Abstract
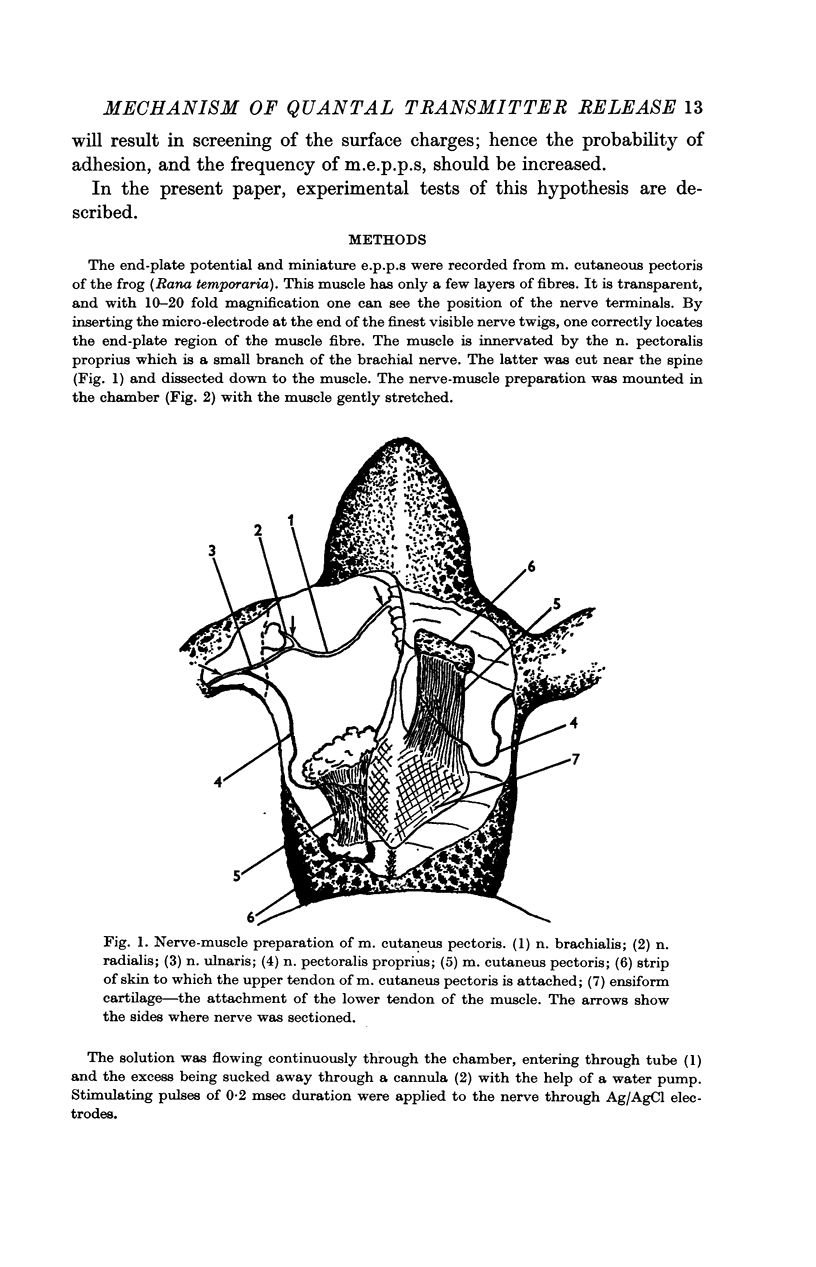
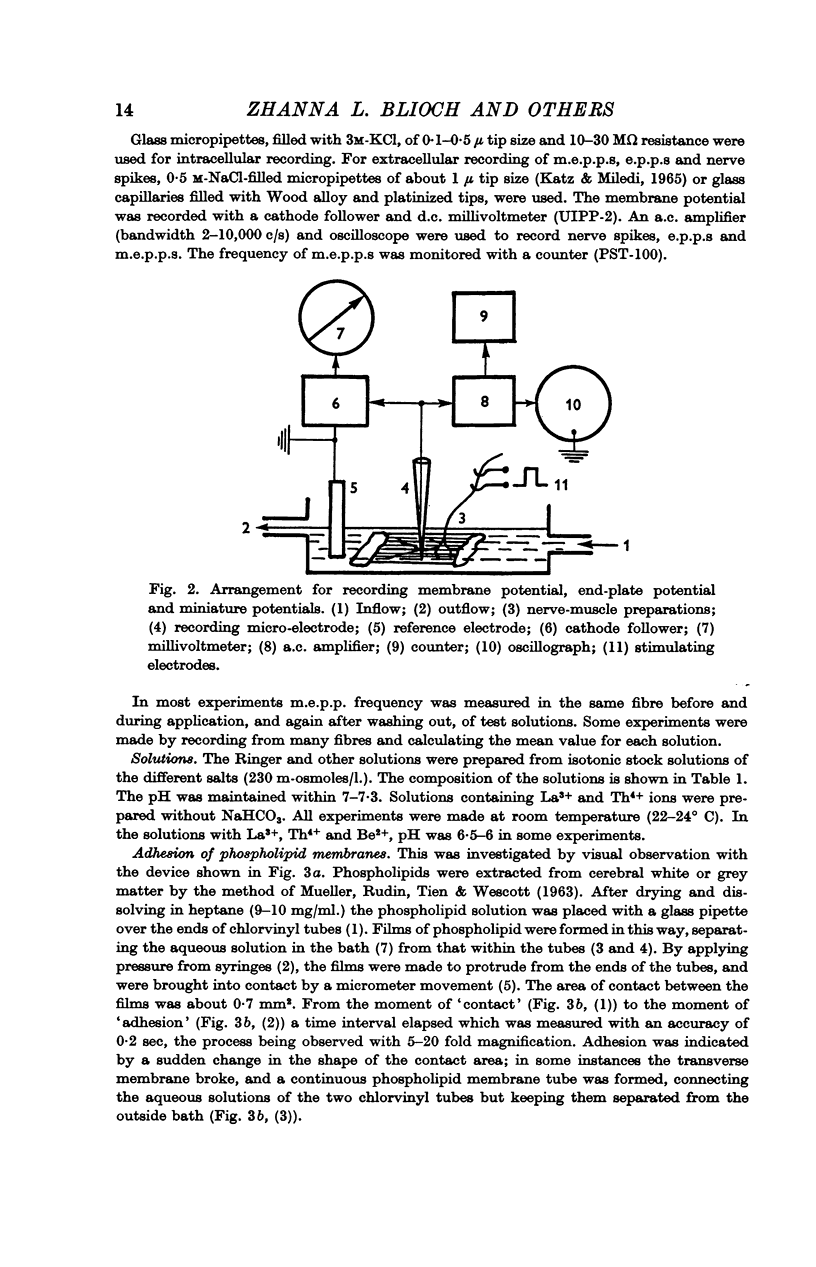
1. The nerve-muscle preparation of the cutaneous pectoris of the frog has been used to study quantal transmitter release.
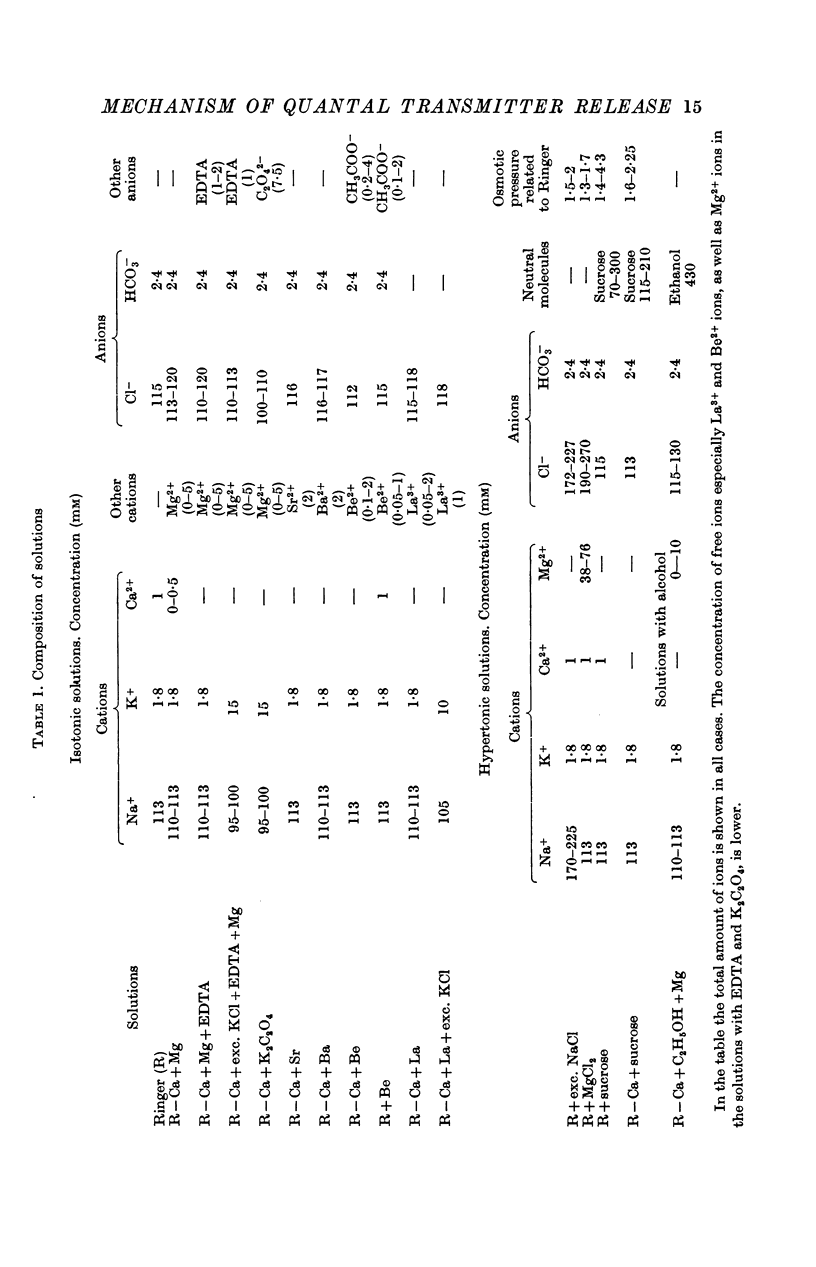
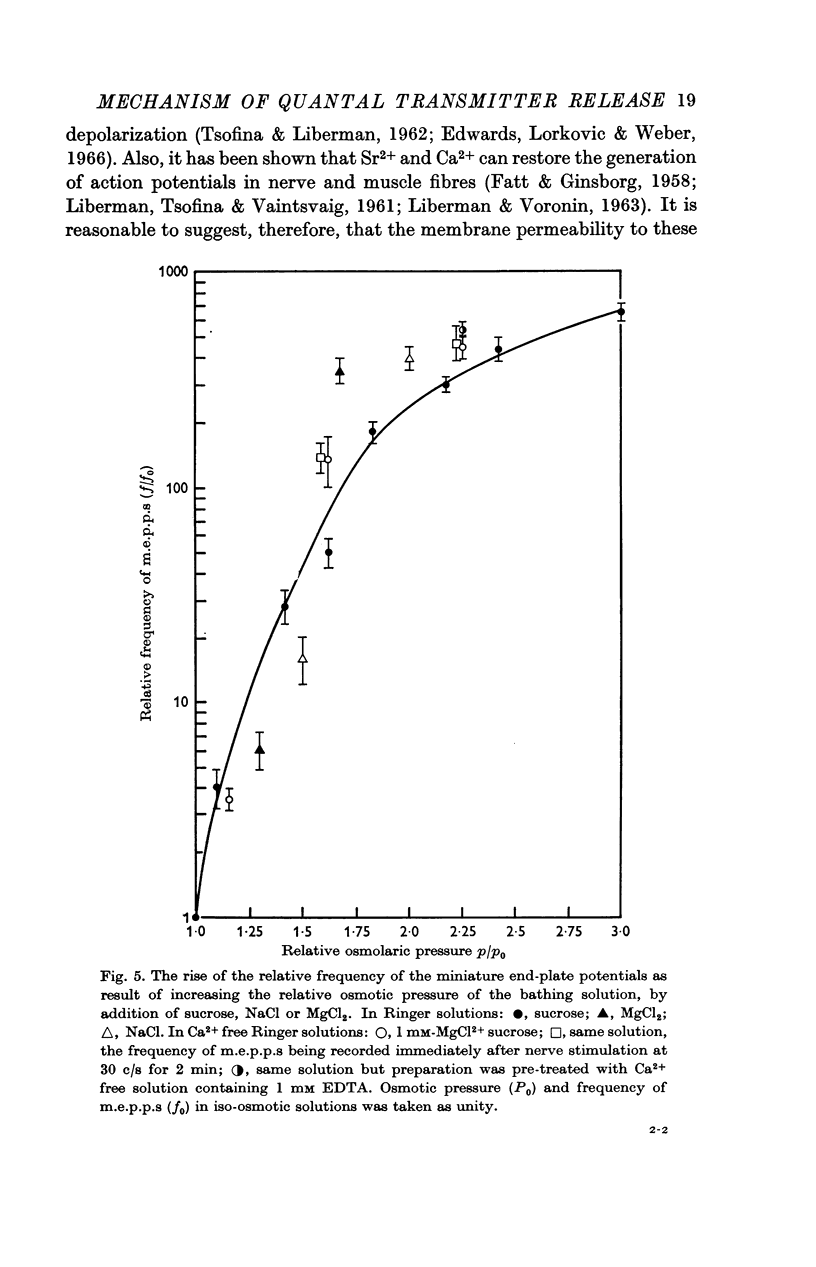
2. When the osmotic pressure of the external solution is raised 1·5-2 fold, the frequency of miniature end-plate potentials (m.e.p.p.s) rises by 1·5-2 orders of magnitude. This effect is independent of the presence of Ca2+ ions and of the nature of the substances by which the osmotic pressure has been increased.
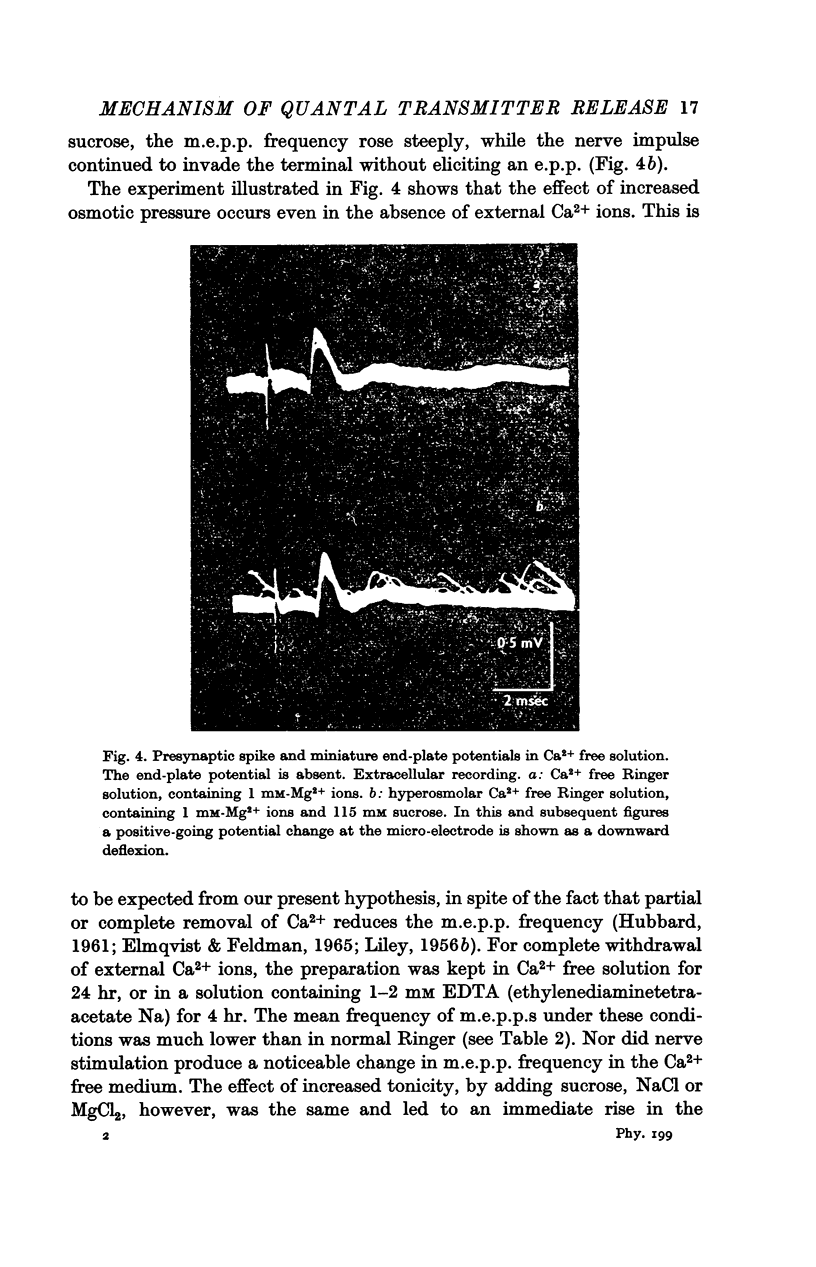
3. In Ca2+ free hypertonic solution the nerve impulse still invades the nerve terminals but does not alter the frequency of the m.e.p.p.s.
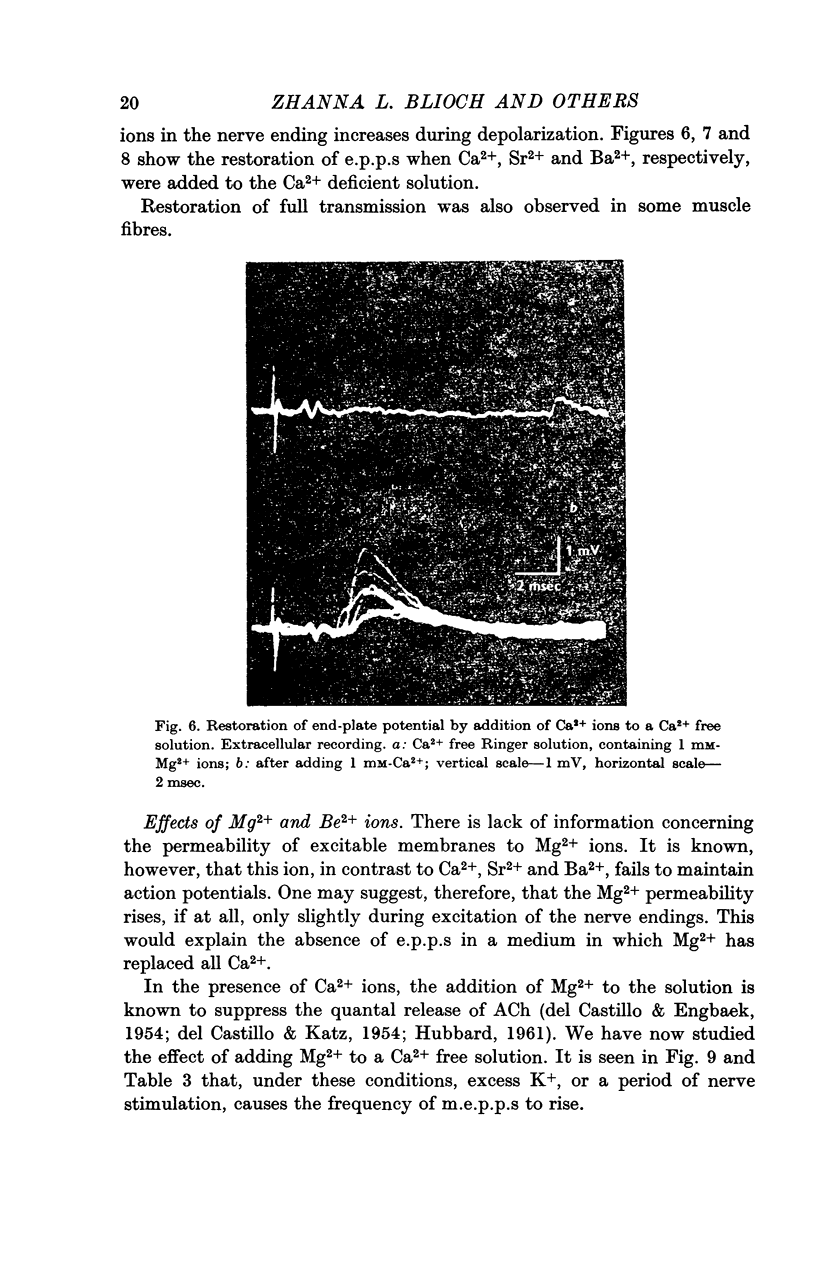
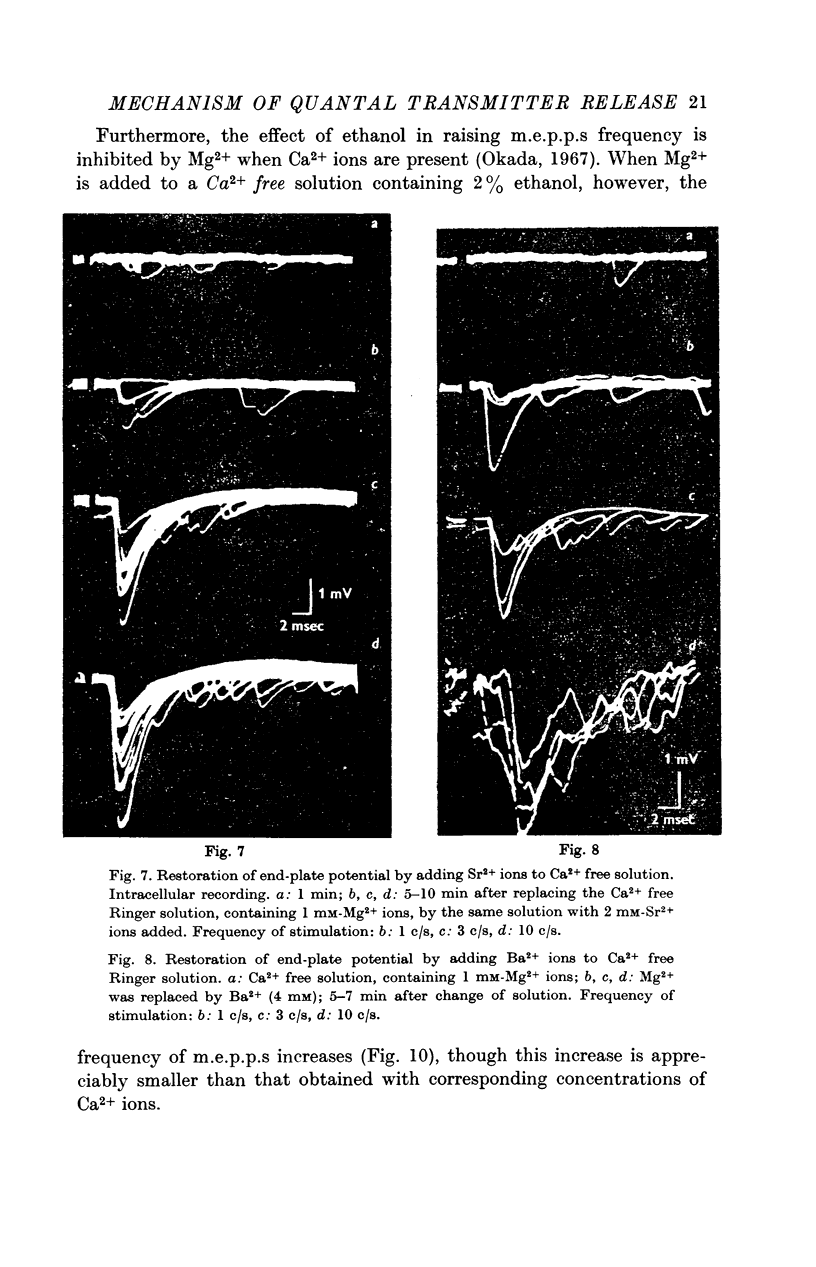
4. The arrival of the impulse in the terminals causes an immediate increase in the rate of quantal release, provided divalent cations are present whose passage through the axon membrane is facilitated by excitation (Ca2+, Sr2+, Ba2+).
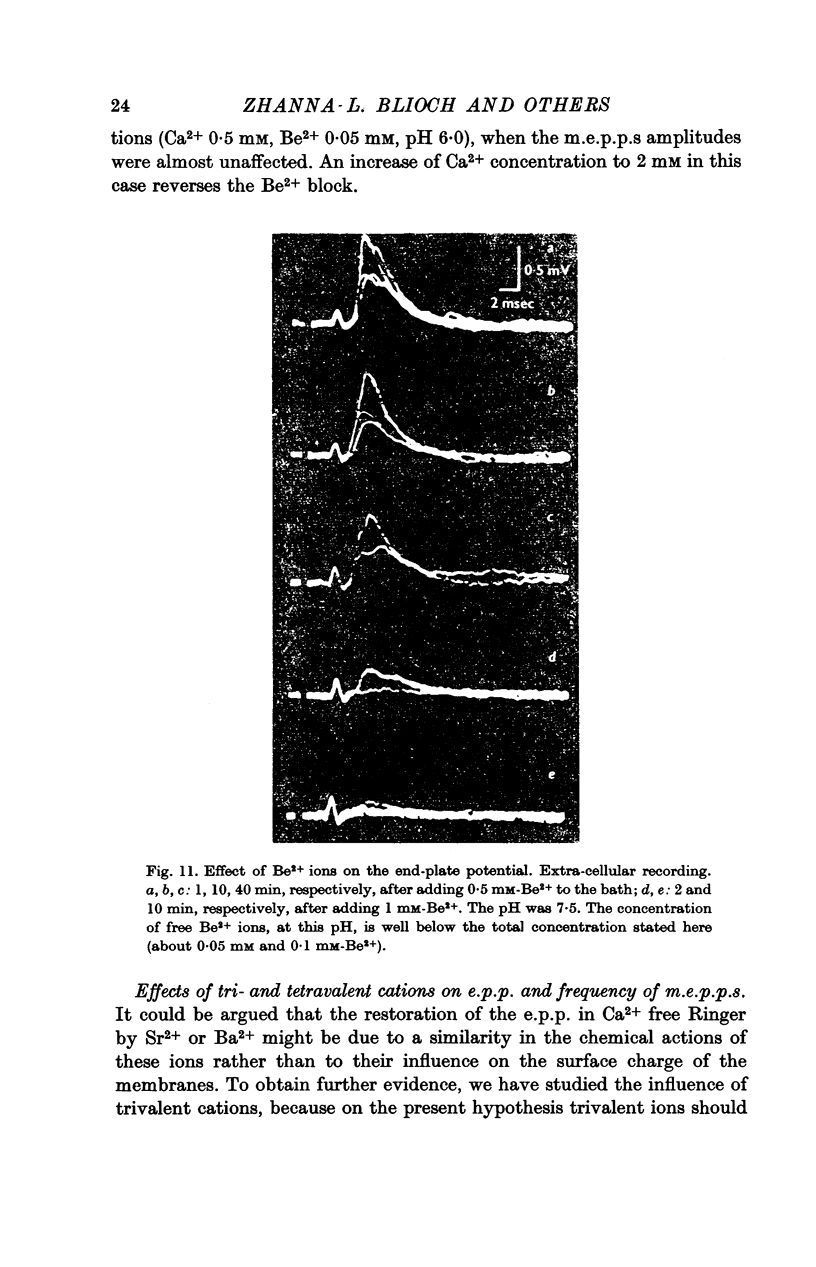
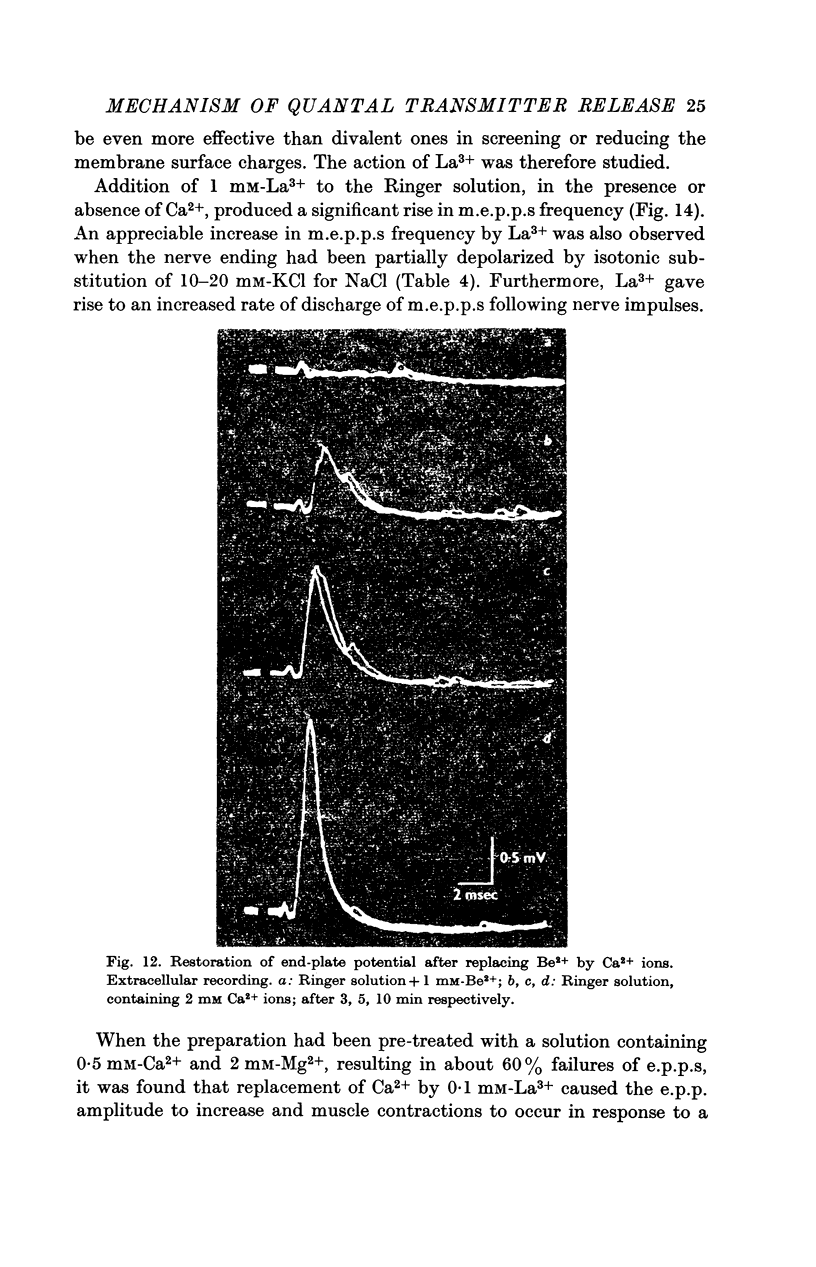
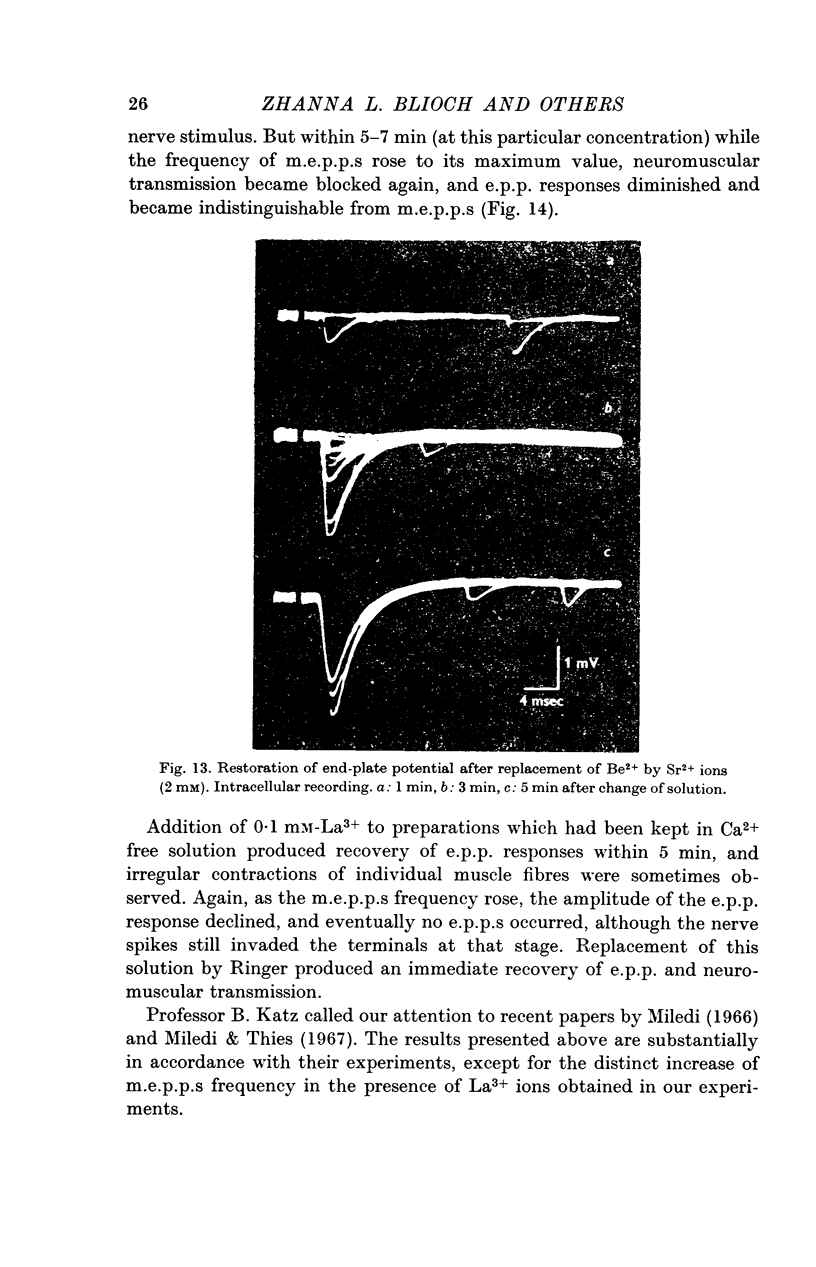
5. Divalent cations which penetrate only slightly (Mg2+, Be2+) lower the frequency of m.e.p.p.s and suppress the end-plate potential (e.p.p.) evoked by an impulse, in the presence of Ca2+ ions. Be2+ is a more effective inhibitor than Mg2+.
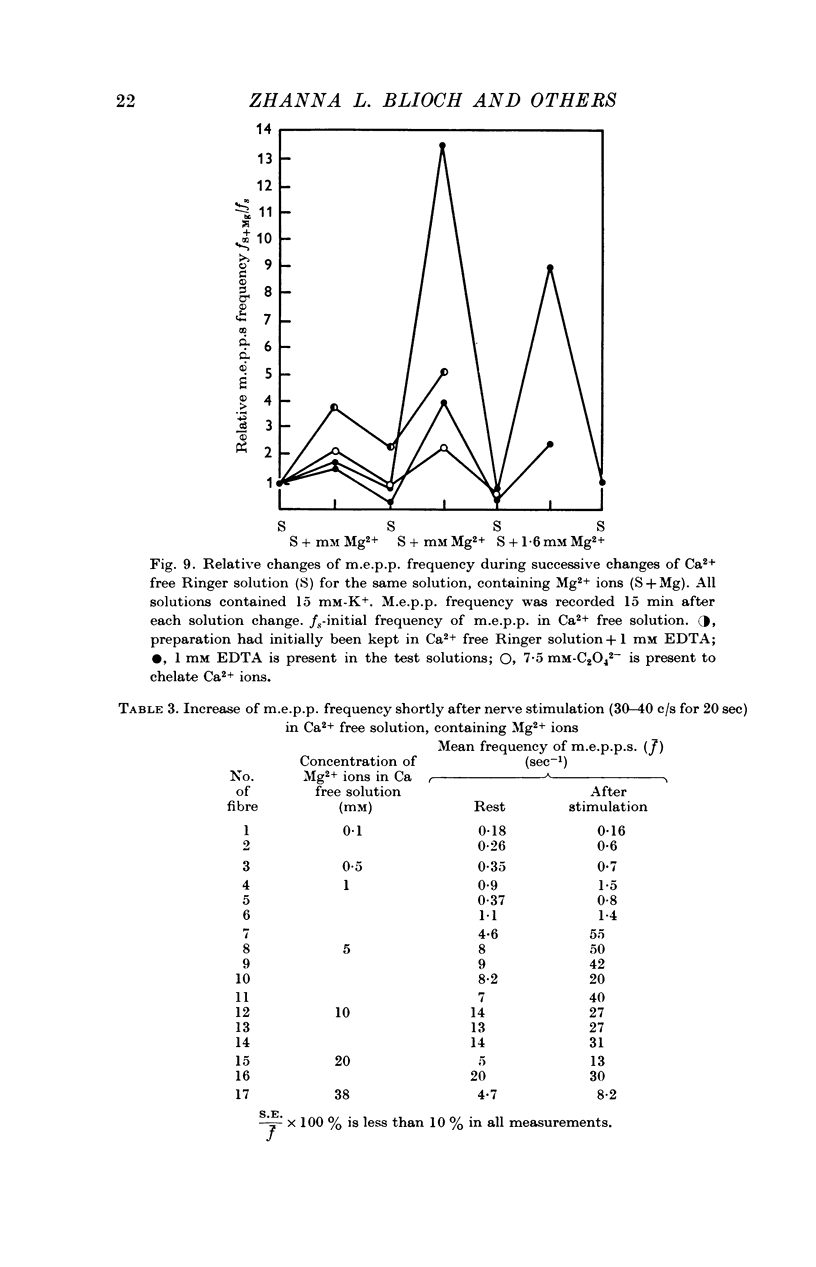
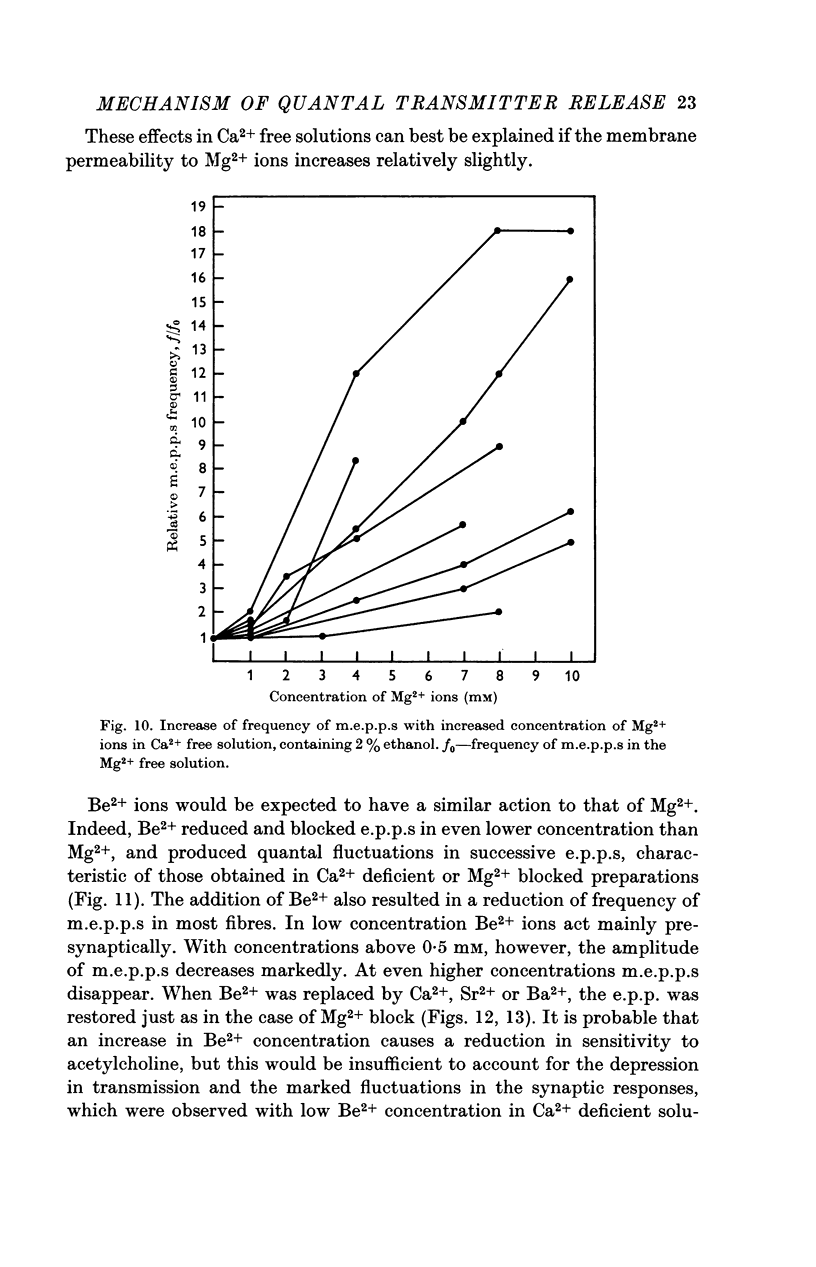
6. In Ca2+ free solutions, adding Mg2+ causes an increase in the frequency of m.e.p.p.s evoked by depolarization of the nerve endings or by treatment with ethanol.
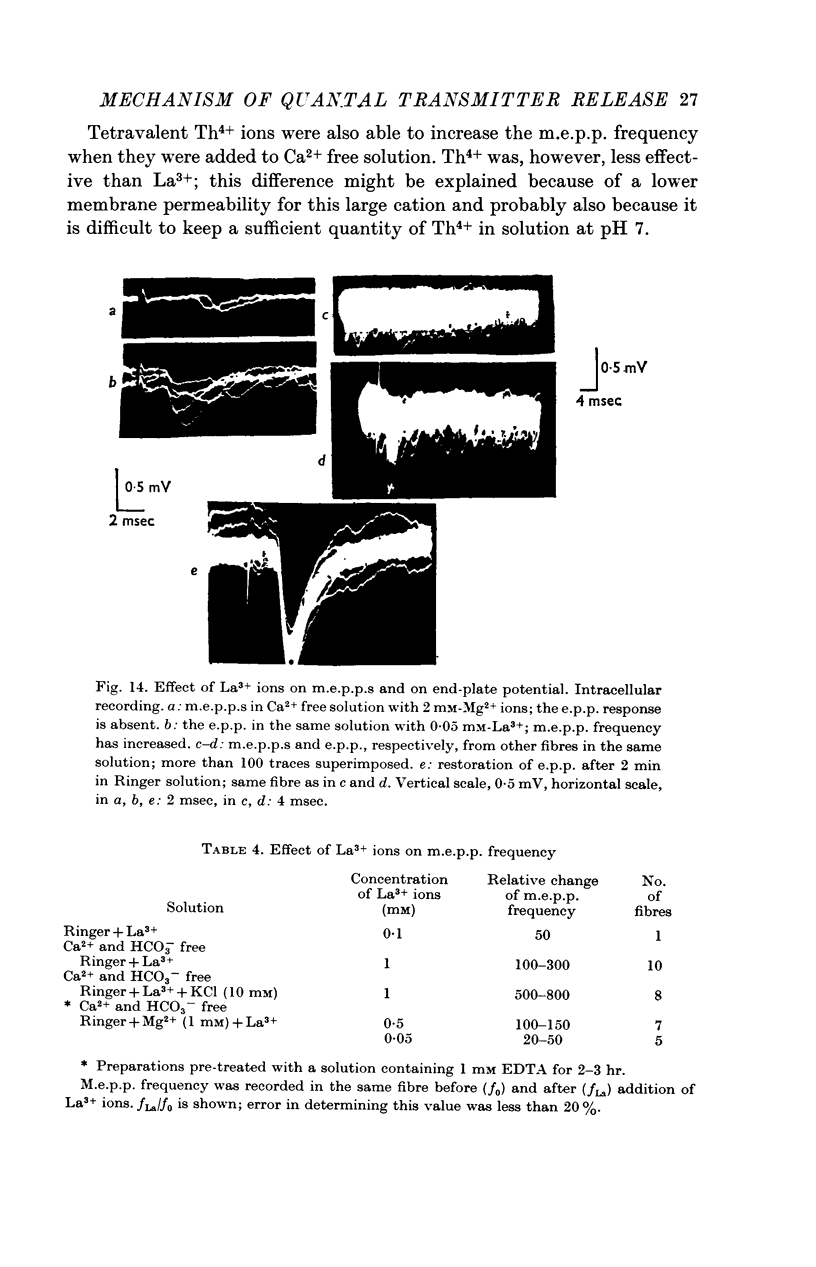
7. The trivalent cation La3+ is more effective than divalent cations are in increasing the frequency of m.e.p.p.s. The tetravalent cation Th4+ also raises the m.e.p.p. frequency.
8. The observations summarized in paragraphs 2-7 indicate that the frequency of m.e.p.p.s at a constant temperature depends only on the concentration of uni-, di- and trivalent cations inside the nerve ending. It is suggested that the internal cation concentration influences the adhesion between synaptic vesicles and the membrane of the nerve ending.
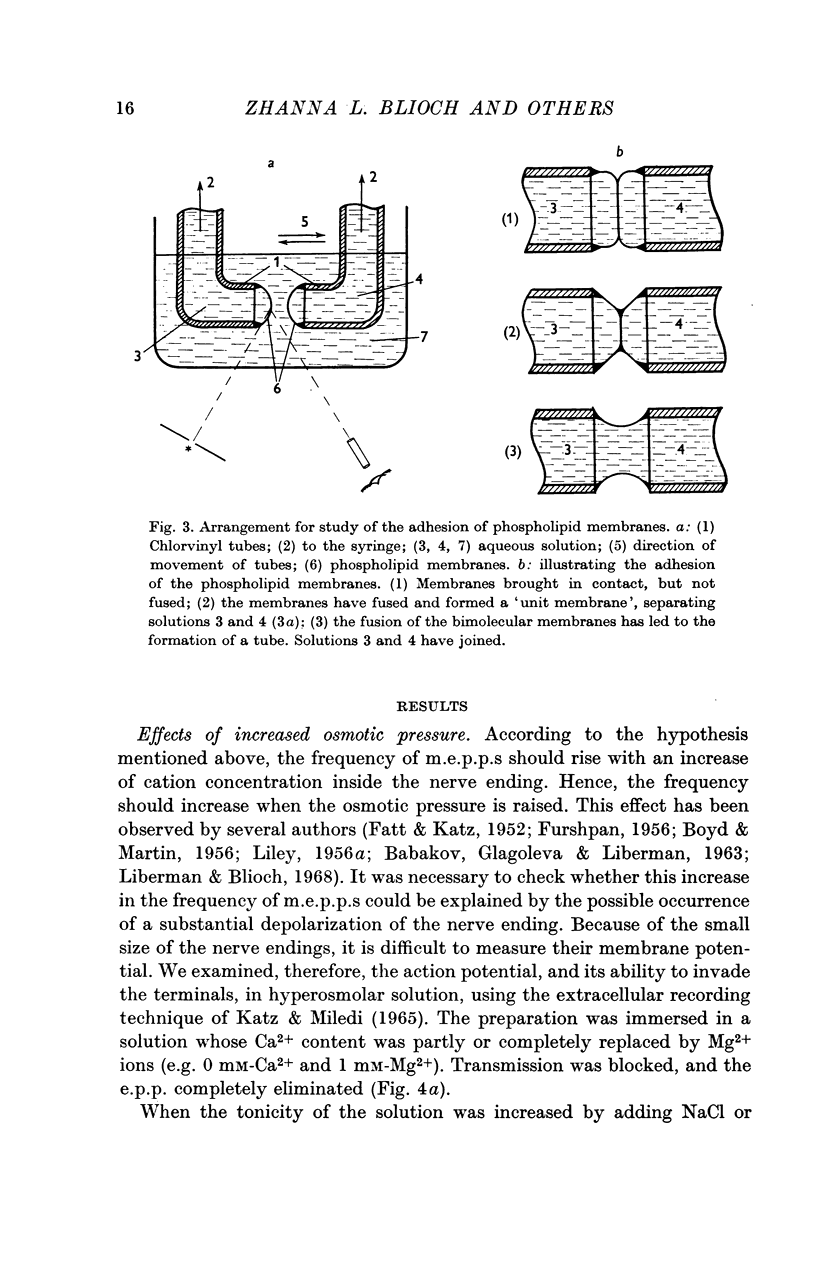
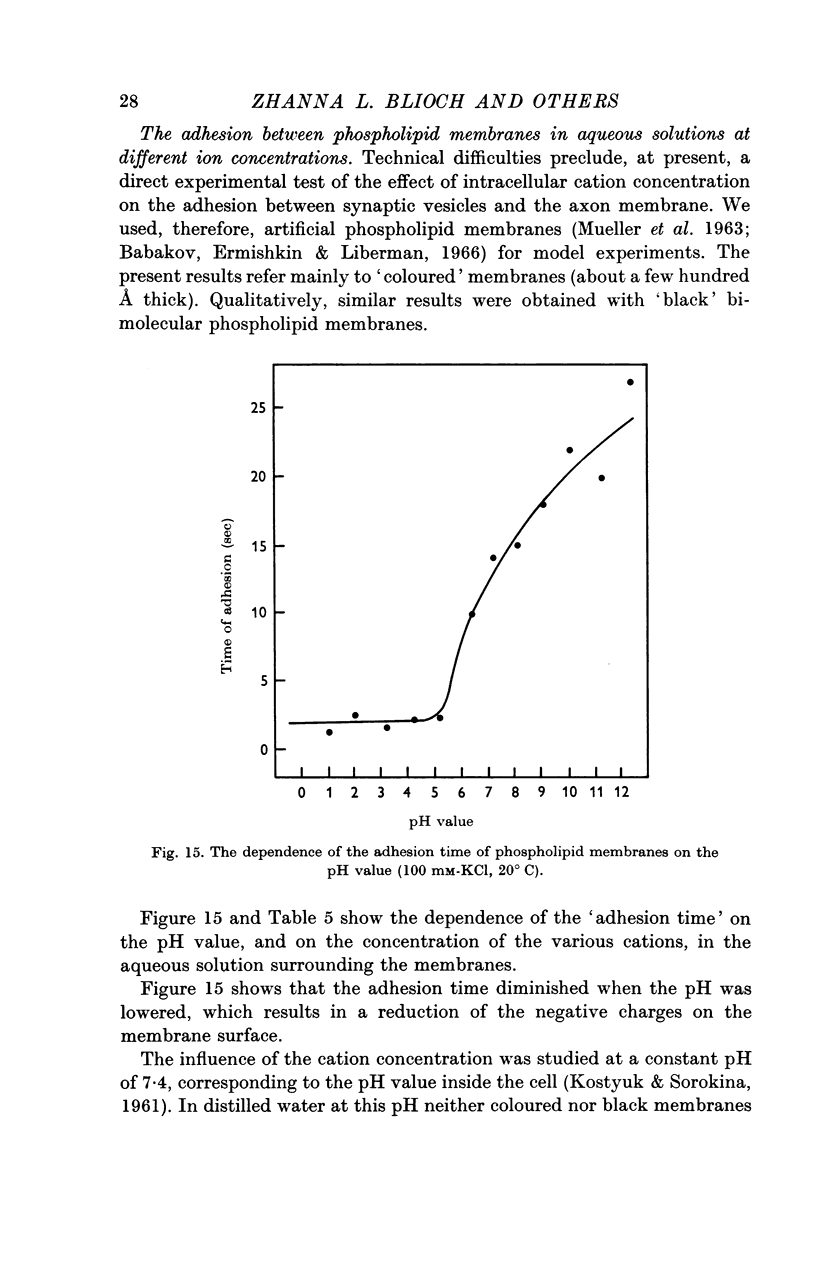
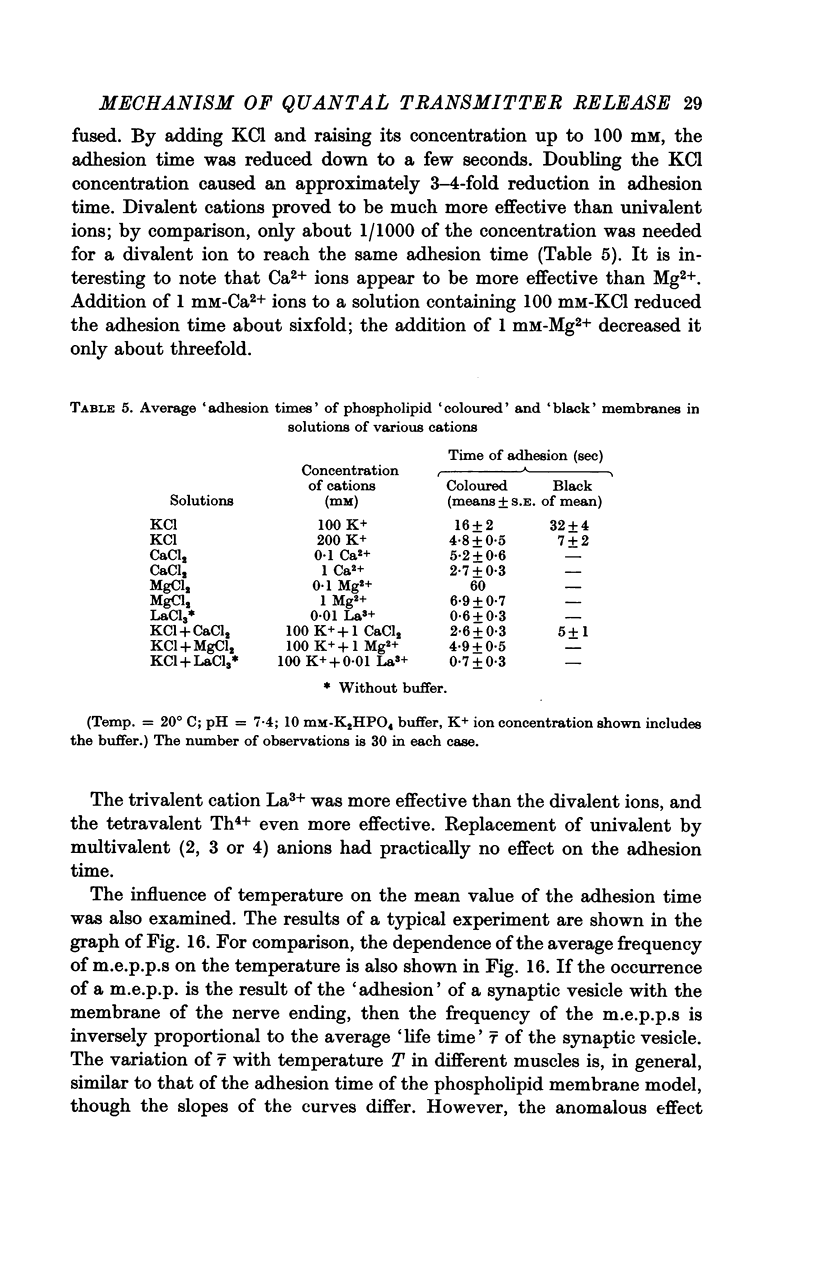
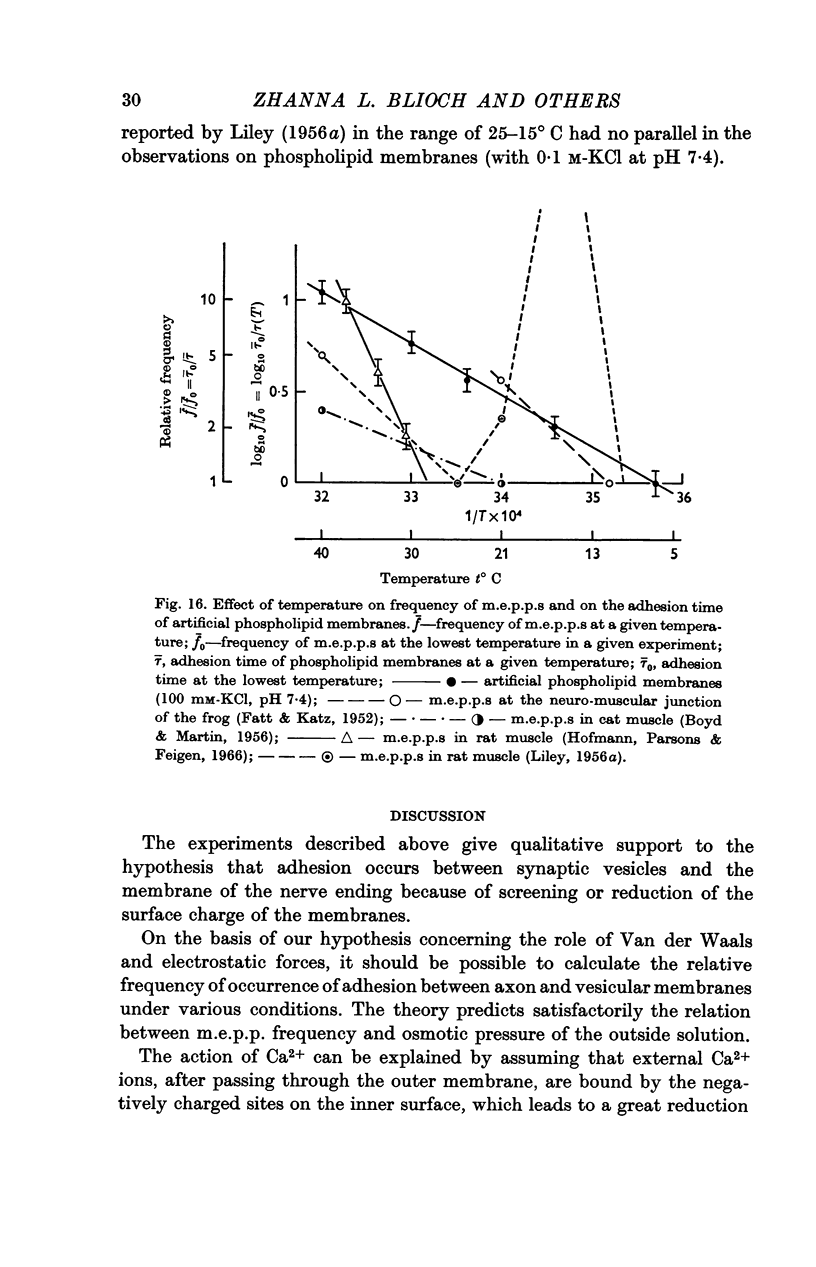
9. For a model experiment, artificial phospholipid membranes have been used to study the effect of uni-, di-, tri- and tetravalent cations on the adhesion process. At pH 7-7·4, the time required for adhesion to take place decreases with increasing cation concentration in the bath. Ca2+ ions are 100-1000 times more effective than K+ ions; La3+ and Th4+ ions are still more effective. The `adhesion time' decreases when the pH is lowered; it increases greatly with lowering of temperature.
10. The hypothesis is put forward that the mutual adhesion of artificial vesicles made of phospholipid membranes, and the adhesion between synaptic vesicles and the membrane of the nerve ending arise by a common mechanism. In both cases, the important factor is the influence of cations on the electric double layer at the membrane surface.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIANCHI C. P., SHANES A. M. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959 Mar 20;42(4):803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babakov A. V., Ermishkin L. N., Liberman E. A. Influence of electric field on the capacity of phospholipid membranes. Nature. 1966 May 28;210(5039):953–955. doi: 10.1038/210953b0. [DOI] [PubMed] [Google Scholar]
- CURTIS A. S. Cell contact and adhesion. Biol Rev Camb Philos Soc. 1962 Feb;37:82–129. doi: 10.1111/j.1469-185x.1962.tb01605.x. [DOI] [PubMed] [Google Scholar]
- DE ROBERTIS E., RODRIGUEZ DE LORES ARNAIZ G., PELLEGRINO DE IRALDI A. Isolation of synaptic vesicles from nerve endings of the rat brain. Nature. 1962 May 26;194:794–795. doi: 10.1038/194794a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., Lorković H., Weber A. The effect of the replacement of calcium by strontium on excitation-contraction coupling in frog skeletal muscle. J Physiol. 1966 Oct;186(2):295–306. doi: 10.1113/jphysiol.1966.sp008035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Influence of ionic environment on acetylcholine release from the motor nerve terminals. Acta Physiol Scand. 1966 May;67(1):34–42. doi: 10.1111/j.1748-1716.1966.tb03284.x. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956 Dec 28;134(3):689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Competition between sodium and calcium ions in transmitter release at mammalian neuromuscular junctions. J Physiol. 1966 Jul;185(1):95–123. doi: 10.1113/jphysiol.1966.sp007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W. W., Parsons R. L., Feigen G. A. Effects of temperature and drugs on mammalian motor nerve terminals. Am J Physiol. 1966 Jul;211(1):135–140. doi: 10.1152/ajplegacy.1966.211.1.135. [DOI] [PubMed] [Google Scholar]
- Kimizuka H., Nakahara T., Uejo H. Cation-exchange properties of lipid films. Biochim Biophys Acta. 1967 Jun 6;137(3):549–556. [PubMed] [Google Scholar]
- LIBERMAN E. A., TSOFINA L. M., VAINTSVAIG M. N. [Role of mono- and bi-valent ions in regeneration of the action potential]. Biofizika. 1961;6:45–51. [PubMed] [Google Scholar]
- LIBERMAN E. A., TSOFINA L. M. [Measurement of the flow of NA and Ca ions through the surface of crustacean muscle fibers during excitation]. Biofizika. 1962;7:201–202. [PubMed] [Google Scholar]
- LIBERMAN E. A., VORONIN L. L. POTENTSIALY DE ISTVIIA MYSHECHNYKH VOLOKON RAKA POSLE DLITEL'NOGO PREBYVANIIA V RASTVORAKH S IONAMI BA. Biofizika. 1963;8:579–587. [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman E. M., Palmer R. F., Collins G. H. Calcium ion uptake by crustacean peripheral nerve subcellular particles. Exp Cell Res. 1967 May;46(2):412–418. doi: 10.1016/0014-4827(67)90077-8. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K. Effects of calcium and magnesium ions on the frequency of miniature end-plate potential discharges in amphibian muscle in the presence of ethyl alcohol. Experientia. 1967 May 15;23(5):363–364. doi: 10.1007/BF02144517. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- TSOFINA L. M., LIBERMAN E. A. [Permeability of crab muscle fibers to Ca and Sr during excitation]. Biofizika. 1962;7:744–748. [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]



