Abstract
We previously reported that if murine leukemia virus particles are produced in the presence of the mild oxidizing agent disulfide-substituted benzamide-2, they fail to undergo the normal process of virus maturation. We now show that treatment of these immature particles with a reducing agent (dithiothreitol) induces their maturation in vitro, as evidenced by proteolytic cleavage of Gag, Gag-Pol, and Env proteins and by their morphology. The identification of partial cleavage products in these particles suggests the sequence with which the cleavages occur under these conditions. This may be a useful experimental system for further analysis of retroviral maturation under controlled conditions in vitro.
Retrovirus particles are initially formed by budding from the plasma membrane of the host cell. They all contain a minimum of three protein species, i.e., the Gag polyprotein, the Gag-Pol polyprotein, and the Env proteins. However, these particles are not infectious until they undergo viral maturation. Maturation consists of a series of cleavages of the Gag and Gag-Pol polyproteins (and, in some retroviruses, in the transmembrane component of the Env protein as well). These cleavages are catalyzed by the viral protease (PR). The Gag polyprotein is always cleaved into at least three fragments, termed matrix (MA), capsid (CA), and nucleocapsid (NC), and the Gag-Pol polyprotein is cleaved, in addition, into PR, reverse transcriptase (RT), and integrase (IN) (in alpharetroviruses, PR is a cleavage product of the Gag polyprotein, and in several retrovirus groups Gag-PR is distinct from both Gag and Gag-PR-Pol) (reviewed in reference 17).
While maturation is an essential step in the retroviral life cycle, many aspects of this phenomenon are very poorly understood. Thus, we have no insight at present into how PR activity is regulated so that maturation is initiated upon the release of the particle. It is also not clear whether there is a single, obligatory sequence of cleavage events in the maturation pathway (17).
We have previously described the effects of hydrophobic mild oxidizing agents on a simple retrovirus, Moloney murine leukemia virus (MMLV) (13). We reported that when MMLV is produced in the presence of one of these agents, i.e., disulfide-substituted benzamide-2 (DIBA-2), it fails to undergo maturation. Similar observations have also been made with HIV-1 (19). Since DIBA-2 is known to induce disulfide cross-linking in the proteins of both MMLV and HIV-1 (13, 14), it seemed possible that this cross-linking was responsible for the inhibition of maturation in these particles. We now report that when immature MMLV, released from cells in the presence of DIBA-2, is treated with the reducing agent dithiothreitol (DTT), the block to maturation is reversed. This observation enables us to induce the controlled maturation of retrovirus particles. In addition, our analysis indicates the probable sequence of cleavage events in Gag during MMLV maturation under these conditions.
MMLV produced in DIBA-2 contains genomic RNA.
As reported previously, MMLV particles fail to undergo maturation if DIBA-2 is present in the culture medium of the virus-producing cells. In principle, this could either be an effect of DIBA-2 upon the viral proteins within the virus-producing cells or upon the newly released virions.
In all retroviruses (except spumaviruses), the NC domain of the Gag polyprotein contains one or two zinc fingers (2). These conserved structures play a crucial role in the encapsidation of viral RNA during virus assembly, since any mutation of cysteine to a residue that does not coordinate zinc results in a profound reduction in RNA encapsidation (2). One known target for the oxidizing action of DIBA-2 in mature MMLV particles is the cysteine thiols of the zinc finger in the NC protein. Oxidation of these groups results in the ejection of zinc from the zinc finger and the disulfide cross-linking of this protein in mature particles (13, 14). Therefore, if DIBA-2 interacted with Gag proteins within the virus-producing cell, i.e., before assembly of the particle, then it would be expected to disrupt the zinc finger; in turn, such oxidized Gag proteins might fail to package viral RNA. Alternatively, DIBA-2 might not react with Gag proteins until the particle is fully assembled and released. In this case, RNA packaging would be normal.
We tested MMLV particles produced in DIBA-2 for the presence of viral RNA as follows. Culture fluids were collected from NIH 3T3 cells that were chronically infected with MMLV; some flasks were treated with 100 μM DIBA-2 (obtained from the Developmental Therapeutics Program of the National Cancer Institute [NCI]) during the interval over which virus was collected. The fluids were filtered through 0.45-μm-pore-size filters and the virus particles were collected by centrifugation for 50 min at 25,000 rpm in an SW28 rotor (Beckman) at 4°C. RNA was extracted from the pelleted virions as described previously (6), and denaturing Northern analysis (7) was performed with a 32P-labeled probe representing the full-length, infectious MMLV genomic plasmid designated pRR88 (described in reference 7).
The results of this analysis are shown in Fig. 1. The figure shows that the virions produced in the presence of DIBA-2 contained approximately the same amount of genomic RNA as the control particles. (The treated sample [lane 2] appears to contain more viral RNA than the control sample [lane 1]. While DIBA-2 does not have a major effect on virus production [13], the amounts of virus in the two samples were not compared in this experiment. Thus, the simplest interpretation of the inequality in RNA levels in the two lanes is that it reflects a small difference in the amounts of virus analyzed.) This finding strongly suggests that DIBA-2 does not act on the viral proteins until RNA packaging has been irreversibly initiated. Similar results have been previously reported with a related compound and human immunodeficiency virus type 1 (HIV-1) (3).
FIG. 1.
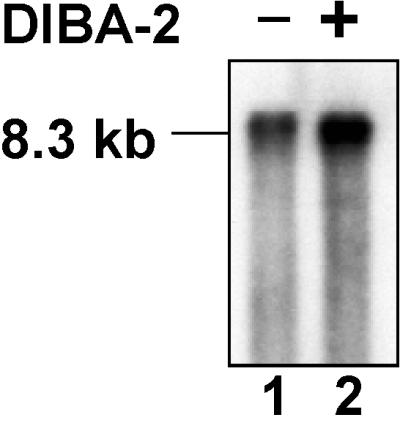
Presence of viral RNA in MMLV particles produced in DIBA-2. MMLV particles produced in the absence (lane 1) or presence (lane 2) of DIBA-2 were pelleted from equal volumes of culture fluid. RNA extracted from the pellets was analyzed by Northern blotting as described in Materials and Methods.
The internal milieu of mammalian cells is known to be a reducing environment (9). Since DIBA-2 is quite hydrophobic, it can probably penetrate into cells. However, it is only a mild oxidizing agent (14) and is presumably immediately neutralized in the intracellular environment. These considerations, together with the results shown in Fig. 1, are most consistent with the hypothesis that the effect of DIBA-2 on viral maturation is due to reactions occurring during or immediately after the release of the particle from the host cell.
Reversal of the block to viral maturation by DTT treatment.
As noted above, the maturation of MMLV particles is blocked by the presence of DIBA-2 in the culture medium into which the particles are released. Since DIBA-2 is known to be capable of oxidizing viral proteins in immature MMLV (13), it seemed possible that oxidation of one or more of these proteins was responsible for the block in maturation. In that case, reduction of the oxidized proteins might allow maturation to proceed in vitro. To test this possibility, we treated the immature particles produced in DIBA-2 with the reducing agent DTT, incubated them at 37°C to allow PR to act, and examined the particles for evidence of maturation.
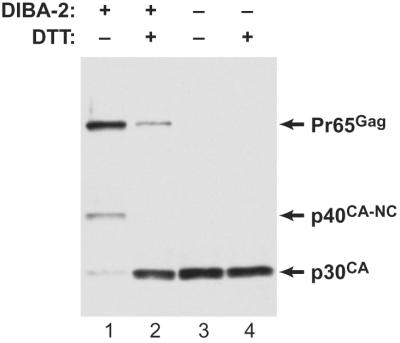
Our initial experiments analyzed cleavage of Gag using immunoblotting with rabbit antiserum against p30CA (a gift of the AIDS Vaccine Program, NCI—Frederick). As shown in Fig. 2 (lane 1), the most abundant protein in the particles released in DIBA-2 was Pr65Gag; a 40-kDa protein and p30CA were also detectable. Treatment of these particles with DTT (lane 2) resulted in the nearly complete cleavage of Pr65Gag and the 40-kDa protein, with the production of the normal cleavage product p30CA. (In some experiments, the cleavage induced by DTT treatment was incomplete; the extent to which maturation was blocked when virus was produced in DIBA-2 was also somewhat variable.)
FIG. 2.
DTT induces cleavage of Pr65Gag in MMLV produced in DIBA-2. NIH 3T3 cells chronically infected with MMLV were seeded at 106 cells per 10-cm-diameter tissue culture dish. Four hours later, the medium was changed to fresh culture medium containing 100 μM DIBA-2. Control dishes lacking DIBA-2 contained the same amount of the dimethyl sulfoxide solvent as the DIBA-2-treated dishes. The culture fluid containing the virus was collected 24 h later and filtered through a 0.45-μm-pore-size filter. MMLV produced in the presence (lanes 1 and 2) or absence (lanes 3 and 4) of DIBA-2 was collected by centrifugation, resuspended in phosphate-buffered saline (PBS), and incubated with (lanes 2 and 4) or without (lanes 1 and 3) 10 mM DTT for 2 h at 37°C. It was then analyzed by immunoblotting with antiserum against p30CA. Immunoblotting was performed as described previously (13) using enhanced chemiluminescence (Amersham).
Similar tests were performed with antisera against other MMLV proteins, including p12, p10NC, PR, RT, IN, and p15(E). These assays showed that all of the cleavages known to be catalyzed by PR occurred when the DIBA-blocked virus was incubated in DTT (see below; data not shown).
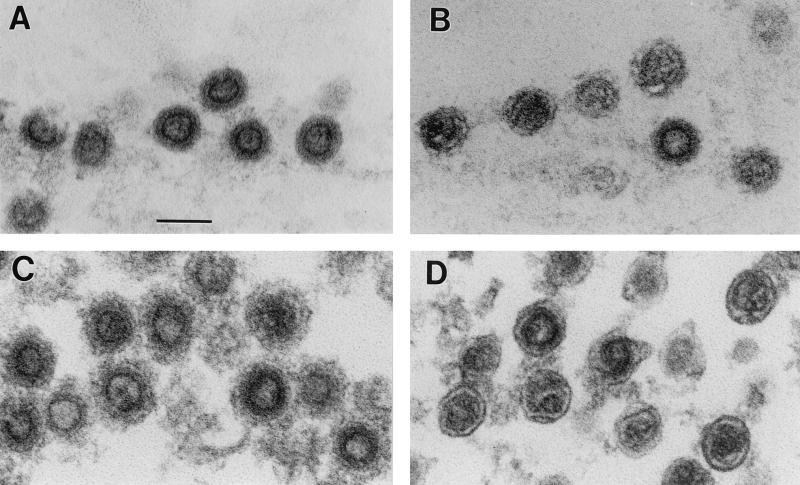
Retroviral maturation is normally accompanied by a dramatic change in the morphology of the virus particle. In the case of MMLV, a hollow, spherical shell in the interior of the particle appears to collapse into a condensed core structure (5, 8, 17, 22). To determine whether the cleavage of Pr65Gag was accompanied by this change in particle structure, we also examined the particles in the electron microscope before and after DTT treatment. As shown in Fig. 3C, the majority of MMLV particles produced in the presence of DIBA-2 appeared to have a hollow center and were very similar to immature particles lacking PR activity (Fig. 3A). However, when the particles produced in DIBA-2 were treated with DTT, a significant fraction of them were converted into mature virions, containing condensed rather than hollow cores (Fig. 3D). Many of these particles were indistinguishable in electron micrographs from the control particles produced in the absence of DIBA-2 (Fig. 3B).
FIG. 3.
DTT converts MMLV particles produced in DIBA-2 from immature to mature morphology. Virus particles were collected onto beads by immunoprecipitation as follows. After incubation in the presence or absence of DTT as for Fig. 2, virus was diluted with PBS and mixed with goat anti-MMLV gp70SU plus protein A-Sepharose beads (Amersham). The mixture was gently rotated overnight at 4°C. The beads were then washed three times with PBS, pelleted, and fixed with 2.5% glutaraldehyde plus 4% paraformaldehyde in PBS. (A) Immature particles produced by transient transfection of 293T cells with a mutant MMLV genome containing the D32L mutation at the active site of PR (6). (B) Wild-type particles, most of which are morphologically mature. (C) Particles produced in DIBA-2, most of which are morphologically immature. (D) Particles produced in DIBA-2 and treated with DTT before attachment to beads. The majority of these particles are morphologically mature. Scale bar = 100 nm.
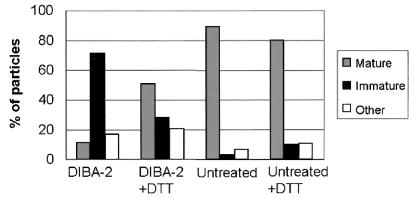
We also monitored the maturation induced by DTT treatment in quantitative terms. As shown in Fig. 4, approximately 72% of the particles produced in the presence of DIBA-2 could be classified as immature, while only 11% appeared mature (16% of these samples could not be classified). However, after these particles were treated with DTT, the percentage of particles with a mature morphology increased to 51%. DTT had no detectable effect on the morphology of particles produced without DIBA-2 (data not shown).
FIG. 4.
Quantitation of the effect of DTT on viral morphology. MMLV samples produced in the presence of DIBA-2 were incubated with or without DTT; a control sample produced without DIBA-2 was also analyzed. Particles were examined after collection on protein A-Sepharose beads as described above. They were considered mature if they contained an electron-dense interior (core) and no visible ring around it. They were considered immature if they contained a complete, unbroken ring surrounding an electron-lucent interior. Particles that did not fit either of these descriptions, such as particles with an incomplete ring, were classified as “other.” The scoring was blind in the sense that the samples were coded before classification. Two completely separate experiments were performed with similar results; the percentages presented here were obtained by summing the counts obtained for the two experiments. A total of at least 150 particles was scored in each of the four experimental categories.
We attempted to determine whether the MMLV particles that had undergone maturation as a result of DTT treatment after production in DIBA-2 had acquired infectivity. No infectious virus in these samples was detected by the S+L− focus assay (1), while a normal virus preparation would be expected to contain well over 104 focus-inducing units/ml. However, we also observed that DTT inactivated control preparations of MMLV that had been produced in the absence of DIBA-2 (data not shown). It seems likely that DTT reduces disulfide bonds not only in Gag and/or Gag-Pol proteins, leading to maturation of DIBA-2-treated virus, but also in the surface glycoprotein gp70SU, in which these bonds are essential for normal function (10). The inactivation of virus by DTT makes it very difficult to determine whether the Gag and/or Gag-Pol proteins in particles produced in DIBA-2 can perform all of their replicative functions after DTT treatment.
Studies on the mechanism of induction of maturation by DTT.
It was of interest to determine the speed with which cleavages occurred in particles grown in DIBA-2 and treated with DTT. We therefore exposed the particles to 10 mM DTT at 37°C for different periods of time. At the end of each incubation period the particles were placed in loading dye and then analyzed by immunoblotting with anti-p30CA antiserum as for Fig. 1. We found that the DTT-induced cleavage was virtually complete after only 15 min (data not shown). When similar experiments were performed at 32°C, the cleavage was only slightly slower than at 37°C; in contrast, almost no cleavage was detected if the samples were incubated in DTT at 0°C (data not shown).
Pr65Gag can be cross-linked into large disulfide-bonded aggregates in MMLV particles, since it migrates extremely slowly in nonreducing sodium dodecyl sulfate-polyacrylamide gels if it is isolated from particles released in DIBA-2 (data not shown) or from DIBA-2-treated PR− MMLV (13). Thus, inhibition of PR-induced cleavage in virus produced in DIBA-2 might be due to cross-linking of Gag and Gag-Pol, the substrates of PR. On the other hand, MMLV PR contains a cysteine residue (16); thus, an alternative possibility is that cross-linking of PR (or the PR domain of Gag-Pol) is responsible for the inhibition of cleavage.
To test the hypothesis that oxidation of PR was responsible for the failure of maturation in MMLV produced in DIBA-2, we constructed an MMLV mutant in which the cysteine residue in PR (encoded by codon 82 of the pol open reading frame [16]) was replaced by serine. The mutant was generated in pRR88 using overlap extension PCR (15), and virus particles were generated from these molecular clones by transient transfection in 293T cells. The mutant underwent normal maturation and was fully infectious (data not shown). These results show that the alteration of cysteine to serine did not interfere with the normal functions of PR in MMLV maturation. We then tested the effects of DIBA-2 on maturation in these particles. We found that DIBA-2 partially blocked the maturation of these mutant particles, but the extent of the block was generally less than that seen in wild-type particles treated in parallel (data not shown). For example, in one experiment, particles were analyzed by immunoblotting as for Fig. 2 and the blots were quantitated by densitometry. We found that uncleaved or partially cleaved Gag precursor represented 12% of the Gag protein in the untreated, wild-type sample; 17% in the untreated mutant sample; 85% in the wild-type virus produced in DIBA-2; and 47% in the mutant virus produced in DIBA-2. The partial resistance of the mutant suggests that the effects of DIBA-2 on maturation of wild-type MMLV are due partly to cross-linking of PR and partly to cross-linking of other cysteines in Gag and/or Gag-Pol.
Identification of cleavage intermediates in blocked and unblocked virions.
In many cases, immunoblotting of MMLV produced in DIBA-2 showed the presence of proteins with sizes between that of the initial, uncleaved precursor protein and that of the mature cleavage product against which the antiserum is directed. It seemed likely that these proteins were cleavage intermediates, produced by cleavage at some, but not all, of the normal proteolytic maturation sites. The existence of these bands raised the possibility of determining the order in which the different cleavages occur under these conditions.
In order to identify these bands, we analyzed the virus produced in DIBA-2 (and also virus produced in DIBA-2 and treated with DTT) by sequential immunoblotting, i.e., immunoblotting with one antiserum, stripping the membrane, and then immunoblotting with a second antiserum. Overlaying films generated by probing the same membrane with different antisera proved to be a sensitive way of identifying bands that react with more than one serum. The results of these studies are summarized in Table 1.
TABLE 1.
Cleavage intermediates identified in MMLV produced in DIBA-2a
| Molecular weight (103) | Reaction with antiserum against:
|
Protein assignment | ||||
|---|---|---|---|---|---|---|
| p12 | CA | NC | PR | RT-IN | ||
| 65 | + | + | + | − | − | Pr65Gag |
| 27 | + | − | − | − | − | MA-p12 |
| 40 | − | + | + | − | − | CA-NC |
| 126 | − | + | RT-IN | |||
| 80 | ± | − | Gag-PR | |||
| 53 | + | − | CA-NC-PR | |||
Proteins present in MMLV produced in the presence of DIBA-2 were identified by sequential immunoblotting with monospecific antisera against viral proteins. Fragments of Gag-Pol were difficult to detect with antisera against Gag proteins because they were in much lower quantity than proteins derived from Gag alone.
We found that the immature particles produced in DIBA-2 contain, in addition to Pr65Gag, a 27-kDa species that reacts with anti-p12 antiserum (monoclonal antibody F548, a gift from Bruce Chesebro, National Institute of Allergy and Infectious Diseases) and a 40-kDa species (visible in Fig. 2, lane 1) that reacts with both anti-p30CA and rabbit anti-p10NC antisera (a gift of the AIDS Vaccine Program, NCI—Frederick). The mature proteins p15MA, p12, p30CA, and p10NC are all produced when the maturation block is relieved by treatment with DTT. Thus, under these conditions, the cleavage between p12 and CA precedes both the cleavage between p15MA and p12 and that between p30CA and p10NC.
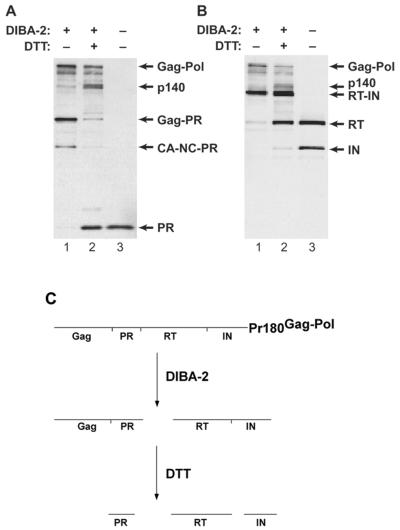
We also analyzed pol gene products by sequential immunoblotting with anti-PR and anti-(RT-IN) antisera. (Data obtained by sequential use of anti-Gag and anti-Pol antisera were difficult to interpret, since the Gag precursor and its cleavage products are present at far higher levels than are Gag-Pol products.) Figure 5 shows the results of analysis with the anti-PR and anti-(RT-IN) antisera: the particles produced in DIBA-2 (lanes 1) contained an ∼80-kDa Gag-PR protein (panel A), an ∼53-kDa CA-NC-PR protein (panel A), and an ∼126-kDa RT-IN fusion product (panel B). Treatment of these particles with DTT (lanes 2) caused the release of the mature proteins PR (14 kDa) (panel A), RT (80 kDa) (panel B), and IN (46 kDa) (panel B). (An ∼140-kDa protein apparently representing the entire pol gene product, i.e., PR-RT-IN, was also produced upon DTT treatment, as shown in lanes 2.) The results indicate that cleavage between PR and RT is less susceptible to inhibition by DIBA-2 than those between Gag and PR or between RT and IN. These inferences are indicated schematically in Fig. 5C.
FIG. 5.
Analysis of pol cleavage products in DIBA-2-treated MMLV. MMLV produced in the presence of DIBA-2 (lanes 1 and 2) was incubated with (lanes 2) or without (lanes 1) DTT; a control sample produced without DIBA-2 (lanes 3) was also analyzed. Immunoblotting was performed with rabbit anti-PR antiserum (A); the membrane was then stripped and reprobed with DC-1, a rabbit anti-(RT-IN) antiserum (B) that was a gift of Judith G. Levin, National Institute of Child Health and Human Development. Autoradiography performed between stripping and reprobing showed that the signal from the first antibody was completely removed by stripping. (C) Inferred sequence of cleavages in Gag-Pol maturation. The figure shows the cleavage intermediates present in MMLV produced in DIBA-2 as well as the final cleavage products found in DTT-treated or control mature virions.
It should be noted that the pathway inferred from these experiments need not be identical to that in normal virus maturation, since the disulfide bonds formed in the presence of DIBA-2 may affect some cleavage events more than others. However, cleavage between p12 and CA is known to precede the other two cleavages in Gag in normal MMLV maturation (12, 21, 22), just as in our present work.
The presence in virions produced in DIBA-2 of Gag-PR (Fig. 5A, lane 1) and RT-IN (Fig. 5B, lane 1) also suggests that cleavage at the C terminus of PR (i.e., between PR and RT) is an early event in maturation. This is quite different from results from other laboratories with HIV-1 and avian retroviruses: in the latter systems, cleavage at the N terminus of PR is apparently a necessary initial step in maturation (4, 18, 20, 24). We do not know whether this difference reflects a real variation in the processing pathway between the different viruses or is due to the use of disulfide cross-linking to artificially arrest virus maturation in our studies on MMLV.
It is striking to note that DTT has already been used to enhance in vitro maturation of the low levels of immature MMLV particles in a normal virus preparation (23). In these studies, wild-type MMLV was isolated under standard conditions, without treatment with a disulfide bond inducer like DIBA-2. While maturation was nearly complete in these virus preparations, the small amount of Pr65Gag remaining in the preparation was cleaved in vitro if the virus was lysed with NP-40. However, cleavage in this situation was arrested at a 40- to 42-kDa intermediate (presumably CA plus NC) unless DTT was also present. It seems likely that the Gag polyprotein in the immature virions in these studies had been spontaneously oxidized during manipulation. The fact that some cleavage occurred in NP-40 alone shows that PR was active under these conditions; thus, the results suggest that disulfide bonds in CA plus NC (rather than in PR) were responsible for the maturation arrest that was reversed by DTT.
In conclusion, we have described a method for inducing the controlled maturation of an entire population of retrovirus particles in vitro. This experimental approach complements previous work using PR inhibitors of HIV-1 (11) and may be useful in detailed biochemical studies of virus maturation in the future.
Acknowledgments
We thank Judith Levin, Bruce Chesebro, the Developmental Therapeutics Program, and the AIDS Vaccine Program for reagents. We also thank Stephen Oroszlan for discussions and Catherine Hibbert for a thoughtful review of the manuscript.
This work was supported in part by contract N01-CO-12400 from the National Cancer Institute.
REFERENCES
- 1.Bassin, R. H., N. Tuttle, and P. J. Fischinger. 1971. Rapid cell culture assay for murine leukaemia virus. Nature 229:564-566. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz, R., J. Fisher, and S. P. Goff. 1996. RNA packaging. Curr. Top. Microbiol. Immunol. 214:177-218. [DOI] [PubMed] [Google Scholar]
- 3.Berthoux, L., C. Pechoux, and J. L. Darlix. 1999. Multiple effects of an anti-human immunodeficiency virus nucleocapsid inhibitor on virus morphology and replication. J. Virol. 73:10000-10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstein, H., D. Bizub, M. Kotler, G. Schatz, V. M. Vogt, and A. M. Skalka. 1992. Processing of avian retroviral Gag polyprotein precursors is blocked by a mutation at the NC-PR cleavage site. J. Virol. 66:1781-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford, S., and S. P. Goff. 1985. A deletion mutation in the 5′ part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J. Virol. 53:899-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu, W., and A. Rein. 1993. Maturation of dimeric viral RNA of Moloney murine leukemia virus. J. Virol. 67:5443-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorelick, R. J., L. E. Henderson, J. P. Hanser, and A. Rein. 1988. Point mutants of Moloney murine leukemia virus that fail to package viral RNA: evidence for specific RNA recognition by a “zinc finger-like” protein sequence. Proc. Natl. Acad. Sci. USA 85:8420-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katoh, I., Y. Yoshinaka, A. Rein, M. Shibuya, T. Odaka, and S. Oroszlan. 1985. Murine leukemia virus maturation: protease region required for conversion from “immature” to “mature” core form and for virus infectivity. Virology 145:280-292. [DOI] [PubMed] [Google Scholar]
- 9.Kosower, N. S., and E. M. Kosower. 1978. The glutathione status of cells. Int. Rev. Cytol. 54:109-160. [DOI] [PubMed] [Google Scholar]
- 10.Linder, M., D. Linder, J. Hahnen, H. H. Schott, and S. Stirm. 1992. Localization of the intrachain disulfide bonds of the envelope glycoprotein 71 from Friend murine leukemia virus. Eur. J. Biochem. 203:65-73. [DOI] [PubMed] [Google Scholar]
- 11.McQuade, T. J., A. G. Tomasselli, L. Liu, V. Karacostas, B. Moss, T. K. Sawyer, R. L. Heinrikson, and W. G. Tarpley. 1990. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science 247:454-456. [DOI] [PubMed] [Google Scholar]
- 12.Naso, R. B., W. L. Karshin, Y. H. Wu, and R. B. Arlinghaus. 1979. Characterization of 40,000- and 25,000-dalton intermediate precursors to Rauscher murine leukemia virus gag gene products. J. Virol. 32:187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rein, A., D. E. Ott, J. Mirro, L. O. Arthur, W. Rice, and L. E. Henderson. 1996. Inactivation of murine leukemia virus by compounds that react with the zinc finger in the viral nucleocapsid protein. J. Virol. 70:4966-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice, W. G., J. G. Supko, L. Malspeis, R. W. Buckheit, Jr., D. Clanton, M. Bu, L. Graham, C. A. Schaeffer, J. A. Turpin, J. Domagala, R. Gogliotti, J. P. Bader, S. M. Halliday, L. Coren, R. C. Sowder II, L. O. Arthur, and L. E. Henderson. 1995. Inhibitors of HIV nucleocapsid protein zinc fingers as candidates for the treatment of AIDS. Science 270:1194-1197. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 16.Shinnick, T. M., R. A. Lerner, and J. G. Sutcliffe. 1981. Nucleotide sequence of Moloney murine leukaemia virus. Nature 293:543-548. [DOI] [PubMed] [Google Scholar]
- 17.Swanstrom, R., and J. W. Wills. 1997. Synthesis, assembly, and processing of viral proteins, p. 263-334. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Plainview, N.Y. [PubMed]
- 18.Tessmer, U., and H. G. Krausslich. 1998. Cleavage of human immunodeficiency virus type 1 proteinase from the N-terminally adjacent p6∗ protein is essential for efficient Gag polyprotein processing and viral infectivity. J. Virol. 72:3459-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turpin, J. A., S. J. Terpening, C. A. Schaeffer, G. Yu, C. J. Glover, R. L. Felsted, E. A. Sausville, and W. G. Rice. 1996. Inhibitors of human immunodeficiency virus type 1 zinc fingers prevent normal processing of Gag precursors and result in the release of noninfectious virus particles. J. Virol. 70:6180-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogt, V. M., R. Eisenman, and H. Diggelmann. 1975. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J. Mol. Biol. 96:471-493. [DOI] [PubMed] [Google Scholar]
- 21.Yoshinaka, Y., and R. B. Luftig. 1977. Characterization of Rauscher leukemia virus (RLV) P40-42, an intermediate cleavage product of the group specific antigen (gag) precursor polypeptide, P65-70. Biochem. Biophys. Res. Commun. 79:319-325. [DOI] [PubMed] [Google Scholar]
- 22.Yoshinaka, Y., and R. B. Luftig. 1977. Murine leukemia virus morphogenesis: cleavage of P70 in vitro can be accompanied by a shift from a concentrically coiled internal strand (“immature”) to a collapsed (“mature”) form of the virus core. Proc. Natl. Acad. Sci. USA 74:3446-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshinaka, Y., and R. B. Luftig. 1977. Partial characterization of a P70 proteolytic factor that is present in purified virions of Rauscher leukemia virus (RLV). Biochem. Biophys. Res. Commun. 76:54-63. [DOI] [PubMed] [Google Scholar]
- 24.Zybarth, G., H. G. Krausslich, K. Partin, and C. Carter. 1994. Proteolytic activity of novel human immunodeficiency virus type 1 proteinase proteins from a precursor with a blocking mutation at the N terminus of the PR domain. J. Virol. 68:240-250. [DOI] [PMC free article] [PubMed] [Google Scholar]