Abstract
The protective mechanisms induced in the mouse upper respiratory tract (URT) after intraperitoneal immunization with G2Na, a recombinant respiratory syncytial virus (RSV) G protein fragment (amino acid residues 130 to 230), were investigated. This protection was recently shown to be mediated by CD4+ T cells and to be critically dependent on the cysteines and amino acids 193 and 194 (H. Plotnicky-Gilquin, A. Robert, L. Chevalet, J.-F. Haeuw, A. Beck, J.-Y. Bonnefoy, C. Brandt, C.-A. Siegrist, T. N. Nguyen, and U. F. Power, J. Virol. 74:3455-3463, 2000). On G2Na, we identified a domain (amino acid residues 182 to 198) responsible for the T-helper-cell activity. This region coincided with a peptide designed AICK (residues 184 to 198) which includes the previously identified murine and human T-helper-cell epitope on the native G protein (P. W. Tebbey, M. Hagen, and G. E. Hancock, J. Exp. Med. 188:1967-1972, 1998). Immunization with AICK, in alum or complete Freund's adjuvant, significantly reduced nasal RSV titers in normal BALB/c mice. However, although lung protection was induced, in contrast to the case with live RSV, neither AICK nor G2Na was able to prevent nasal infection in gamma interferon (IFN-γ)-knockout mice. Anti-IFN-γ neutralizing antibodies partially inhibited URT protection after administration to G2Na-immunized BALB/c mice. Furthermore, while purified CD4+ T cells from BALB/c mice immunized with G2Na or AICK significantly reduced lung and nasal infection of naive recipient mice after adoptive transfer, the cells from IFN-γ-knockout mice had no effect. Together, these results demonstrated for the first time that the T-helper-cell epitope of RSV G protein induces URT protection in mice after parenteral immunization through a Th1-type, IFN-γ-dependent mechanism.
Respiratory syncytial virus (RSV) accounts for most of the annual severe viral respiratory infections which occur in infants, children, and the elderly (14, 34, 35). In adults, RSV infection is also frequent but is generally restricted to the upper respiratory tract (URT) because of progressive accumulation of protective immune responses (13, 16). However, an efficient immunization should be able to protect both the lower respiratory tract (LRT) and the URT in order to prevent the transmission of the virus to less immunocompetent individuals.
To date, no RSV vaccine candidate has successfully passed phase III clinical trials. Major obstacles encountered by the different approaches relate to the lack of immunogenicity and/or protective efficacy in the vaccinees and, most importantly, the risk, through induction of aberrant T-cell responses (20, 24, 41), of immunopotentiating the disease at the time of natural infection.
Among RSV proteins, G protein, the highly glycosylated attachment protein, has been clearly implicated in such adverse immunopathologic responses (15, 41). This protein is highly immunogenic and confers lung protection in animal models through induction of RSV-specific antibodies (Abs) (8, 32). However, the protection tends to be strain specific due to the high degree of variability that characterizes the protein (7, 39). In addition, purified G protein or vaccinia virus vectors expressing this protein prime for a Th2 immune response which is responsible for an aberrant T-cell activation and lung eosinophilia after RSV challenge (1, 15, 18, 37).
Interestingly, none of these pathological responses were induced in animal models after immunization with a recombinant, nonglycosylated RSV G protein fragment (called G2Na) produced in Escherichia coli (9, 26, 28). G2Na comprises residues 130 to 230, including the conserved central domain of RSV G protein (residues 164 to 176) (12). It also contains the region located between amino acid residues 184 and 198, which was recently associated with Th2-type immune responses and priming for lung eosinophilia in mice (38). In rodents, G2Na fused to BB, a carrier protein (23) (BBG2Na), induces a rapid, potent, and long-lasting lung and nasal protection against both RSV-A and -B challenge (29).
In a previous study, we showed that protection of the LRT and URT after intraperitoneal (i.p.) immunization with BBG2Na relies on separate immune mechanisms (27). While circulating Abs account for protection of lungs against RSV, CD4+ T cells are required for the URT. In addition, the use of site-specific and deletion mutants allowed the identification of a region containing critical amino acids for URT protection, which is located between amino acid residues 173 and 194 (27).
In the present study, we first mapped this region and identified a domain responsible for the induction of the T-helper-cell activity. We then demonstrated, for the first time, that a peptide encompassing the T-helper-cell epitope of RSV G protein is able to induce lung and nasal RSV protection in BALB/c mice. Finally, we showed that IFN-γ plays an essential role in the control of URT infection.
MATERIALS AND METHODS
Production and purification of G2Na.
Gene assembly, vector constructions, expression, and first-step protein purification of G2Na were done as previously described (10, 29). After freeze-drying, the protein was further purified to homogeneity by reversed-phase high-performance liquid chromatography on a preparative Vydak (Hesperice, Calif.) C4 column (250 by 22 mm [inner diameter], 300 Å [pore size], 10 μm [particle size]) with a triethylammonium formate buffer (TEAF)-acetonitrile gradient, using 40 mM TEAF (pH 3.0) (solvent A) and a mixture of 40 mM TEAF (pH 3.0) and acetonitrile (10:90) (solvent B). The flow rate was 8 ml/min, and the gradient consisted of a 0- to 37.5-mm linear gradient from 5 to 80% solvent B (2%/min). Fractions were collected for analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. G2Na-containing fractions were pooled individually and freeze-dried. Protein content was determined by the bicinchoninic acid method, and the protein was analyzed for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 15% homogeneous gel under reducing conditions.
Peptide synthesis.
A set of 90 overlapping dodecamers offset by one residue and covering the whole G2Na sequence was synthesized on 2-hydroxyethyl metacrylate-grafted polyethylene pins with a Chiron Technologies Multipin DKP cleavable-peptide kit (31). A coupling rate of two amino acids per day was used. After removal of the final terminal amino acid 9-fluorenylmethoxy carbonyl group and the side chain protecting groups, peptides were cleaved from the support by sonication with an ammonium bicarbonate buffer (0.1 M, pH 8.4) with 40% (vol/vol) acetonitrile as a cosolvent, leading to diketopiperazine formation of the Lys-Pro C-terminal moiety (3). The peptide solutions were then freeze-dried. The two control decapeptides PGPSDTPILP and ERVYIHPFHL were analyzed by reverse-phase high-performance liquid chromatography. The chromatograms which were obtained were similar to those of the supplier.
Peptides AICKRIPNKKPGKKT (named AICK) and AICGRGPNGKPGKKT (named AICG), corresponding to peptide 19 and a mutated sequence at three anchor positions used by Tebbey et al. (38), were also synthesized on a Applied Biosystems 433A peptide synthesizer by using conventional 9-fluorenylmethoxy carbonyl-tbutyl chemistry on a rink amide resin.
Virus preparation, challenge procedure, and titration.
RSV-A (Long strain; ATCC VR-2; American Type Culture Collection, Manassas, Va.) was propagated in HEp-2 cells (ECACC 86030501; European Collection of Animal Cell Culture, Porton Down, Salisbury, United Kingdom) as previously described (29). The virus stock was prepared from the supernatant of a 48- to 72-h culture and stored at −196°C until use. Mice were anesthetized by intramuscular injection of 2.5 ml of a 4/1 (vol/vol) mixture of ketamine (Imalgène 500; Rhône Mérieux, Lyon, France) and xylazine (2% Rompun; Bayer, Puteaux, France) per kg and challenged intranasally (i.n.) with 50 μl of a virus preparation containing 105 tissue culture infective doses (TCID50) of RSV-A. For virus titration in lungs and nasal tracts, mice were sacrificed 5 days after challenge following anesthesia and total intracardiac puncture. Removal and processing of lungs and nasal tract lavage fluids (NTL) and virus titrations were done as previously described (29). The limit of detection of virus in lung tissues and NTL were log10 1.45/g and 0.45/ml, respectively.
Mice.
Specific-pathogen-free female BALB/c, SCID CB17 (CB17 PrkdeSCID on a BALB/c background), and IFN-γ-knockout (BALB/c-Infg tm1Ts) inbred mice, age 8 to 9 weeks, were purchased from IFFA CREDO (l'Arbresle, France) and kept under specific-pathogen-free conditions. They were given sterilized mouse maintenance diet AO4 (Usine d'Alimentation Rationnelle, Villemoissin-sur-Orge, France) and water ad libitum and manipulated according to French and European guidelines. BALB/c and IFN-γ-knockout mice were confirmed to be seronegative for RSV-A before inclusion in the experiments.
ELISA.
G2Na- and RSV-A-specific immunoglobulin G (IgG) titers were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (29). ELISA titers were expressed as the reciprocal of the last dilution that had an optical density of >0.15 and at least twofold that of the control well to which no sample was added.
Localization of the T-helper-cell epitope on G2Na.
Six-week-old pathogen-free BALB/c mice were used for the experiments. Groups of 10 mice were immunized either with 15 μg of G2Na per mouse or phosphate-buffered saline (PBS) in complete Freund's adjuvant (CFA) (Sigma, St. Quentin Fallavier, France) in the base of the tail. Ten days after immunization, inguinal lymph nodes were removed and pooled within experimental groups into RPMI 1640 (Gibco, Cergy Pontoise, France). A single-cell suspension was made by teasing the nodes apart. The cells were washed and resuspended in medium to give a suspension of 106 cells/ml.
Cells were stimulated in vitro by incubating 3.5 × 105 cells/well in 96-well round-bottom plates (Costar, Brumath, France) in triplicate with 10 to 50 μg of the various peptides. As positive control, cells were activated with 50 μg of G2Na per ml. Cultures were incubated for 72 h at 37°C with 5% CO2. Eighteen hours before the end of the culture, the cultures were pulsed with 1 μCi of [methyl-3H]thymidine (Amersham, Les Ulis, France). The cells were harvested onto filters by using an semiautomatic harvester (Skaton, Lier, Norway), and the incorporated [3H]thymidine was determined with a Packard 1900CA-Tricarb beta counter. Results were expressed in counts per minute and were calculated from the geometric means of triplicate cultures.
Immunization procedures for protection studies.
Mice were immunized by two or three i.p. injections of a 200-μl preparation containing either 20 μg of G2Na; 12, 32, or 40 μg of peptide; or 105 TCID50 of live RSV at 2-week intervals. G2Na was always administered with 20% (vol/vol) of Al(OH)3 (alum; Superfos BioSector, Vedbaek, Denmark), and peptides were administered in either alum or 50% (vol/vol) CFA. Control mice (immunized with PBS or peptide AICG) were treated with CFA or alum. Since similar results were obtained in both cases, and only the results obtained with mice treated with CFA are shown. A serum puncture was performed before RSV challenge, 3 weeks after the last immunization, to document the seroconversion.
In vivo neutralization of IFN-γ was performed on the same day as the RSV challenge by one i.p. injection (per mouse) of 100 μg of goat anti-mouse IFN-γ-neutralizing or control IgG Abs (AF-485-NA and AB-108C, respectively; R & D Systems) diluted in 200 μl of PBS, followed for 2 days by daily i.n. administrations of 10 μg in 20 μl.
Cell preparation and adoptive cell transfer.
BALB/c and IFN-γ-knockout mice were sacrificed 10 days after three i.p. immunizations with G2Na or peptides. Their spleens and mesenteric lymph nodes were removed, processed into single-cell suspensions in RPMI 1640 with 10% fetal calf serum, and incubated on a nylon wool column for 90 min at 37°C. The nonadherent cells were carefully eluted and washed in PBS. CD4+ T lymphocytes were obtained by purification using the Magnetic Activated Cell Separation System (Miltennyi Biotec, Bergisch-Glodbach, Germany). Cells were incubated for 45 min at 4°C with microbeads coated with anti-CD19 and anti-CD8 Abs (Miltennyi Biotec). Attached B and CD8+ cells were eliminated by passage through a magnetic column according to the recommendations of the manufacturer. The nonadherent CD4+ T cells (>95% viability) were more than 97% pure, as controlled by flow cytometry. They were transferred into H-2-identical SCID mice at 20 × 106 cells per mouse to investigate their protective efficacy, 2 to 3 h after previous i.n. challenge of the mice with 105 TCID50 of RSV-A.
Statistical analyses.
Statistical analyses were done using the t test and Kolmogorov-Smirnov tests (for low sample numbers) of the Statigraphic software program (Manugistics, Rockville, Md.).
RESULTS
Localization of the T-cell determinant of G2Na by using 12-mer peptides.
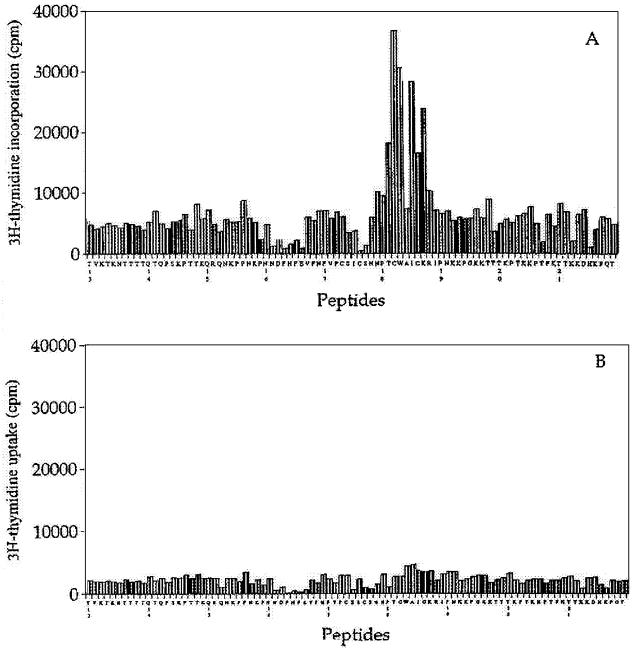
In order to localize T-cell epitopes on G2Na, 90 peptides of 12 amino acids in length covering the complete sequence of G2Na were synthesized. Proliferation of inguinal lymph nodes from either naive or G2Na-immunized mice was investigated. The proliferation of cells from G2Na-immunized mice was dose dependent, with a maximal effect at 50 μg of peptide/ml (data not shown). This proliferation was observed only with peptides covering the region from amino acid 182 to 198 of G2Na, corresponding to the sequence CWAICKRIPNKKPGKKT (Fig. 1A). The results were confirmed with different groups of mice as well as different batches of peptides (data not shown), while no proliferation was consistently obtained with cells from naive mice (Fig. 1B). Previous experiments in our laboratories demonstrated that depletion of CD4-positive cells abolished the T-cell proliferation observed after stimulation of immune cells with G2Na (9). Similarly, cell proliferation of G2Na-immunized mice induced by the peptides covering the region from amino acid 182 to 198 was completely eliminated after depletion of CD4-positive cells (data not shown), confirming that it was CD4 mediated. Together, these results showed that the recombinant G protein fragment from position 130 to 230 of the human RSV contains a T-helper-cell determinant located between amino acid residues 182 and 198. Indeed, this region coincides with the murine and human T-cell epitope identified on the native G protein by Tebbey et al. and located within a peptide, named AICK, encompassing residues 184 to 198 (38).
FIG. 1.
Proliferation of cells from G2Na-immunized mice with 12-mer peptides. Cells were harvested from inguinal lymph nodes of G2Na-immunized (A) or PBS-immunized (B) mice and stimulated for 48 h with 50-μg/ml concentrations of the 90 peptides derived from G2Na. They were pulsed with 1 μCi of [3H]thymidine for 18 h. The results are expressed as means of triplicate determinations and are representative of those from four different experiments.
RSV G protein T-helper-cell epitope induces the protection of the URT.
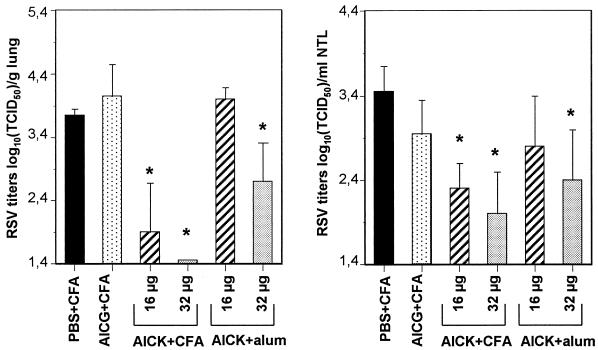
Previous work performed in mice with protein fragments derived from G2Na suggested that the T-helper-cell epitope located on the G protein plays a critical role in the protection of the URT against RSV infection (27). To confirm these results, we decided to immunize the mice with the T-helper-cell epitope itself and used the AICK peptide. Control mice were immunized with PBS or AICG peptide. AICG, also described by Tebbey et al. (38), includes residues 184 to 198, with K-to-G mutations at positions 187 and 192 and an I-to-G mutation at position 189. These mutations resulted in complete inhibition of the T-helper-cell activity, as demonstrated by the absence of response in a T-cell proliferation assay (data not shown). AICK and AICG were injected i.p. at 16 or 32 μg per injection, either in the presence of alum (i.e., under the experimental conditions previously used to show the importance of the RSV G protein T-helper-cell epitope in URT protection, [27]) or in CFA (which is a stronger adjuvant than alum in mice). Control mice were immunized with alum or CFA, with similar results (not shown). As reported in Table 1 (experiment I), almost all mice treated with AICK developed an IgG response, confirming an earlier observation (25) that the region from amino acid 184 to 198 of RSV G protein includes a B-cell epitope. These IgGs were detected against both G2Na and RSV antigens. In contrast, AICG-immunized mice remained seronegative (ELISA titers of <1.95 log10), comparing favorably with mice immunized with PBS in adjuvant. In accordance with this humoral response, lung RSV titers were reduced, with the highest effect being obtained in mice immunized with the highest doses of AICK in CFA (Fig. 2) (P > 0.05). Similarly, nasal RSV titers were reduced after administration of AICK but not AICG, with the highest effect being obtained with 32 μg of AICK in CFA (2 ± 0.5 log10 TCID50/ml of NTL, versus 3.45 ± 0.3 in PBS-immunized mice). These results demonstrated that the T-helper-cell epitope of RSV G protein is able to induce a protective activity against RSV infection in both the mouse LRT and URT.
TABLE 1.
Serum IgG titers after immunizations with peptidesa
| Expt | Mice | Immunization | IgG ELISA titer (log10)b
|
|
|---|---|---|---|---|
| G2Na | RSV | |||
| I | BALB/c | PBS + CFA | <1.95 | <1.95 |
| 32 μg of AICG + CFA | <1.95 | <1.95 | ||
| 16 μg of AICK + CFA | 4.3 ± 0.6 | 3.3 ± 0.5 | ||
| 32 μg of AICK + CFA | 4.4 ± 0.56 | 3.4 ± 0.6 | ||
| 16 μg of AICK + alum | <1.95 | <1.95 | ||
| 32 μg of AICK + alum | 3 ± 1.07 | 2 ± 0.19 | ||
| II | BALB/c | 40 μg of AICK + CFA | <1.95 | <1.95 |
| 40 μg of AICK + CFA | 2.9 ± 1.12 | 2.8 ± 0.85 | ||
| 20 μg of G2Na + alum | 5.58 ± 0.26 | 4.8 ± 0.48 | ||
| 105 TCID50 of RSV | 3.3 ± 0.62 | 4.1 ± 0.26 | ||
| IFN-γ knockout | 40 μg of AICG + CFA | <1.95 | <1.95 | |
| 40 μg of AICK + CFA | 2.6 ± 0.58 | 2.6 ± 0.89 | ||
| 20 μg of G2Na + alum | 5.6 ± 0.42 | 5.2 ± 0.40 | ||
| 105 TCID50 of RSV | 3.6 ± 0.26 | 3.9 ± 0.21 | ||
BALB/c and IFNγ-knockout mice were immunized i.p. three times (experiment I) or two times (experiment II) at 2-week intervals with AICG, AICK, or G2Na, adjuvanted in either alum or CFA, or with 105 TCID50 of live RSV, as indicated in Materials and Methods. Seroconversions were documented by ELISA 3 weeks after the last immunization, using G2Na and RSV antigens.
The results are expressed as the means ± standard deviations of IgG titers (log10) calculated from six mice per group.
FIG. 2.
Lung and nasal tract protection following immunization with AICK. BALB/c mice were immunized three times with different doses of AICK or AICG in either alum or CFA. Control mice received PBS in CFA. The mice were challenged and sacrificed after 5 days for titration of RSV in lungs and nasal tracts. Results are expressed as means ± standard deviations of lung and nasal RSV titers calculated from six mice per group. ∗, P < 0.05 compared with mice immunized with AICG in CFA.
IFN-γ is essential for the protection of the URT.
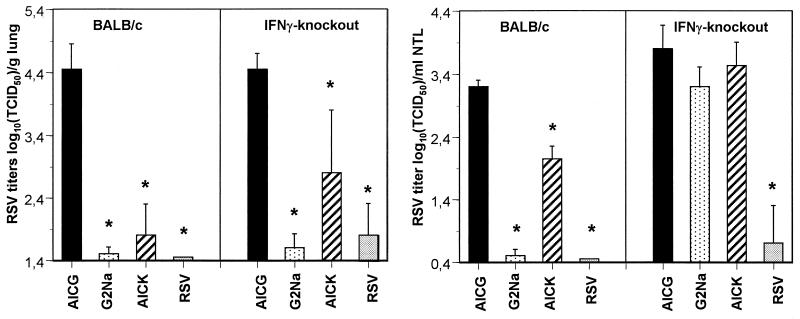
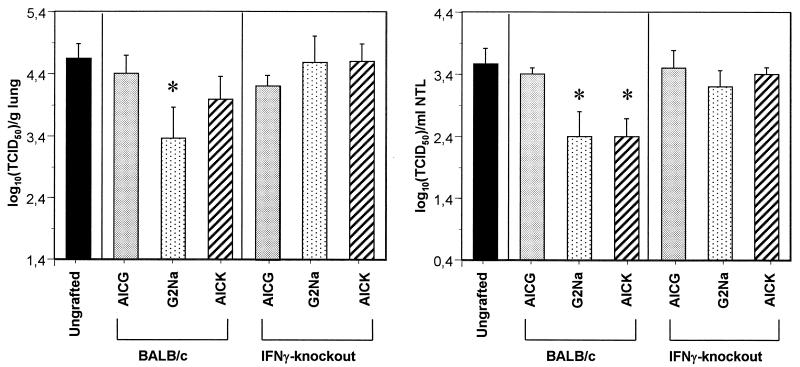
Since G2Na-induced URT protection was recently shown to rely on CD4+ and not CD8+ T cells (27) and since cytotoxic T cells were never observed after immunization with RSV G protein in animal models and AICK was able to decrease URT infection, we hypothesized that G2Na-specific CD4+ T helper cells generated after AICK immunization display a protective activity by the release of antiviral cytokines. To test the role of IFN-γ in URT protection, BALB/c and IFN-γ-knockout mice were immunized twice with 20 μg of G2Na in alum (i.e., under conditions used previously for induction of URT protection [27]), 40 μg of AICK in CFA (i.e., the most efficient formulation used for immunization with the peptide). or 105 TCID50 of live RSV. Control mice for infection received AICG in CFA. The mice, with the exception of those immunized with AICG, developed anti-G2Na and anti-RSV-A Abs, as indicated in Table 1 (experiment II). In both strains of mice, lung RSV titers were significantly reduced after immunization with G2Na, AICK, and RSV compared with those of the mice treated with AICG, although this reduction was less important in IFN-γ-knockout mice than in BALB/c mice (Fig. 3) (2.8 ± 1 versus 1.8 ± 0.5 log10 TCID50/g of lung for BALB/c and IFN-γ-knockout mice, respectively, following immunization with AICK; P < 0.05). In contrast, in the nasal tract, while live RSV reduced infection in both BALB/c and IFN-γ-knockout mice, G2Na and AICK were protective in BALB/c mice only. These results showed that IFN-γ is essential for URT protection induced by G2Na and by the CD4+ T-helper-cell epitope.
FIG. 3.
Effects of G2Na and AICK in BALB/c and IFN-γ-knockout mice. BALB/c and IFN-γ-knockout mice were immunized twice i.p. with 20 μg of G2Na in alum, 40 μg of AICK or AICG in CFA, or 105 TCID50 of live RSV. Control mice were treated with PBS in CFA. Three weeks later, they were challenged for titration of RSV in lungs and nasal tracts. The results are expressed as means ± standard deviations of RSV titers calculated from five or six BALB/c or IFN-γ-knockout mice per group. ∗, P < 0.05 compared with mice immunized with AICG in CFA.
In vivo neutralization of IFN-γ reduces the protection of the URT.
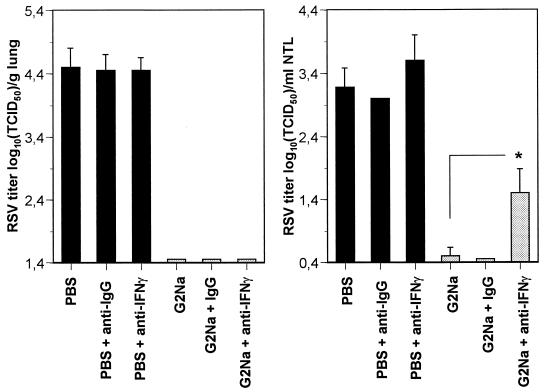
To determine whether secreted IFN-γ is directly involved in the protection of the URT against RSV infection, BALB/c mice previously immunized with G2Na or PBS in alum were treated i.p. and i.n. with anti-IFN-γ neutralizing Abs or with control IgGs and challenged with 105 TCID50 of RSV. Lung and nasal tract RSV titers were determined after 5 days. As shown in Fig. 4, the anti-IFN-γ neutralizing Abs had no effect on lungs, which remained completely protected from RSV infection. In contrast, they induced a small but significant increase of RSV titers in the URT (1.5 ± 0.38 log10 TCID50/ml of NTL in G2Na-immunized mice treated with the anti-IFN-γ Abs versus 0.5 ± 0.13 log10 TCID50/ml NTL in untreated mice; P < 0.05), while neither lung nor nasal RSV titers were modified after treatment with control IgGs. Thus, the production of secreted IFN-γ is directly involved in the control of RSV infection in the URT after immunization with G2Na.
FIG. 4.
: In vivo neutralization of IFN-γ. BALB/c mice were immunized twice with 20 μg of G2Na or PBS in alum and challenged after 3 weeks. These mice were treated with control IgGs or anti-IFN-γ neutralizing antibody administered i.p. and i.n. as indicated in Materials and Methods. Five days after challenge, the mice were sacrificed. The results are expressed as means ± standard deviations of RSV titers measured in lungs and NTL, calculated from two experiments with 9 or 10 mice per group. ∗, P < 0.05.
RSV clearance in the URT after adoptive transfer of CD4+ T cells.
To examine in detail the immune mechanisms responsible for RSV clearance in the URT and confirm the role of CD4+ T cells and IFN-γ in this process, an adoptive cell transfer experiment was performed. BALB/c and IFN-γ-knockout mice were immunized twice with G2Na in alum or with AICK or AICG in CFA. Splenic CD4+ T cells were prepared from the different groups and transferred into H-2-compatible SCID mice. The mice were previously challenged with 105 TCID50 of RSV. Ungrafted and challenged mice were used as controls for RSV infection. Nine days after cell transfer, the mice were bled and sacrificed. In accordance with the elimination of B cells from the grafted cell preparations, all of the mice remained seronegative to G2Na and RSV (data not shown). As shown in Fig. 5, lung and nasal RSV titers of the mice grafted with T cells from AICG-immunized mice compared favorably with those of the ungrafted animals (4.4 ± 0.29 log10 TCID50/g of lung and 3.4 ± 0.1 log10 TCID50/ml of NTL). CD4+ T cells from BALB/c mice were able to reduce lung RSV infection of the recipient mice after previous immunization with G2Na (3.36 ± 0.5 versus 4.64 ± 0.24 log10 TCID50/g in the ungrafted mice; P < 0.05), as well as RSV infection of the URT after immunization with either G2Na or AICK (2.4 ± 0.4 and 2.4 ± 0.29 log10 TCID50/ml of NTL in the G2Na- and AICK-primed mice, respectively, versus 3.56 ± 0.25 log10 TCID50/ml of NTL in ungrafted mice). In contrast, CD4+ T cells from IFN-γ-knockout mice had no effect at all, whatever the immunogen used to prime the T cells was. These results confirmed the ability of CD4+ T cells to control RSV infection in the URT and demonstrated that this protective effect is dependent on the ability of the transferred cells to produce IFN-γ.
FIG. 5.
RSV protection after adoptive CD4+ T-cell transfer. BALB/c and IFN-γ-knockout mice were immunized three times i.p. with 20 μg of G2Na in alum or 40 μg of AICK or AICG in CFA. Ten days after the last immunization, splenic CD4+ T cells were isolated and transferred to H-2-compatible SCID mice previously challenged with 105 TCID50 of RSV. Ungrafted and challenged SCID mice were used as controls for infection. Nine days later, the mice were sacrificed for titration of RSV in lungs and nasal tracts. The results are expressed as means ± standard deviations of RSV titers measured in lungs and NTL, calculated from six or seven mice per group. ∗, P < 0.05 compared with mice grafted with cells of AICG-immunized mice.
DISCUSSION
While most of the previous reports associated RSV G protein-specific T lymphocytes with adverse pathological immune responses, we demonstrated in previous work that these cells are important in the control of URT infection in mice after parenteral immunization with either RSV or a recombinant RSV G protein fragment (27). The implicated cells were identified as CD4+ T lymphocytes; their protective activity was antibody independent and critically linked to the presence of the conserved cysteines as well as amino acid residues 193 and 194. For the first time, here, we demonstrate that these cells control the nasal infection, through the induction of a Th1-type, IFN-γ-dependent, immune response.
In an earlier study, we found that at least one T-helper-cell epitope was located on G2Na and responsible for the generation of G2Na-specific T-cell proliferation (9). In order to localize the corresponding T-cell determinants on the recombinant G2Na fragment, we used a panel of 12-mer overlapping peptides spanning amino acid residues 130 to 230 of the RSV G protein. Cell proliferation of BBG2Na- and G2Na-primed mice was consistently induced upon stimulation with peptides covering the region from amino acid 182 to 198 of RSV G protein, and it was mediated by CD4+ T cells, as demonstrated by depletion experiments. These results are consistent with earlier observations of Tebbey et al. (38), who identified a single T-helper-cell epitope on the native RSV G protein (between amino acid residues 184 and 198) that is recognized by both murine and human cells. They are in accordance with those of Varga et al., who showed that CD4+ T lymphocytes infiltrating lungs of mice previously immunized with a vaccinia virus expressing the G protein and challenged with RSV recognize a single I-Ed-restricted epitope with a core sequence mapping to amino acids 185 to 193 (40). Thus, we confirmed that immunization with BBG2Na or G2Na was able to induce CD4+ T cells specific for the T-helper-cell epitope located on the RSV G protein.
The induction of protective mechanisms by the T-helper-cell epitope was demonstrated upon immunization with the peptide AICK (amino acid residues 184 to 198) injected with either CFA or alum. These adjuvants are well known for their respective abilities to favor the development of predominant Th1- or Th2-type immune responses (2, 4-6). In addition, since URT protection was always obtained with BBG2Na or G2Na adsorbed to alum (27), the antiviral response was not imposed by the adjuvant used in immunizations but was solely dependent on the priming to the T-helper-cell epitope. However, since immunizations with peptides were more efficient in the induction of URT protection in CFA than in alum, CFA was chosen to perform the experiments with AICK and AICG.
Interestingly, AICK includes not only a T-cell epitope but also a B-cell epitope, as demonstrated by the seroconversion of mice against G2Na and RSV antigens. Both BALB/c and IFN-γ-knockout mice developed IgGs. Consistent with our earlier report demonstrating that lung protection relies primarily on G2Na-specific Abs (27), AICK-specific IgGs were able to protect lungs against RSV infection after passive transfer into naive mice (unpublished observation). Therefore, it was not surprising that lung protection was maintained in IFN-γ-knockout mice following immunization with G2Na or AICK, because of the presence of RSV-specific Abs. Similarly, it was expected that the in vivo neutralization of IFN-γ had no effect on lung protection in G2Na-immunized BALB/c mice, since these mice developed high levels of RSV-specific Abs.
IFN-γ is a cytokine with pleiotropic effects, including the stimulation of macrophages and NK cells, the regulation of helper and cytotoxic T-cell responses, and the control of Ig isotypes switching. Although the lack of IFN-γ does not prevent the development of specific immune responses (30), this cytokine is essential for natural resistance to bacteria such as Listeria monocytogenes or Mycobacterium bovis (11, 17, 19). It was also shown to play an important role in the outcome of infections with vaccinia virus, rhinovirus, and, to a lesser extent, lymphocytic choriomeningitis virus (22, 33).
A specific anti-RSV effect of IFN-γ in mice was previously described by Kumar et al. (21). Those authors showed that transferring a plasmid expressing an IFN-γ cDNA in the URTs of mice significantly reduced infection and eosinophil infiltration in lungs upon RSV challenge. This transfer was associated with a shift of the local cytokine production from a Th2-like to a Th1-like profile. These findings agree with ours showing the increase of nasal RSV titers upon in vivo neutralization of IFN-γ, the inability of G2Na or AICK to protect IFN-γ-knockout mice, and the lack of protective activity of IFN-γ-incompetent cells after adoptive transfer. In fact, we extend the results of Kumar et al. (21) to the nasal tract and demonstrate that the ability to produce IFN-γ, and to release this cytokine, is a prerequisite for RSV G protein-specific CD4+ T cells to control the viral replication.
The fact that live RSV injected i.p. alone was able to protect the URTs of IFN-γ-knockout mice (in contrast with G2Na or AICK) showed that elimination of RSV in the nasal tracts of RSV-primed mice is not solely dependent on CD4+ T cells. While these cells are responsible for elimination of nasal RSV in G2Na- or AICK-immunized mice, they participate only in the control of URT infection in mice primed with live RSV (27). Other effector mechanisms less dependent or not dependent on the production of IFN-γ can mediate this effect. In fact, this hypothesis was confirmed by earlier experiments showing that elimination of CD8+ T cells (like depletion of CD4+ T cells) was also able to significantly reduce RSV clearance in the nasal tracts of RSV-primed mice (27).
At present, it is clear that IFN-γ-competent, RSV G protein-specific CD4+ T cells are able to display an antiviral activity independently of CD8+ and B lymphocytes, since this effect can be transferred to T- and B-cell-deficient SCID mice, in both lungs and the nasal tract. However, we cannot exclude the participation of local macrophages and NK cells.
Furthermore, since Th1-type and Th2-type cytokine responses can develop simultaneously after RSV challenge in mice primed with RSV G protein (36) or with formalin-inactivated RSV (26), it remains to be determined if, in our case, the protective Th1-type response in the URT develops on its own or if this response is concomitant with a Th2-type one. Interestingly, previous studies in our laboratories demonstrated that in contrast to the case with formalin-inactivated RSV, although the recombinant G2Na fragment induces a predominant Th2-type T-cell response upon immunization, no production of IL-4, IL-5, or IgE was detected either in sera or in lungs of G2Na-primed mice after live RSV challenge (9, 26). These results indicated that the Th2-type memory T cells were not recalled either in lungs or in the periphery in these mice. They suggest, with the protection observed in the URT, that reactivation of RSV G protein-specific Th1 responses can be achieved without reactivation of the Th2-type response. This hypothesis is in agreement with results from Varga et al. (40) indicating that the single epitope of RSV G protein primes for memory lymphocytes able to produce Th1 and Th2 cytokines in lungs after RSV challenge and that, although the CD4+ T cells predominantly use Vβ14 T-cell receptor, Th1 and Th2 cytokines are probably produced by different effector cells.
Together, these findings suggest that an RSV subunit vaccine such as G2Na could not only protect both the LTR and UTR against RSV infection but also control the Th orientation of the immune responses induced upon RSV infection. They suggest that the final outcome of the cellular response might be dictated by presently unknown factors, which indeed justifies further investigation. Identification of these factors would allow prevention of the development of the unacceptable RSV-associated immunopathologic responses previously observed with the formalin-inactivated virus and thus has important implications for the development of a safe RSV vaccine.
Acknowledgments
We thank Francis Derouet for expert technical help.
REFERENCES
- 1.Alwan, W. H., W. J. Kozlowska, and P. J. M. Openshaw. 1994. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 179:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Audibert, F. M., and L. D. Lise. 1993. Adjuvants: current status, clinical perspectives and future prospects. Immunol. Today 14:281-284. [DOI] [PubMed] [Google Scholar]
- 3.Bray, A. M., L. M. Lagniton, R. M. Valerio, and N. J. Maeji. 1994. Sonication-assisted cleavage of hydrophobic peptides. Application in multipin peptide synthesis. Tetrahedron Lett. 35:9079-9082. [Google Scholar]
- 4.Brewer, J. M., M. Conacher, A. Satoskar, H. Bluethmann, and J. Alexander. 1996. In interleukin-4-deficient mice, alum not only generate T helper 1 responses equivalent to Freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur. J. Immunol. 26:2062-2066. [DOI] [PubMed] [Google Scholar]
- 5.Brewer, J. M., M. Conacher, C. A. Hunter, M. Mohrs, F. Brombacher, and J. Alexander. 1999. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J. Immunol. 163:6448-6454. [PubMed] [Google Scholar]
- 6.Chuang, Y. H., B. L. Chiang, C. C. Chou, and K. H. Hsieh. 1997. Immune effector cells induced by complete Freund's adjuvant exert an inhibitory effect on antigen-specific type 2 T helper responses. Clin. Exp. Allergy 27:315-324. [PubMed] [Google Scholar]
- 7.Collins, P. L., K. McIntosch, and R. M. Chanock. 1996. Respiratory syncytial virus, p 1313-1351. In B. N. Fields, D. N. Knipe, and P. M. Howley (ed.), Virology. Lippincott-Raven, Philadelphia, Pa.
- 8.Connors, M., P. L. Collins, C. Y. Firestone, and B. R. Murphy. 1991. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J. Virol. 65:1634-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corvaia, N., P. Tournier, T. N. Nguyen, J. F. Haeuw, U. F. Power, H. Binz, and C. Andreoni. 1997. Challenge of BALB/c mice with respiratory syncytial virus does not enhance the Th2 pathway induced after immunization with a recombinant G fusion protein, BBG2Na, in aluminum hydroxide. J. Infect. Dis. 176:560-569. [DOI] [PubMed] [Google Scholar]
- 10.Dagouassat, N., V. Robillard, J.-F. Haeuw, H. Plotnicky-Gilquin, U. F. Power, N. Corvaïa, Thien Nguyen, J.-Y. Bonnefoy, and A. Beck. 2001. A novel bipolar mode of attachment to aluminum-containing adjuvants by BBG2Na, a recombinant subunit hRSV vaccine. Vaccine 19:4143-4152. [DOI] [PubMed] [Google Scholar]
- 11.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 12.Garcia, O., M. Martin, J. Dopazo, J. Arbiza, S. Frabasile, J. Russi, M. Hortal, P. Perez-Brena, I. Martinez, B. Garcia-Barreno, et al. 1994. Evolutionary pattern of human respiratory syncytial virus (subgroup A): cocirculating lineages and correlation of genetic changes in the G glycoprotein. J. Virol. 68:5448-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall, C. B., E. E. Walsh, C. E. Long, and K. C. Schnabel. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163:693-698. [DOI] [PubMed] [Google Scholar]
- 14.Han, L. L., J. P. Alexander, and L. J. Anderson. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. Infect. Dis. 179:25-30. [DOI] [PubMed] [Google Scholar]
- 15.Hancock, G. E., D. J. Speelman, K. Heers, E. Bortell, J Smith, and C. Cosco. 1996. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J. Virol. 70:7783-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson, F. W., A. M. Collier, W. A. Clyde, Jr., and F. W. Denny. 1979. Respiratory-syncytial-virus infections, reinfections and immunity: a prospective, longitudinal study in young children. N. Engl. J. Med. 300:530-534. [DOI] [PubMed] [Google Scholar]
- 17.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-γ receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, T. R., J. E. Fischer, and B. S. Graham. 2001. Construction and characterization of recombinant vaccinia viruses co-expressing a respiratory syncytial virus protein and a cytokine. J. Gen. Virol. 82:2107-2116. [DOI] [PubMed] [Google Scholar]
- 19.Kamijo, R., J. Le, D. Shapiro, E. A. Havell, S. Huang, M. Aguet, M. Bosland, and J. Vilèek. 1993. Mice that lack the interferon-γ receptor have profoundly altered responses to infection with Bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J. Exp. Med. 178:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott,. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 21.Kumar, M., A. K. Behera, H. Matsuse, R. F. Lockey, and S. S. Mohapatra. 2000. Intranasal IFN-γ gene transfer protects BALB/c mice against respiratory syncytial virus infection. Vaccine 18:558-567. [DOI] [PubMed] [Google Scholar]
- 22.Leist, T. P., M. Eppler, and R. M. Zinkernagel. 1989. Enhanced virus replication and inhibition of lymphocytic choriomeningitis virus disease in anti-gamma interferon-treated mice. J. Virol. 63:2813-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libon, C., N. Corvaïa, J.-F. Haeuw, T. N. Nguyen, S. Stahl, J.-Y. Bonnefoy, and C. Andréoni. 1999. The serum albumin-binding region of Streptococcal protein G (BB) potentiates the immunogenicity of the G133-230 RSV-A protein. Vaccine 17:406-414. [DOI] [PubMed] [Google Scholar]
- 24.Murphy, B. R., S. L. Hall, A. B. Kulkarni, J. E. Crowe, Jr., P. L. Collins, M. Connors, R. A. Karron, and R. M. Chanock. 1994. An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Res. 32:13-36. [DOI] [PubMed] [Google Scholar]
- 25.Plotnicky-Gilquin, H., L. Goetsch, T. Huss, T. Champion, A. Beck, J.-F. Haeuw, T. N. Nguyen, J.-Y. Bonnefoy, N. Corvaïa, and U. F. Power. 1999. Identification of multiple epitopes (protectopes) in the central conserved domain of a prototype human respiratory syncytial virus G protein. J. Virol. 73:5637-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plotnicky-Gilquin, H., T. Huss, J.-P. Aubry, J.-F. Haeuw, A. Beck, J.-Y. Bonnefoy, T. N. Nguyen, and U. F. Power. 1999. Absence of lung immunopathology following respiratory syncytial virus (RSV) challenge in mice immunized with a recombinant RSV G protein fragment. Virology 258:128-140. [DOI] [PubMed] [Google Scholar]
- 27.Plotnicky-Gilquin, H., A. Robert, L. Chevalet, J.-F. Haeuw, A. Beck, J.-Y. Bonnefoy, C. Brandt, C.-A. Siegrist, T. N. Nguyen, and U. F. Power. 2000. CD4+ T-cell-mediated antiviral protection of the upper respiratory tract in BALB/c mice following parenteral immunization with a recombinant respiratory syncytial virus G protein fragment. J. Virol. 74:3455-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power, U. F., T. Huss, V. Michaud, H. Plotnicky-Gilquin, T. N. Nguyen, and J.-Y. Bonnefoy. 2001. Differential histopathology and chemokine gene expression in lung tissues following respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV- or BBG2Na-immunized mice. J. Virol. 75:12421-12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Power, U. F., H. Plotnicky-Gilquin, T. Huss, A. Robert, M. Trudel, S. Stahl, M. Uhlen, T. N. Nguyen, and H. Binz. 1997. Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fusion protein containing a respiratory syncytial virus G protein fragment. Virology 230:155-166. [DOI] [PubMed] [Google Scholar]
- 30.Price, G. E., A. Gaszewska-Mastarlarz, and D. Moskophidis. 2000. The role of alpha/beta and gamma interferon in development of immunity to influenza A virus in mice. J. Virol. 74:3996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodda, S. J., N. J. Maeji, and G. Tribbick. 1996. Epitope mapping using multipin peptide synthesis. Methods Mol. Biol. 66:137-147. [DOI] [PubMed] [Google Scholar]
- 32.Routledge, E. G., M. M. Willcocks, A. C. R. Samson, L. Morgan, R. Scott, J. J. Anderson, and G. L. Toms. 1988. The purification of four respiratory syncytial virus proteins as protective agents against experimental infection in BALB/c mice. J. Gen. Virol. 69:293-303. [DOI] [PubMed] [Google Scholar]
- 33.Sethi, S. K., A. Bianco, J. T. Allen, R. A. Knight, and M. A. Spiteri. 1997. Interferon-gamma down-regulates the rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) on human airway epithelial cells. Clin. Exp. Immunol. 110:362-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shay, D. K., R. C. Holman, R. D. Newman, L. L. Liu, J. W. Stout, and L. J. Anderson. 1999. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 282:1440-1446. [DOI] [PubMed] [Google Scholar]
- 35.Shay, D. K., R. C. Holman, G. E. Roosevelt, M. J. Clarke, and L. J. Anderson. 2000. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979-1997. J. Infect. Dis. 183:16-22. [DOI] [PubMed] [Google Scholar]
- 36.Srikiatkhachorn, A., W. Chang, and T. J. Braciale. 1999. Induction of Th-1 and Th-2 responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J. Virol. 73:6590-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stott, E. J., G. Taylor, L. A. Ball, K. K.-Y. Young, A. M. Q. King, and G. W. Wertz. 1987. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J. Virol. 61:3855-3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tebbey, P. W., M. Hagen, and G. E. Hancock. 1998. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J. Exp. Med. 188:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trudel, M., F. Nadon, C. Seguin, and H. Binz. 1991. Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide from the G glycoprotein. Virology 185:749-757. [DOI] [PubMed] [Google Scholar]
- 40.Varga, S. M., E. L. Wissinger, and T. J. Braciale. 2000. The attachment (G) glycoprotein of Respiratory Syncytial Virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J. Immunol. 165:6487-6495. [DOI] [PubMed] [Google Scholar]
- 41.Waris, M. E., C. Tsou, D. D. Erdman, S. R. Zaki, and L. J. Anderson. 1996. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 70:2852-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]