Abstract
1. Insulin secretion from pieces of rabbit pancreas incubated in vitro was studied in media of different ionic compositions.
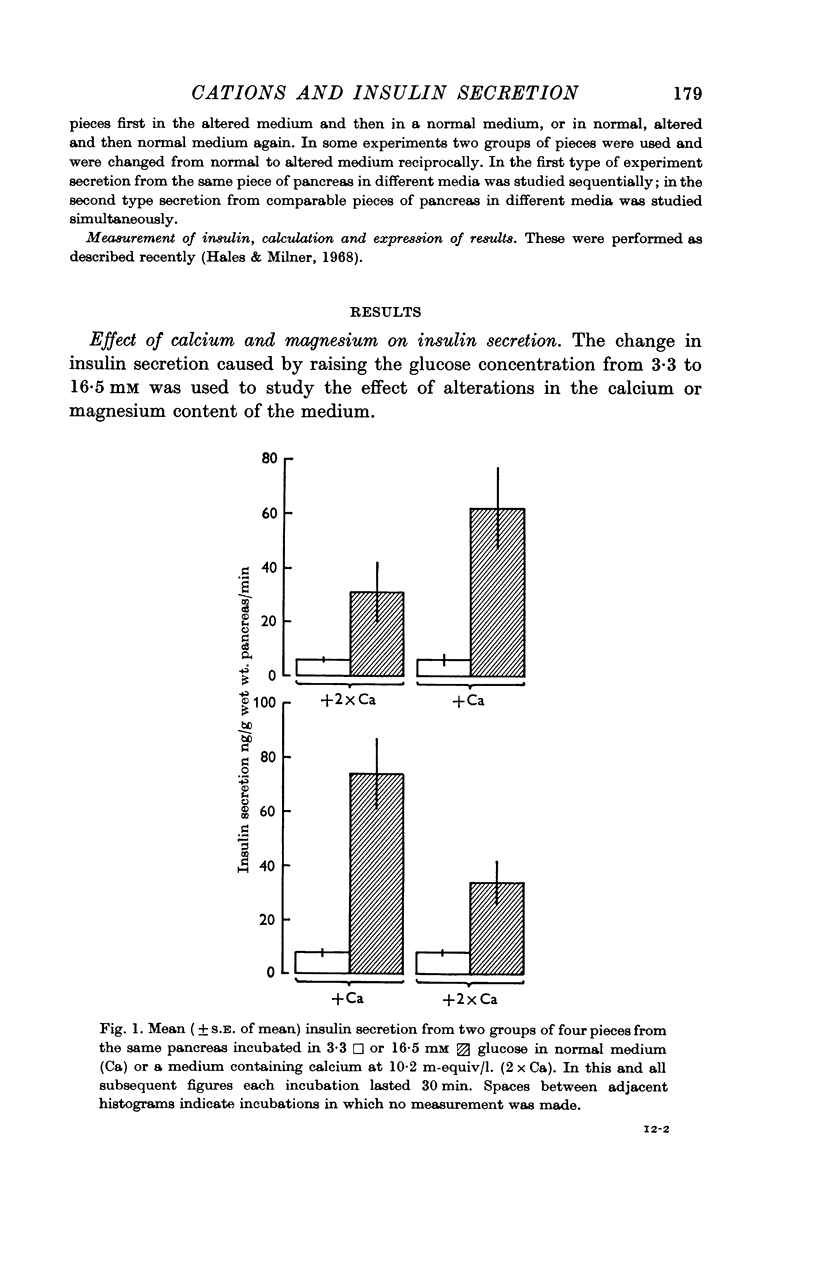
2. Insulin secretion stimulated by glucose was abolished by removal of calcium from the incubation medium and inhibited by a twofold rise in the calcium concentration to 10·2 m-equiv/l. Removal of magnesium from the medium did not affect glucose-stimulated secretion but a tenfold rise in the extracellular magnesium concentration to 24 m-equiv/l. abolished secretion.
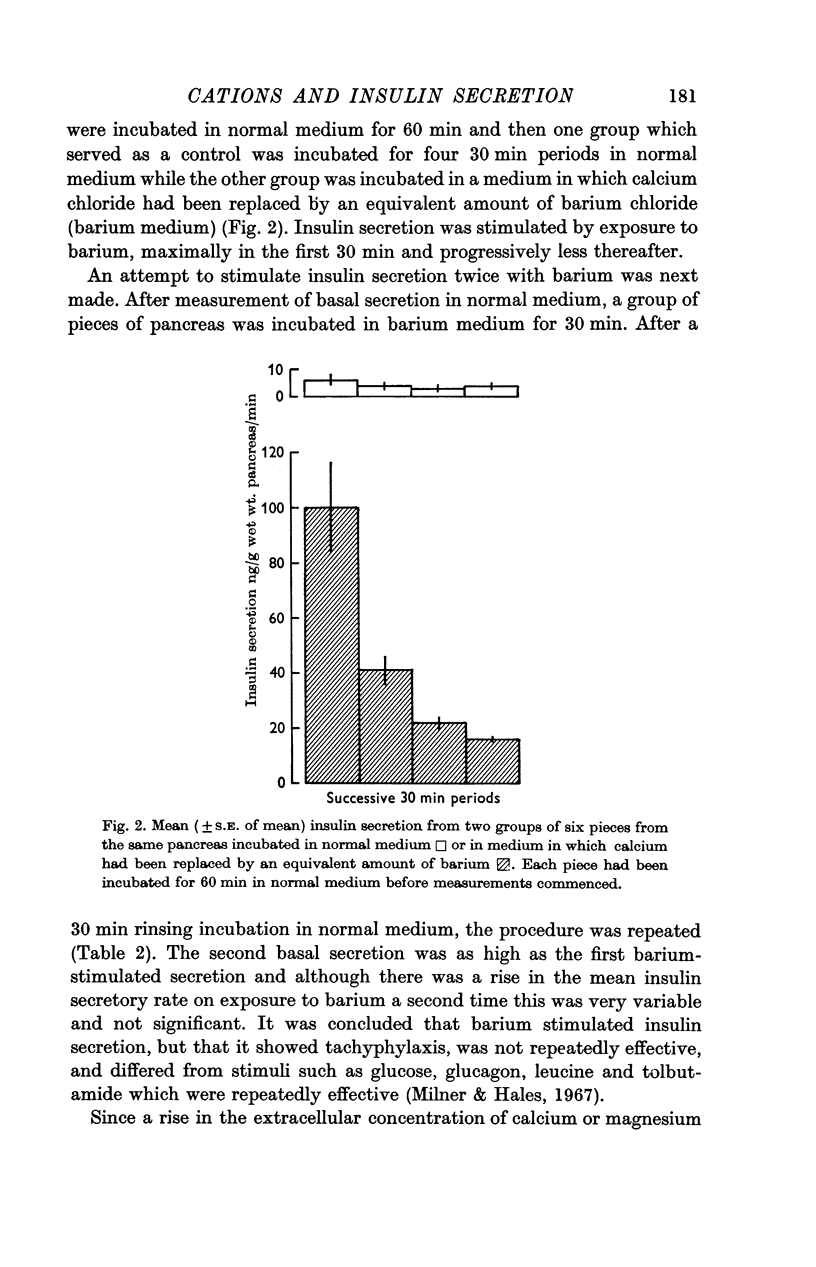
3. The replacement of calcium by an equivalent amount of barium (5·1 m-equiv/l.) stimulated insulin secretion. Barium stimulation declined with time and was inhibited by the presence of calcium, 5·1 m-equiv/l., or abolished by the presence of magnesium, 24 m-equiv/l., in the incubation medium.
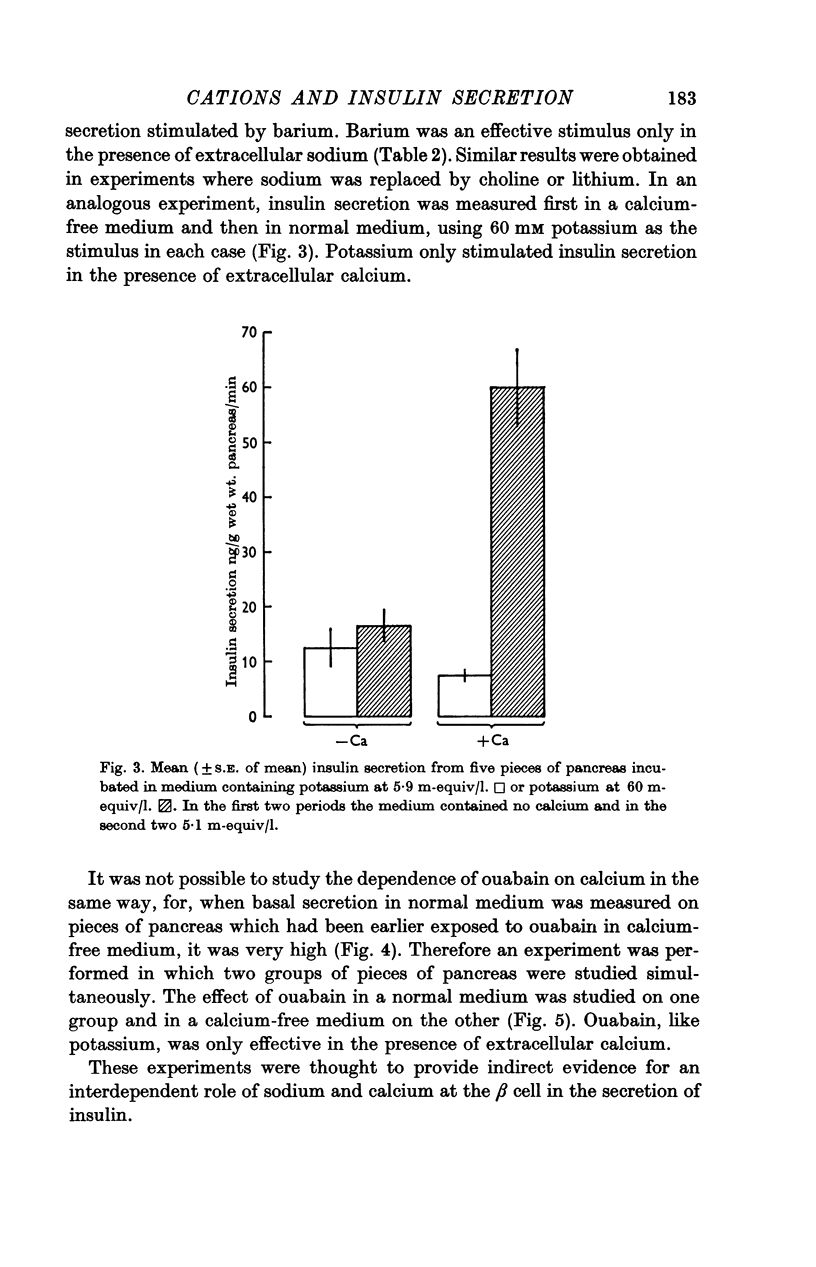
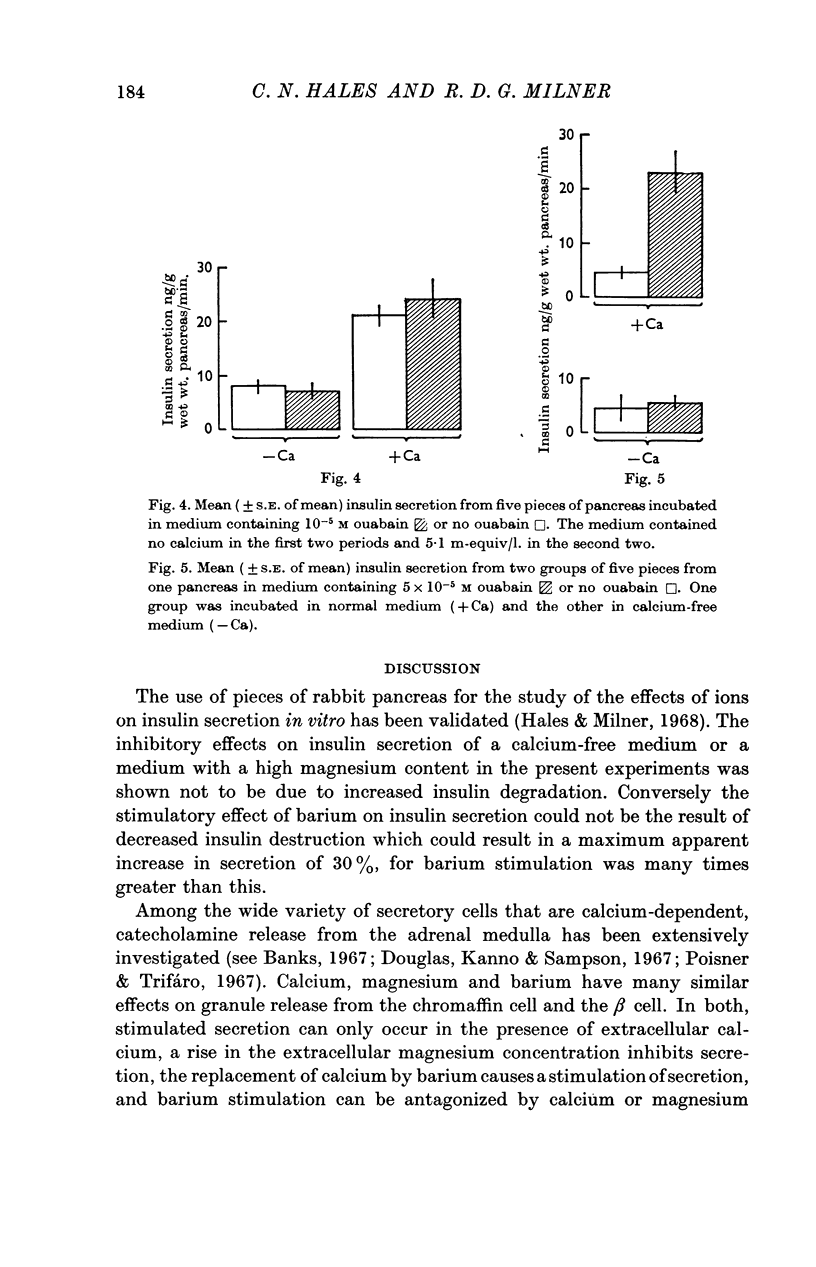
4. The interactions of mono- and divalent cations on insulin secretion were studied by using ouabain or potassium as tools to raise intracellular sodium concentration and barium as a calcium analogue. Ouabain and potassium were effective stimuli only in the presence of calcium, and barium only stimulated insulin secretion in the presence of sodium.
5. The results of these experiments suggest that both calcium and sodium must act at the β cell membrane or enter the cell before insulin release can occur in response to a variety of stimuli.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Banks P. The effect of ouabain on the secretion of catecholamines and on the intracellular concentration of potassium. J Physiol. 1967 Dec;193(3):631–637. doi: 10.1113/jphysiol.1967.sp008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Requirement for calcium ion in insulin secretion by the perfused rat pancreas. Am J Physiol. 1968 Jan;214(1):174–178. doi: 10.1152/ajplegacy.1968.214.1.174. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., STARK L. The effect of calcium ions on the motor end-plate potentials. J Physiol. 1952 Apr;116(4):507–515. doi: 10.1113/jphysiol.1952.sp004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. STIMULANT ACTION OF BARIUM ON THE ADRENAL MEDULLA. Nature. 1964 Jul 18;203:305–307. doi: 10.1038/203305a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker S. E. Release of vasopressin and oxytocin from isolated pituitary glands of adult and new-born rats. J Physiol. 1966 Jul;185(2):429–444. doi: 10.1113/jphysiol.1966.sp007994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. The role of sodium and potassium in insulin secretion from rabbit pancreas. J Physiol. 1968 Feb;194(3):725–743. doi: 10.1113/jphysiol.1968.sp008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. M., Macintosh F. C. Calcium and synaptic transmission in a sympathetic ganglion. J Physiol. 1940 Jan 15;97(3):408–416. doi: 10.1113/jphysiol.1940.sp003818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. Cations and the secretion of insulin. Biochim Biophys Acta. 1968 Jan 3;150(1):165–167. doi: 10.1016/0005-2736(68)90022-9. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. The role of calcium and magnesium in insulin secretion from rabbit pancreas studied in vitro. Diabetologia. 1967 Mar;3(1):47–49. doi: 10.1007/BF01269910. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Trifaró J. M. The role of ATP and ATPase in the release of catecholamines from the adrenal medulla. I. ATP-evoked release of catecholamines, ATP, and protein from isolated chromaffin granules. Mol Pharmacol. 1967 Nov;3(6):561–571. [PubMed] [Google Scholar]
- Samli M. H., Geschwind I. I. Some effects of energy-transfer inhibitors and of Ca++-free or K+-enhanced media on the release of luteinizing hormone (LH) from the rat pituitary gland in vitro. Endocrinology. 1968 Feb;82(2):225–231. doi: 10.1210/endo-82-2-225. [DOI] [PubMed] [Google Scholar]
- Vale W., Burgus R., Guillemin R. Presence of calcium ions as a requisite for the in vitro stimulation of TSH-release by hypothalamic TRF. Experientia. 1967 Oct 15;23(10):853–855. doi: 10.1007/BF02146887. [DOI] [PubMed] [Google Scholar]



