a central segment of constant maximum sensitivity (uniform centre) and sloping outer segments of exponentially decreasing sensitivity (exponential annulus). The dimensions of the uniform centre (horizontal axis × vertical axis) varied from 0·1° × 0·1° to 2·5° × 2·2°, the half width of the exponential annulus ranged from 0·1° to 0·63°.
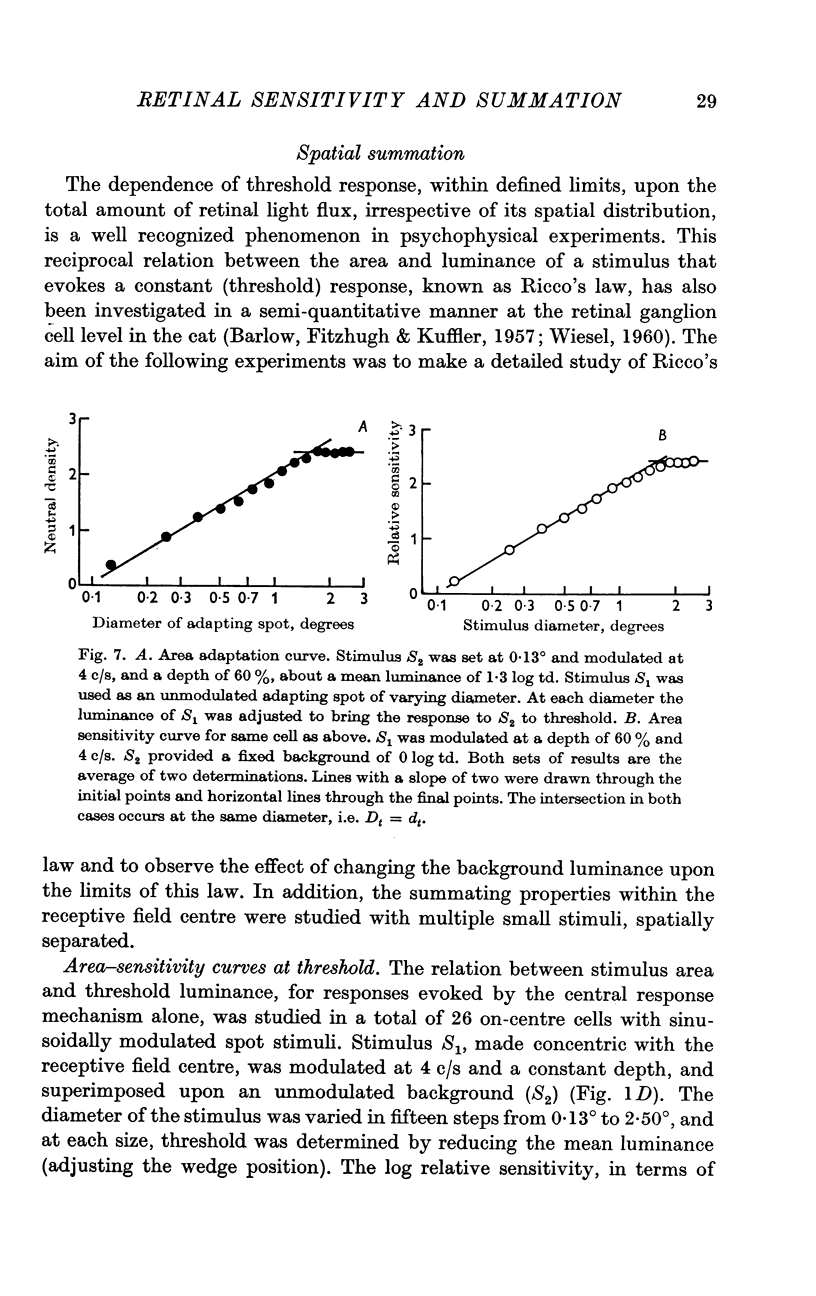
4. Adapting spots of varying diameter were placed concentric with the receptive field and the (unmodulated) luminance, at each diameter, that reduced a small central (sinusoidal) stimulus to threshold, was determined. The resulting area—adaptation curve, (adapting luminance plotted against diameter) showed that within defined limits the state of adaptation is determined by the flux independent of its distribution.
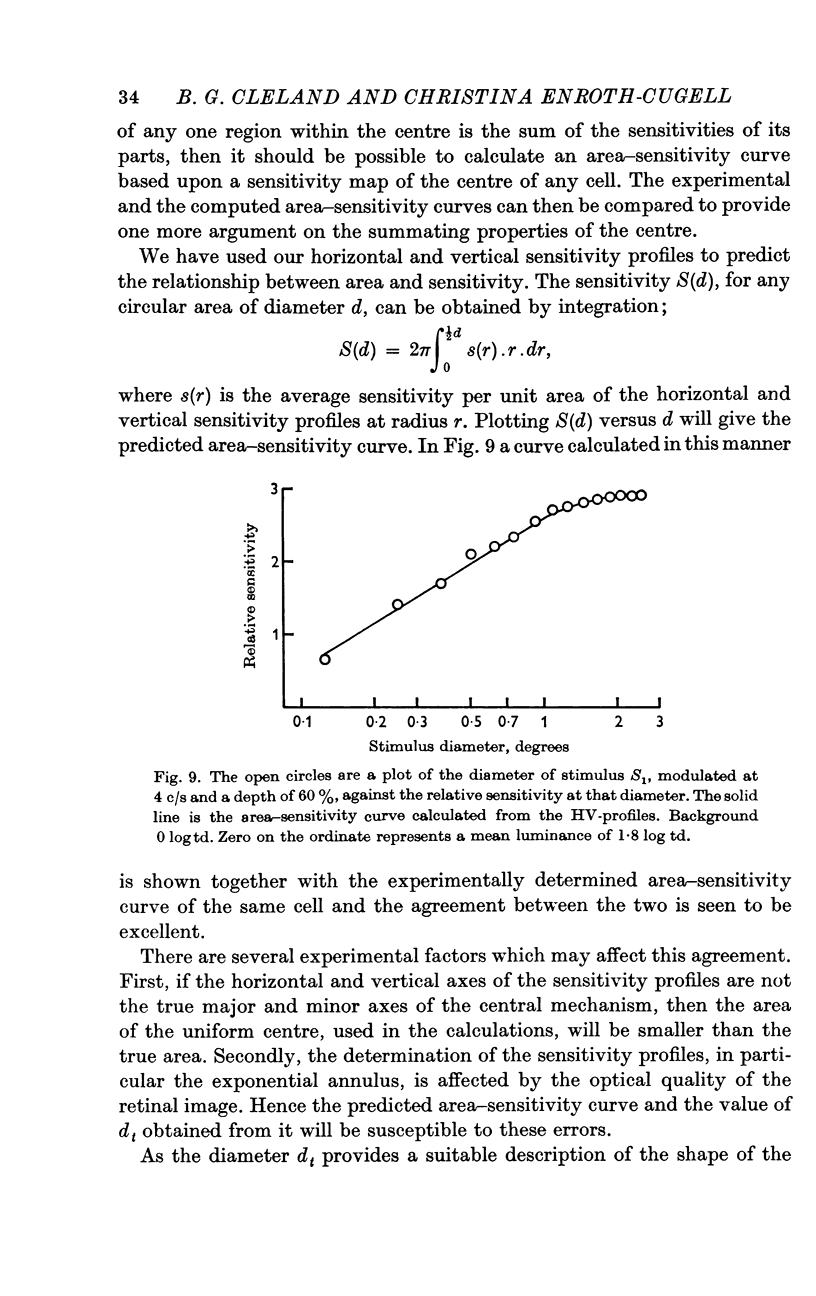
5. Sinusoidal stimuli of varying diameter were placed concentric with the receptive field and the threshold luminance at each diameter was determined. Suprathreshold square-wave stimuli indicated that the central mechanism alone contributed to the response. These area-sensitivity curves did not show any decrease in sensitivity at larger diameters.
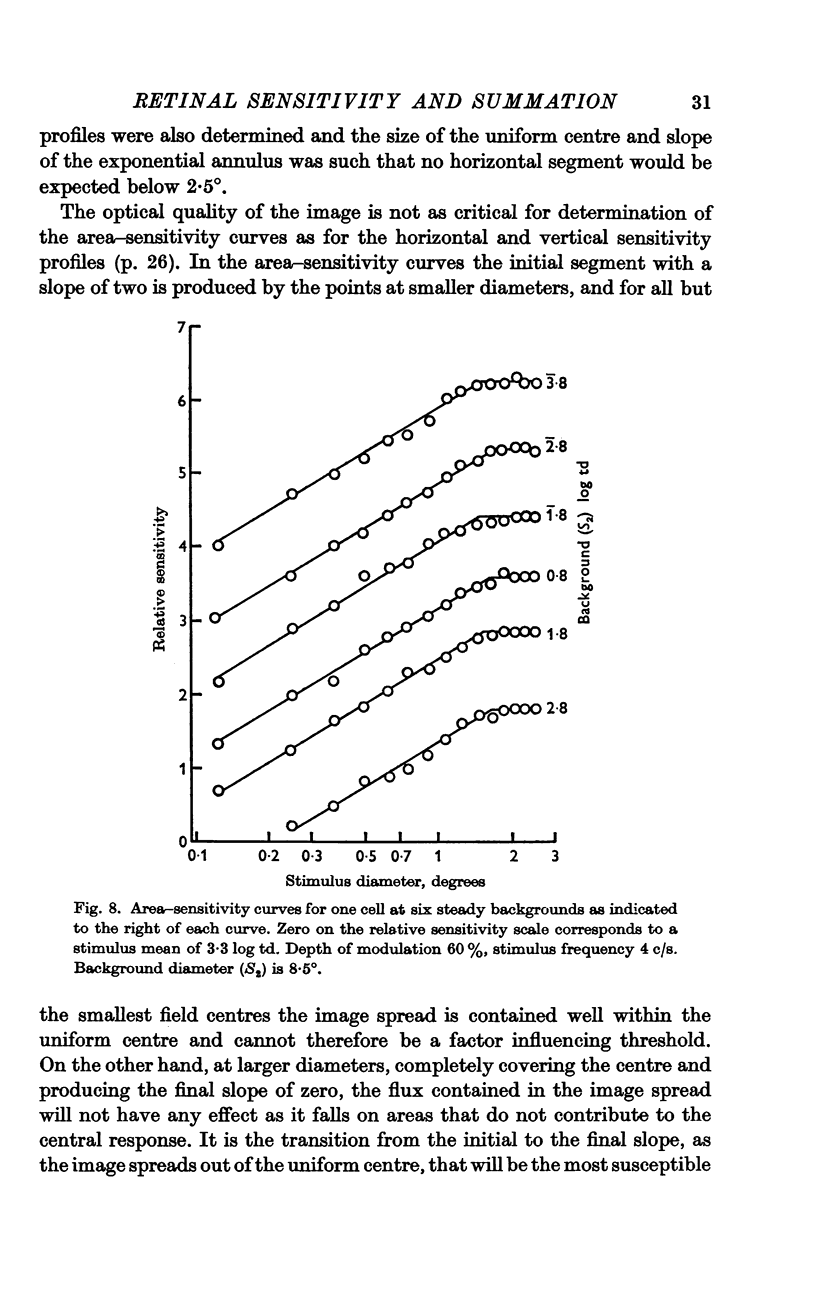
6. The shape of the area—sensitivity curve, and hence the extent of the summating area, was found to be independent of the state of adaptation.
7. For any one cell the shapes of the area—adaptation and area—sensitivity curves were shown to be identical, indicating that adapting flux and stimulus flux are independent of distribution over the same defined limits.
8. The sensitivity of combinations of small disconnected areas of the receptive field was found to be equal to the sum of their individual sensitivities.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Temporal and spatial summation in human vision at different background intensities. J Physiol. 1958 Apr 30;141(2):337–350. doi: 10.1113/jphysiol.1958.sp005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Neurons in the retina; organization, inhibition and excitation problems. Cold Spring Harb Symp Quant Biol. 1952;17:281–292. doi: 10.1101/sqb.1952.017.01.026. [DOI] [PubMed] [Google Scholar]
- PIRENNE M. H., DENTON E. J. Accuracy and sensitivity of the human eye. Nature. 1952 Dec 20;170(4338):1039–1042. doi: 10.1038/1701039a0. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. The difference spectrum and the photosensitivity of rhodopsin in the living human eye. J Physiol. 1956 Oct 29;134(1):11–29. doi: 10.1113/jphysiol.1956.sp005622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Rushton W. A. Bleached rhodopson and visual adaptation. J Physiol. 1965 Dec;181(3):645–655. doi: 10.1113/jphysiol.1965.sp007789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli D. N. Visual receptive fields in the cat's retina: complications. Science. 1966 Jun 24;152(3730):1768–1768. doi: 10.1126/science.152.3730.1768. [DOI] [PubMed] [Google Scholar]
- WAGNER H. G., MACNICHOL E. F., Jr, WOLBARSHT M. L. Functional basis for "on"-center and "off"-center receptive fields in the retina. J Opt Soc Am. 1963 Jan;53:66–70. doi: 10.1364/josa.53.000066. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]



