Abstract
1. The oxygen consumption of isolated anterior byssus retractor muscle (ABRM) of Mytilus edulis was measured during tonic contraction induced by acetylcholine (ACh).
2. The respiration was measured with an oxygen electrode during 95 min, divided into one period of 5 min and six successive periods of 15 min.
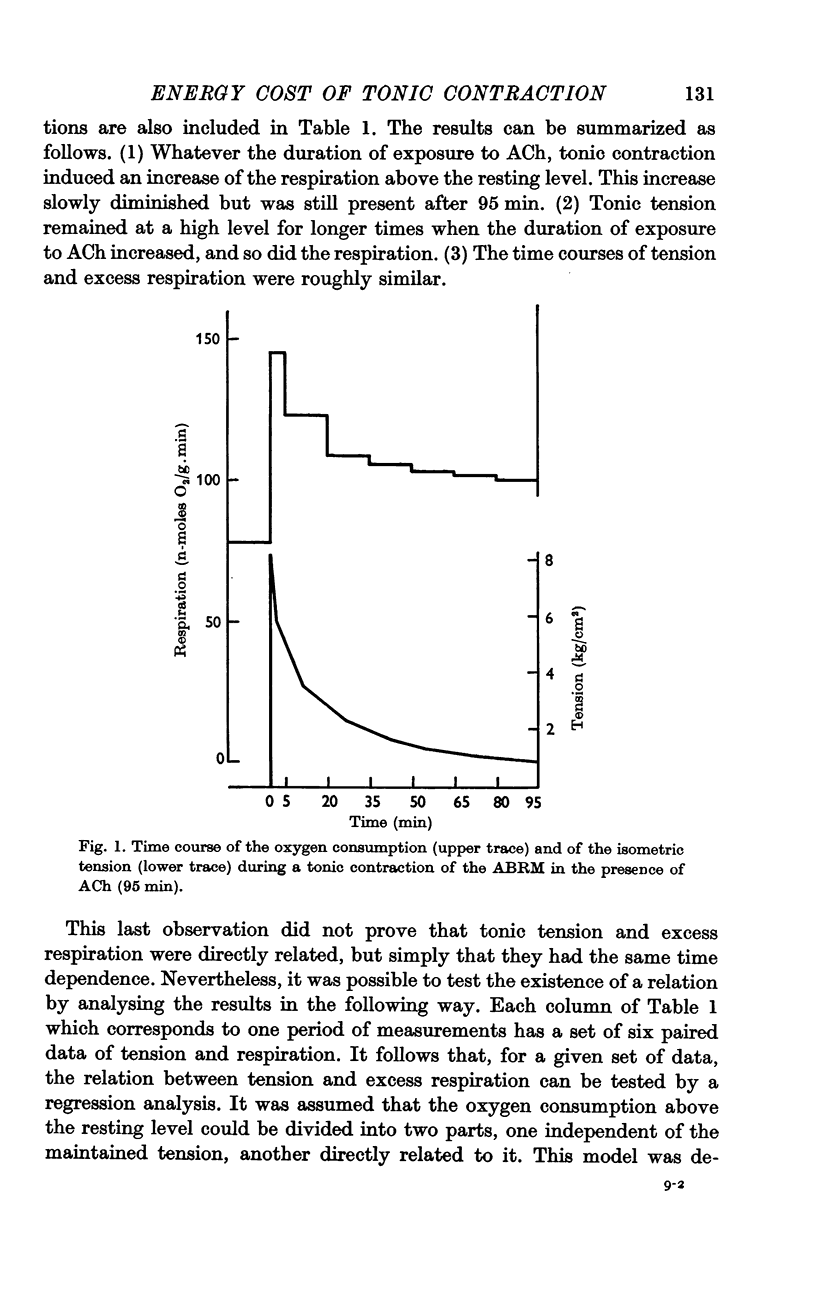
3. Tonic contraction induced a prolonged increase of the basal respiration that slowly diminished with a time course roughly similar to that of the tonic tension.
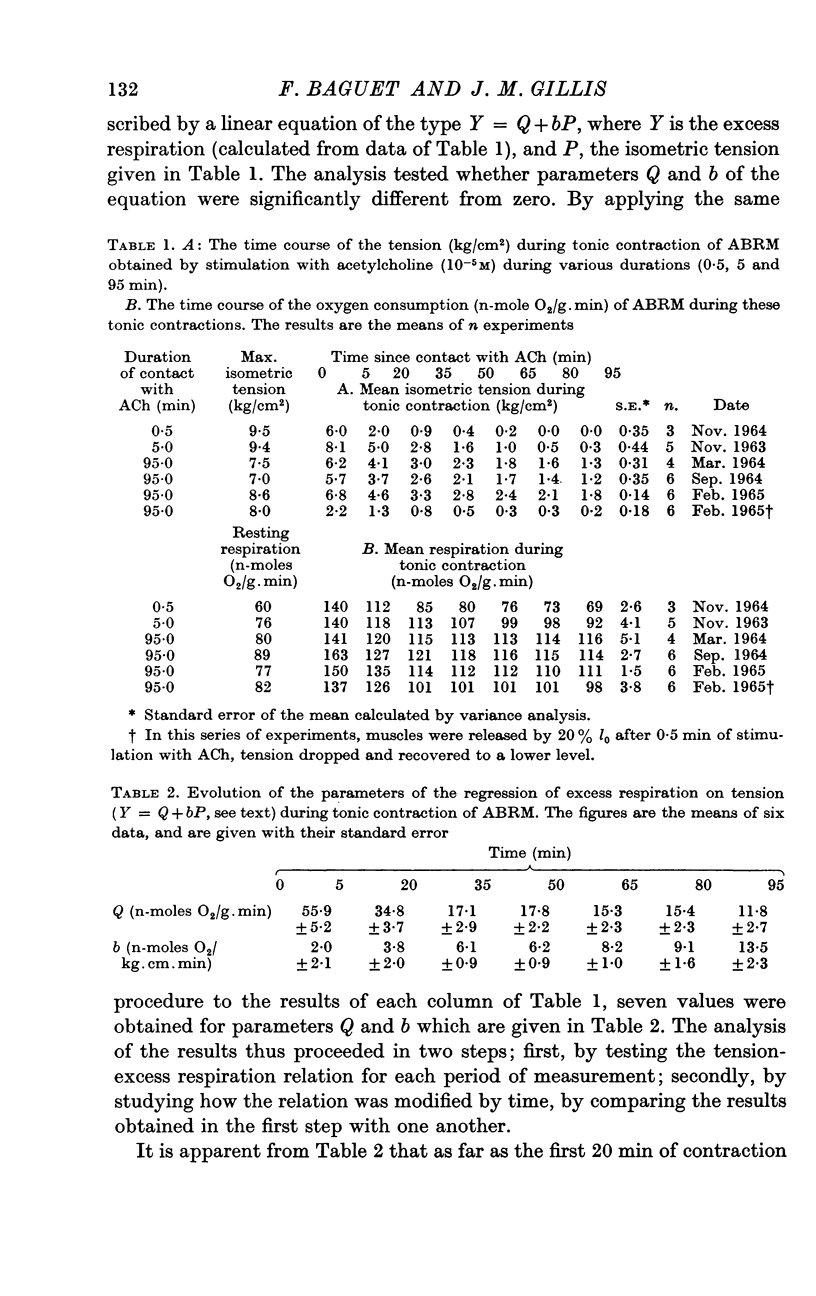
4. For each period of measurement, the excess respiration over the resting level could be analysed into a constant amount and an amount that depended on the maintained tonic tension. The analysis was performed by fitting regression equations of the type Y = Q+bP, where Y is the excess respiration in n-moles O2/g.min, and P, the isometric tension (kg/cm2); term b of the equation expresses the amount of oxygen consumption directly proportional to the tonic tension.
5. During the first 20 min of contraction, terms b of the equations are not significant, and most of the excess respiration (terms Q) is independent of the tension. The oxygen consumed during this time is supposed to reflect the recovery metabolism for the energy cost of the development of the tension.
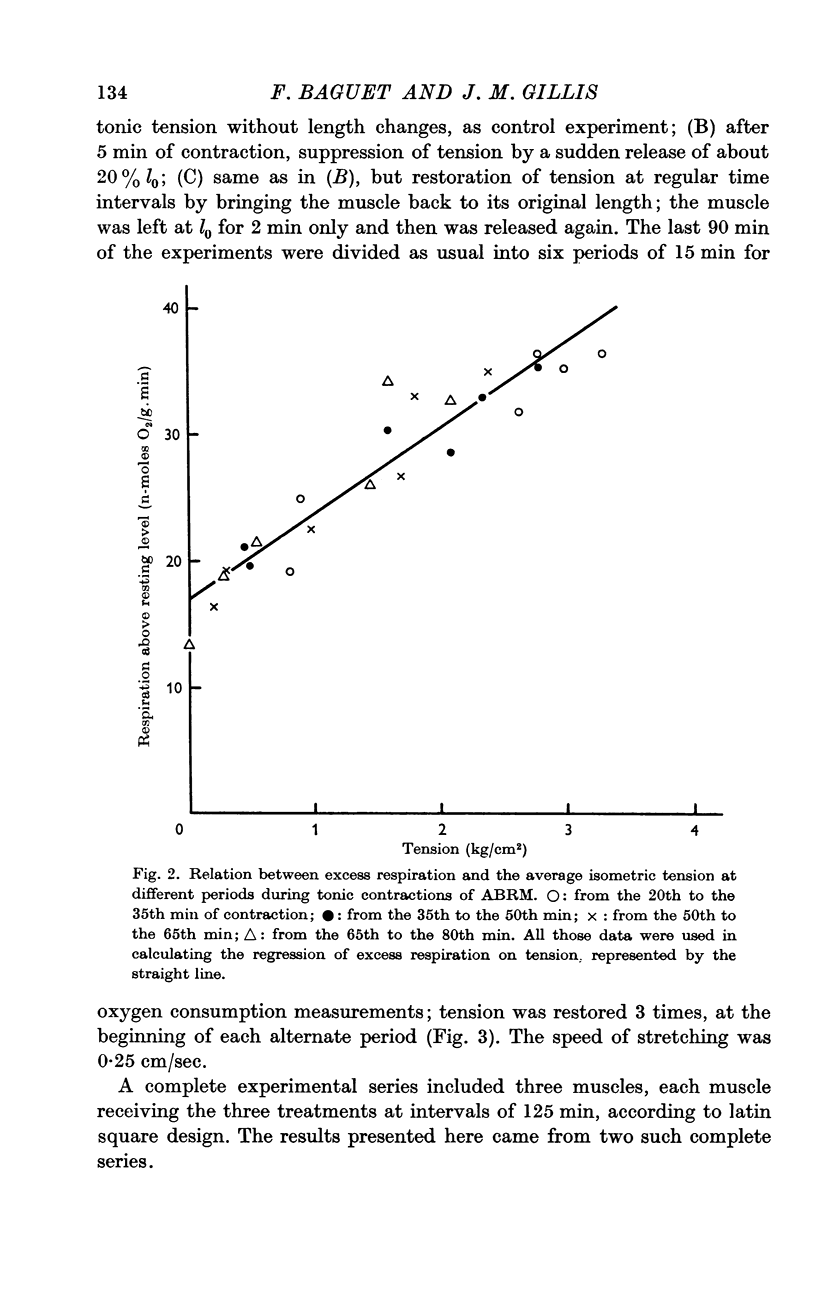
6. From the 20th to the 80th min of contraction, terms Q are reduced and terms b are significant and constant. The excess respiration during this period is equal to 16·9 (±0·5) n-moles O2/g.min + P × 6·8 (±0·5) n-moles O2/kg.cm.min (±S.E. of the means, n = 24).
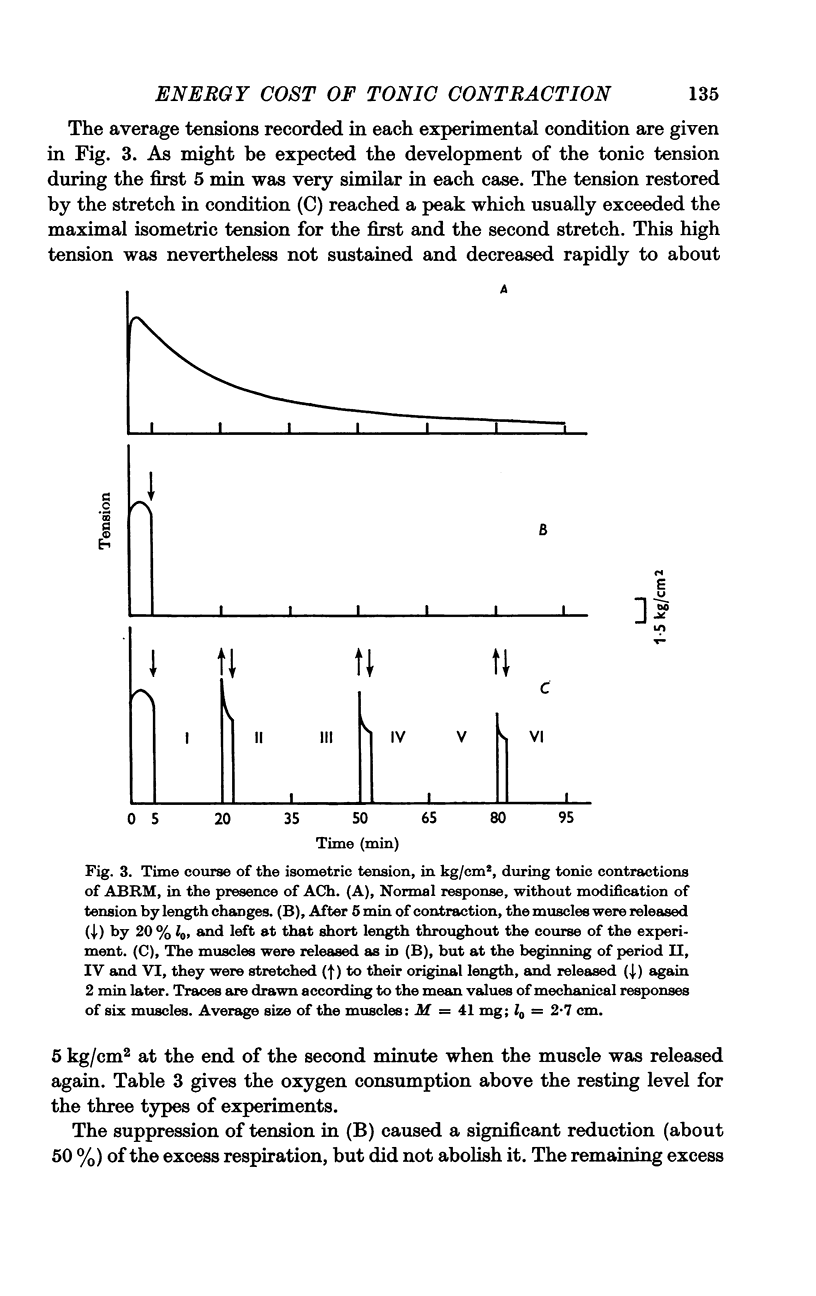
7. During a tonic contraction suppression of tension by a release reduced the oxygen consumption which increased again when tension was restored by stretching the muscle back to its original length. This confirmed the role of tension in determining the intensity of respiration during the catch.
8. The oxygen consumption related to this tension restored by stretching the muscle, varied from 8·0 to 12·3 n-moles O2/kg.cm.min. These figures are of the same order of magnitude as the coefficient b obtained in the case of tonic contraction without modification of tension by length changes.
9. These results are taken as a demonstration that the maintenance of tonic tension is an `active' phenomenon with a metabolic counterpart.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY K. Invertebrate tropomyosin. Biochim Biophys Acta. 1957 Jun;24(3):612–619. doi: 10.1016/0006-3002(57)90255-x. [DOI] [PubMed] [Google Scholar]
- BRECHT K., UTZ G., LUTZ E. Uber die Atmung quergestreifter und glatter Muskeln von Kaltblütern in Ruhe, Dehnung, Kontraktion und Kontraktur. Pflugers Arch. 1955;260(6):524–537. doi: 10.1007/BF00363669. [DOI] [PubMed] [Google Scholar]
- BULBRING E. Measurements of oxygen consumption in smooth muscle. J Physiol. 1953 Oct;122(1):111–134. doi: 10.1113/jphysiol.1953.sp004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguet F., Gillis J. M., Dainoff G. Energétique du relâchement phasique d'un muscle lisse de lamellibranche. Arch Int Physiol Biochim. 1967 Jun;75(3):523–527. [PubMed] [Google Scholar]
- Baguet F., Gillis J. M. The respiration of the anterior byssus retractor muscle of Mytilus edulis (ABRM) after a phasic contraction. J Physiol. 1967 Jan;188(1):67–82. doi: 10.1113/jphysiol.1967.sp008124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL B. R. The nature of the phasic and the tonic responses of the anterior byssal retractor muscle of Mytilus. J Physiol. 1959 Dec;149:154–177. doi: 10.1113/jphysiol.1959.sp006332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON W. H., KAHN J. S., SZENTGYORGYI A. G. Paramyosin and contraction of catch muscles. Science. 1959 Jul 17;130(3368):160–161. doi: 10.1126/science.130.3368.160. [DOI] [PubMed] [Google Scholar]
- LOWY J., HANSON J. Ultrastructure of invertebrate smooth muscles. Physiol Rev Suppl. 1962 Jul;5:34–47. [PubMed] [Google Scholar]
- Litarczek G. Oxidation processes occurring in the system plasma (serum)-potassium ferricyanide. J Physiol. 1928 Mar 30;65(1):1–14. doi: 10.1113/jphysiol.1928.sp002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLMAN B. M. CONTRACTION IN THE OPAQUE PART OF THE ADDUCTOR MUSCLE OF THE OYSTER (CRASSOSTREA ANGULATA). J Physiol. 1964 Sep;173:238–262. doi: 10.1113/jphysiol.1964.sp007455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TWAROG B. M. Responses of a molluscan smooth muscle to acetylcholine and 5-hydroxytryptamine. J Cell Physiol. 1954 Aug;44(1):141–163. doi: 10.1002/jcp.1030440112. [DOI] [PubMed] [Google Scholar]
- Winton F. R. The changes in viscosity of an unstriated muscle (Mytilus edulis) during and after stimulation with alternating, interrupted and uninterrupted direct currents. J Physiol. 1937 Jan 18;88(4):492–511. doi: 10.1113/jphysiol.1937.sp003455. [DOI] [PMC free article] [PubMed] [Google Scholar]



