Abstract
Hepatitis delta virus (HDV) causes both acute and chronic liver disease throughout the world. Effective medical therapy is lacking. Previous work has shown that the assembly of HDV virus-like particles (VLPs) could be abolished by BZA-5B, a compound with farnesyltransferase inhibitory activity. Here we show that FTI-277, another farnesyltransferase inhibitor, prevented the production of complete, infectious HDV virions of two different genotypes. Thus, in spite of the added complexity and assembly determinants of infectious HDV virions compared to VLPs, the former are also sensitive to pharmacological prenylation inhibition. Moreover, production of HDV genotype III virions, which is associated with particularly severe clinical disease, was as sensitive to prenylation inhibition as was that of HDV genotype I virions. Farnesyltransferase inhibitors thus represent an attractive potential class of novel antiviral agents for use against HDV, including the genotypes associated with most severe disease.
Hepatitis delta virus (HDV) is a small RNA virus responsible for acute and chronic liver disease worldwide (2, 24, 25, 39, 46). As yet, no effective medical therapy exists against this pathogen. The HDV virion is composed of three general elements: an RNA genome, delta antigens (encoded by the genome), and a surrounding lipid envelope. The lipid envelope is embedded with hepatitis B virus (HBV) surface antigen (HBsAg) proteins-L, M, and S (13)—which are provided by a coinfecting hepatitis B virus (1). They provide a means of exit and presumably entry for HDV, and this explains why HDV infections are always found in the presence of a coexisting HBV infection (20, 40). Once inside a cell, however, HDV can replicate its genome in the absence of any HBV gene products (17, 23). The HDV genome is a 1.7-kb single-stranded circular RNA molecule (47). Sequencing of isolates from around the world has led to a classification into three genotypes based on sequence variation—I, II, and III—the last genotype being associated with particularly severe clinical disease (3, 6, 32, 34). There are two major isoforms of delta antigen found in complete virions, termed small and large (24). They are identical in sequence except that the large delta antigen has an extra 19 amino acids at its carboxyl terminus, the result of a specific RNA editing event which occurs during replication of the HDV genome (5, 30).
This larger delta antigen isoform displays unique properties, including the ability to inhibit genome replication and induce assembly and secretion of both complete HDV virions and virus-like particles (VLPs), which consist of just large delta antigen and the smallest of the three HBV surface antigen proteins (8). The determinants of assembly may be different for these two types of HDV particles, as they involve different compositions of envelope proteins, and packaging of the RNA genome to form fully infectious particles appears to involve structural features provided by small delta antigen (21, 49).
At least for genotype I, one critical interaction between HDV and HBV proteins has been shown to depend on the presence of the last four amino acids of the large delta antigen, Cys-Arg-Pro-Gln-COOH, which comprise a CXXX box motif (where C = cysteine, and X = any amino acid) (18, 27, 31, 42, 50). This amino acid sequence is necessary for the protein to be posttranslationally modified by farnesyltransferase, an enzyme which covalently attaches a prenyl lipid (farnesyl) to the cysteine of the CXXX box (37). Mutation of the CXXX box cysteine abolishes both prenylation and the ability of large delta antigen to form VLPs with HBV surface antigen.
It has not been clear if the same determinants of assembly are shared by all HDV genotypes. Indeed, considerable sequence variation exists between the latter, such that while there is over 94% identity among genotype III isolates, there is only 60 to 70% homology between genotypes III and I (3). This sequence variation, however, is not distributed evenly throughout the HDV genome. In particular, there are regions of the delta antigen reading frame where there is almost no homology between genotypes I and III, such as in the carboxyl-terminal region, which is known to be critical for assembly of genotype I virus particles and in the CXXX box. Therefore, it cannot be assumed that prenylation plays a similar role in the assembly of both genotypes or whether a postulated requirement for prenylation in genotype III assembly might be offset by some structural feature unique to genotype III. This is especially true as there are to date no studies on genotype III assembly.
Because oncogenic forms of ras are farnesylated (7, 19) and dependent on such prenylation for transforming activity (11) a variety of compounds have been developed to inhibit farnesyltransferases for potential use as anticancer agents (41). We previously demonstrated that one such compound, BZA-5B, was also potent against the production of HDV genotype I VLPs devoid of genomic RNA (16). This suggested that targeting of prenylation-dependent virus assembly might form the basis of a novel anti-HDV strategy. Before these results can be potentially translated into a practical clinical therapy, however, several critical questions must be answered. These include the following. (i) Can BZA-5B's effects on HDV VLP production be generalized to other farnesyltransferase inhibitors (FTIs)? (ii) Is the assembly of complete, genome-containing, infectious HDV virions similarly sensitive to pharmacological prenylation inhibition? (iii) Are other genotypes of HDV susceptible to such a therapeutic strategy?
In this work, we sought to address these questions. Specifically, we first set up an in vitro culture system for producing genotype I HDV virions which are capable of infecting primary human hepatocytes. We then used this system to determine the effect of FTI-277 (28)-a drug which also inhibits farnesyltransferase yet is structurally quite different from BZA-5B-on the production of these HDV virions. Finally, we applied a similar approach to study the effect of FTI-277 on the production of HDV genotype III virions. We found not only that micromolar concentrations of FTI-277 prevented the production of HDV genotype I virions in a dose-dependent and specific manner but that HDV genotype III virion production was also inhibited by this compound. FTIs thus represent a potential novel class of antiviral agents for use against HDV, including the genotypes associated with most severe disease.
MATERIALS AND METHODS
Production of HDV virions in cultured cells.
Huh7 cells were plated on 10-cm2 dishes (6 × 106 to 8.5 × 106 cells per plate), and cultured in Huh7 medium (RPMI 1640-Dulbecco's modified Eagle medium [1:1], 10% fetal bovine serum, penicillin [100 U/ml], streptomycin [100 μg/ml]) for 18 h prior to transfection with either Lipofectamine or Lipofectamine 2000 according to the manufacturer's (GibcoBRL) directions. For HDV type I virion production, we performed double transfections with pSVLD3 (23), which contains a head-to-tail trimer of the HDV genome, and pGEM4ayw.2x, which bears a head-to-tail dimer of the full-length HBV genome (12). When only one construct was used in control experiments, the total amount of DNA was maintained by supplementing with pcDNA3 (Invitrogen, Carlsbad, Calif.). For HDV type III transfections, pCMV · HDVIII(+) (4), which bears a 1.2-mer of antigenomic HDV sequence, was substituted for pSVLD3.
To prepare inocula for primary hepatocytes, the culture medium from 10 dishes was collected during days 6 through 10 after transfection, precleared at low speed to remove cell debris, and concentrated 100-fold on Centricon plus-80 tubes according to the manufacturer's (Millipore, Bedford, Mass.) directions. Aliquots were analyzed for RNA content on Northern blots.
Inhibition of virion production by FTI-277.
Starting the first day after transfection, as described above, medium was replaced every day with Huh7 medium containing 0.2% dimethyl sulfoxide (DMSO), 400 μM dithiothreitol (DTT), and various concentrations of FTI-277 (Calbiochem, La Jolla, Calif.). On day 10, the cells were washed several times with 1× phosphate-buffered saline (PBS), in order to remove traces of DTT, and their viability was measured by an XTT assay as described elsewhere (43). Culture medium HBsAg concentrations were determined using an enzyme-linked immunosorbent assay-based assay (Auszyme Kit; Abbott Laboratories, Abbott Park, Ill.). Cells were then washed twice with 1× PBS and scraped in 2 ml of Trizol reagent (GibcoBRL) in order to purify their RNA content, following the manufacturer's instructions. The supernatants were precleared at low speed, loaded on 2-ml cushions of 20% sucrose in 1× PBS, and ultracentrifuged in an SW41Ti rotor (Beckman) for 15 h at 30,000 rpm at 4°C (44). After removing the supernatants, the pellets were carefully resuspended in water and extracted as described below for Northern analysis.
Northern analysis of HDV RNA.
Viral RNA from inocula or sera was purified using a QIAamp Viral RNA mini kit (QIAGEN, Valencia, Calif.) according to the manufacturer's instructions. Various amounts of RNA were then incubated at 55°C for 50 min in the presence of 1.9 M glyoxal (Fisher Scientific, Fair Lawn, N.J.)-7.1 mM sodium phosphate (pH 6.8)-4.5 mM EDTA-35% DMSO. Samples were then loaded on a 1.5% agarose gel containing 10 mM sodium phosphate, pH 6.8, and subjected to electrophoresis for 4 h at 150 mA. For supernatants, half of the RNA was used, while for cellular RNA, 5 μg was loaded. RNAs were capillary transferred overnight to Zeta-Probe (Bio-Rad, Richmond, Calif.) membranes. After transfer, the membrane was either baked for 2 h at 80°C or UV cross-linked using a Stratalinker (Stratagene) and then covered with boiling Tris-HCl, pH 8 (20 mM), and allowed to reach room temperature with gentle rocking. The blot was then placed in a hybridization oven (Hybaid, Franklin, Mass.), prehybridized 1 h at 70°C in 5× SSPE (0.75 M NaCl, 0.05 M NaH2PO4, 5 mM EDTA [pH 7.0]), 5× Denhardt's solution, 0.5% (wt/vol) sodium dodecyl sulfate (SDS), and denatured yeast tRNA (20 μg/ml; Sigma) and then hybridized 18 h at 70°C in 5 ml of the same solution containing a riboprobe labeled with [α-32P]UTP (3,000 ci/mmol; Amersham) and corresponding to the antigenomic HDV sequence. After hybridization, the blot was washed at 70°C with 400 ml of 2× SSPE-0.1% SDS, then with 400 ml of 1× SSPE-0.1% SDS, and then with 200 ml of 0.1× SSPE-0.1% SDS. The membrane was dried at 70°C and subjected to autoradiography and phosphorimager (Molecular Dynamics) analysis.
Infection of primary hepatocytes with HDV inocula.
Human hepatocyte isolations were performed as previously described with minor modifications (29). Briefly, an approximate apical piece of liver (3 by 3 by 2 cm; obtained under approved institutional review board protocol) was perfused with 100 ml of preperfusion solution (Earle's balanced salt solution without Ca2+ with 0.7 mM EGTA) for 20 min, followed by perfusion with 80 ml of collagenase D (Boehringer Mannheim) at a concentration of 0.5 mg/ml for 20 min. Cells were filtered and hepatocytes were separated from nonparenchymal cells by three rounds of low-speed centrifugation. All hepatocytes were obtained from donors that were HBV, HCV, HDV, and human immunodeficiency virus negative. Cells were plated on six-well Primaria plates (Becton Dickinson, Franklin Lakes, N.J.) or on eight-well Lab-Tek II chamber slides (Nalge Nunc International, Naperville, Ill.). As soon as they settled down, the medium was removed and replaced with HDV-containing inoculum. The following day, the inoculum was replaced with fresh medium and the medium was changed every 3 days thereafter. Cells seeded in six-well plates were processed for RNA contents with Trizol, and their RNA content was analyzed by Northern blot.
Immunofluorescence.
All immunofluorescence steps were done at room temperature. Cells seeded on eight-well slides were washed twice with 1× PBS and then fixed with 4% formaldehyde (Ted Pella, Redding, Calif.) for 20 min. Fixation was followed by a 15-min permeabilization step with 0.2% Triton X-100 and 3% FBS in 1× PBS. Cells were then quenched and blocked for at least 1 h with 50 mM glycine-50 mM ammonium chloride in 1× PBS containing 0.1% Triton X-100 and 3% FBS. An anti-HDV human serum was applied at a 1:500 dilution, in wash solution (1× PBS with 0.1% Triton and 3% FBS) for ≥1 h. After extensive washing, secondary antibody was applied at a dilution of 1:100 (rhodamine-conjugated sheep anti-human; Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.) for at least 20 min. Slides were washed extensively in washing solution, then in distilled water before mounting with Fluoroguard (Bio-Rad).
RESULTS
Previously, we have shown that the release of VLPs in the medium of cells stably expressing large delta antigen and HBV small surface antigen could be inhibited by BZA-5B, a benzodiazepine peptidomimetic which targets farnesyltransferase (16). In this study we sought to determine the effect of prenylation inhibition on the production of complete, infectious HDV virions. To this end, we first set up a cell culture system based on one previously described by others (45, 48) which is capable of producing such infectious particles. In our version of this system, Huh7 cells are transiently transfected with two viral genome-producing constructs: pSVLD3 which bears a head to tail trimer of HDV type I genomic sequence and leads to the initiation of HDV genome replication (23), and pGEM4ayw.2x which contains a head-to-tail dimer of the HBV genome subtype ayw and is capable of generating cytoplasmic replicating species (a similar construct for subtype adw is described in reference 9), as well as the envelope proteins necessary for HDV to be released in the culture medium.
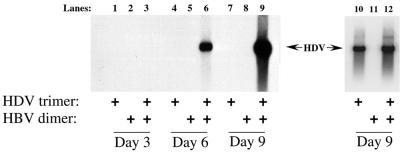
As shown in Fig. 1, although not detectable in the first 3 days (lane 3), virions containing HDV genomic RNA appeared in the culture medium during days 4 to 6 after transfection (lane 6), amounting to about 4 × 109 geq/ml. This amount increased to an estimated 1010 geq/ml during the next 3 days (lane 9). Moreover, while the amounts of intracellular HDV RNA were equivalent in all cells transfected with pSVLD3, HDV RNA was only detectable in the medium of the cells transfected with a source of HBV envelope proteins (lanes 6 and 9).
FIG. 1.
Production of HDV virions. Northern analysis of RNAs from supernatants of Huh7 cells transfected with HDV and/or HBV genome-encoding constructs. Following transfection of Huh7 cells with pSVLD3 alone (lanes 1, 4, 7, and 10), pGEM4ayw.2x alone (lanes 2, 5, 8, and 11), or both constructs (lanes 3, 6, 9, and 12), culture media were collected on day 3 (lanes 1 to 3), day 6 (lanes 4 to 6), and day 9 (lanes 7 to 9) and processed for HDV RNA analysis, as described in Materials and Methods. Lanes 10 to 12 contain RNA isolated from the underlying cells at day 9. Arrows indicate sizes of circular HDV genomic RNA.
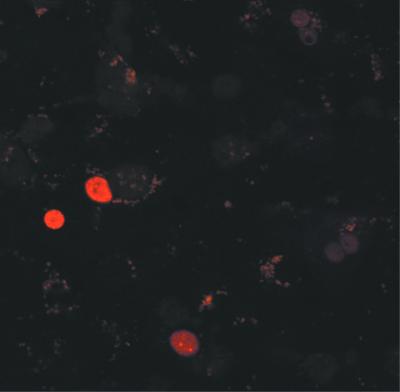
To confirm that these in vitro-produced virions were infectious, we asked whether they could initiate HDV replication in primary human hepatocytes. Supernatants from cells such as those in lanes 7 and 9 were used to prepare control and HDV inocula, respectively, using a Centricon concentration method as described (38). Aliquots were inoculated onto primary human hepatocytes previously plated as monolayers in eight-well slides. After an overnight incubation, the cells were washed and the medium was replaced. One week after infection, immunofluorescence staining against delta antigen revealed the characteristic nuclear localized pattern indicative of HDV replication in ∼5% of the hepatocytes (Fig. 2). A similar staining pattern was observed when inocula of HDV-infected chimpanzee or woodchuck sera were used (data not shown), but not with control inocula.
FIG. 2.
HDV infection of human primary hepatocytes. In vitro-generated HDV inocula were used to infect monolayer cultures of hepatocytes as described in Materials and Methods. One week after inoculation, cells were fixed and stained with a human serum containing antibodies against delta antigen. Epitopes recognized by this serum were visualized with a rhodamine-conjugated anti-human antibody.
We next wanted to determine whether the production of HDV virions in our cultured cells was sensitive to drugs preventing prenylation. Our previous studies on the inhibition of VLP assembly employed the FTI BZA-5B (16). For the present experiments we chose to substitute the latter with FTI-277. Like BZA-5B, FTI-277 (and its analog FTI-276) is a specific inhibitor of farnesyltransferase (28) and this latter compound inhibits large delta antigen prenylation in our previously described in vitro prenylation assay (16) (data not shown). Although FTI-277 targets the same enzyme as BZA-5B, these inhibitors have very different molecular structures. Thus, any observed inhibition of HDV particle production with FTI-277 would underscore that it is the farnesyltransferase-inhibiting activity, rather than some other feature of the inhibiting drug, that is the critical factor for disrupting HDV assembly.
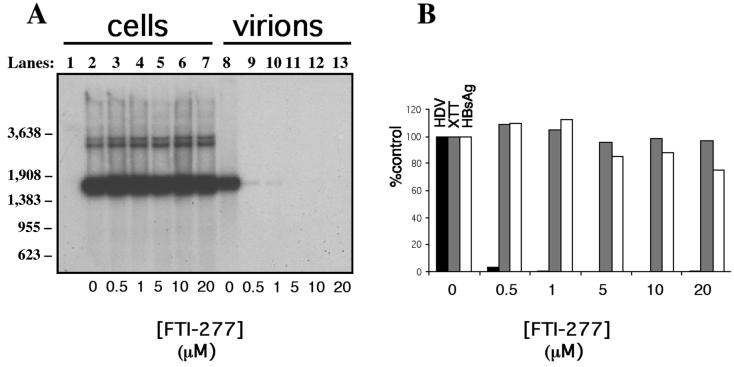
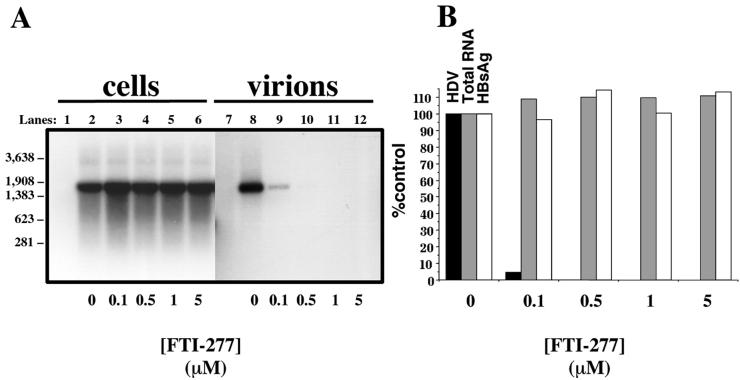
We initiated HDV type I virion production by transfecting Huh7 cells with pSVLD3 and pGEM4ayw.2x as in Fig. 1 (lanes 3, 6, or 9). Cells were then grown in the presence of carrier (DMSO and DTT) alone or carrier plus increasing concentrations of FTI-277. After nine daily medium changes, viability of cells was assessed. Supernatants and cells were then processed for HDV RNA content analysis as described in Materials and Methods (Fig. 3). FTI-277 had no apparent effect on HDV replication (Fig. 3A, lanes 2 to 7), as HDV intracellular RNA could be detected in cells grown in the presence of all tested drug concentrations. Nor did FTI-277 affect the steady-state levels of delta antigen, as similar amounts were detected by Western blots at all tested drug concentrations (data not shown). On the other hand, HDV genomic RNA isolated from the culture medium was quite sensitive to FTI-277 (Fig. 3A, lanes 9 to 13). At 0.5 μM FTI-277, virion production was decreased by 95% compared to treatment with carrier alone (Fig. 3A, lane 9 versus lane 8). With FTI-277 concentrations above 1 μM, virion production could no longer be detected (Fig. 3A, lanes 11 to 13, and B). We evaluated for nonspecific effects of the drug in a variety of ways. Total RNA levels were equivalent among all samples (data not shown). We also performed XTT assays on the treated cells (Fig. 3B). This assay, based on reduction of the tetrazolium salt XTT by cellular dehydrogenases, provides a sensitive means of assessing cell metabolism (43). Finally, we measured the production and release of HBsAg into the media (Fig. 3B) as this protein is not subject to prenylation and should therefore not be affected by the FTI. These latter two assays revealed that FTI-277 had no significant effect on either overall cell viability or general protein synthesis, even at the highest concentrations of drug.
FIG. 3.
FTI-277 inhibits production of HDV genome-containing particles. (A) Following transfection with both HDV and HBV genome-encoding constructs, as described in Fig. 1 and in Materials and Methods, Huh7 cells were maintained in a daily changed medium containing carrier (0.2% DMSO and 400 μM DTT) alone (lanes 2 and 8) or carrier plus 0.5 μM (lanes 3 and 9), 1 μM (lanes 4 and 10), 5 μM (lanes 5 and 11), 10 μM (lanes 6 and 12), or 20 μM (lanes 7 and 13) FTI-277. On day 10 after transfection, cells (lanes 1 to 7) and supernatants (lanes 8 to 13) were processed for Northern analysis of HDV RNA, as described in Materials and Methods. Lane 1 corresponds to total RNA extracted from nontransfected cells subjected to carrier-containing medium. Molecular weight markers are indicated at the left of the figure. (B) The amount of HDV RNA in the culture medium of cells treated with the indicated amount of FTI-277 was quantitated using a phosphorimager and plotted as percentage of the untreated control (0 μM) (black bars). Prior to total RNA extraction, cells were monitored for viability (XTT assay) (grey bars) and supernatants were analyzed for protein expression and secretion (HBV surface antigen released into the medium) (empty bars).
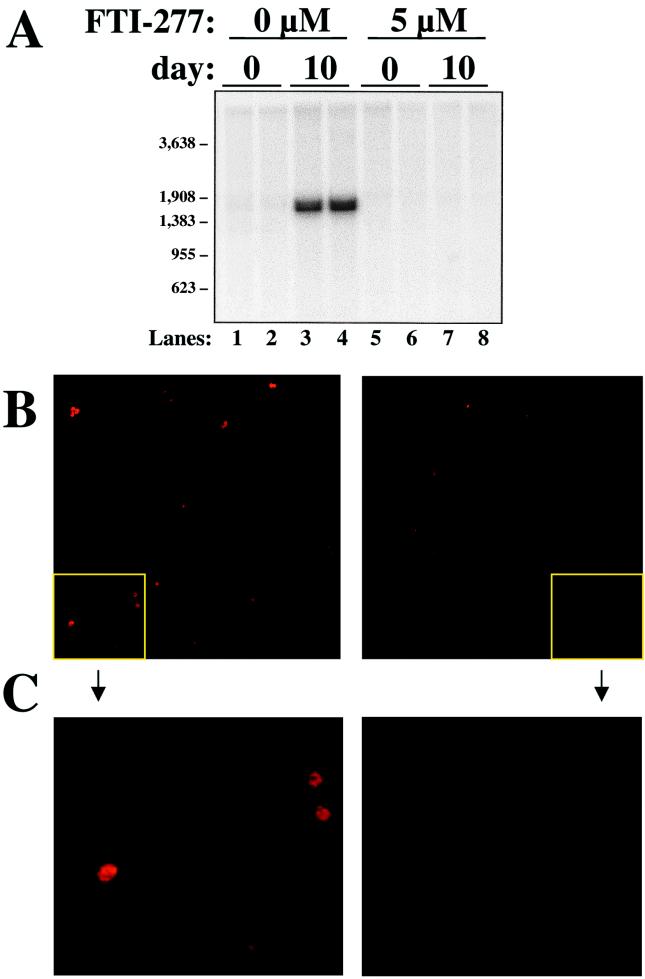
To confirm that FTI-277 was effective in inhibiting the production of not only physical HDV particles but also infectious ones, we subjected aliquots of selected media supernatants to the primary hepatocyte infection assay described in Fig. 2. Supernatants from Huh7 cells transfected with HBV- and HDV-encoding plasmids and treated either with carrier (Fig. 4, left side) or with 5 μM FTI-277 (Fig. 4, right side) were concentrated and layered onto primary hepatocytes. After washing to remove unadsorbed inocula, RNA extracted from hepatocytes harvested at day 0 and day 10 postinoculation was subjected to northern analysis for HDV. Day 10 cells were also fixed and stained for the presence of delta antigen by immunofluorescence (Fig. 4B and C). Infection of hepatocytes could clearly be demonstrated in cells exposed to supernatant obtained from Huh7 cells treated with carrier alone (Fig. 4A, lanes 3 and 4, and B and C, left panels). Hepatocytes subjected to supernatants obtained from cells treated with 5 μM FTI-277, however, had no evidence of detectable infectious particles (Fig. 4A, lanes 7 and 8, and B and C, right panels).
FIG. 4.
FTI-277 inhibits the production of infectious HDV particles. Huh7 cells were transfected as in Fig. 3 and treated with carrier alone or carrier plus 5 μM FTI-277. On day 10 after transfection, culture media were concentrated and inoculated onto duplicate cultures of primary human hepatocytes, as described in Materials and Methods. (A) After overnight exposure to these inocula, hepatocytes were washed with fresh medium and processed for Northern analysis of HDV genomic RNA either immediately (day 0, lanes 1, 2, 5, and 6) or after culture for 10 more days (day 10, lanes 3, 4, 7, and 8). MW markers are indicated at the left of the figure. (B and C) Similarly treated cells were also analyzed on day 10 for large delta antigen by immunofluorescence as in Fig. 2. Panel C shows a higher power magnification of the corresponding quadrants in panel B.
Studies of HDV assembly to date have been limited to HDV genotype I. It has been shown that the 19 C-terminal amino acids of large delta antigen, which includes the CXXX box, are necessary and sufficient for particle assembly with HBsAg (26). Interestingly, although the CXXX box motif is present in both cases, there is almost no sequence homology between HDV genotypes I and III in this region of large delta antigen. We therefore wondered if the determinants of assembly identified for HDV I were the same for HDV III. In particular, we wished to determine whether the production of genotype III virions was as sensitive to drugs preventing prenylation as are HDV I virions. To initiate production of genotype III HDV particles, we employed the same strategy as in Fig. 1 except that Huh7 cells were transfected with pCMV · HDVIII(+), in place of pSVLD3. This plasmid has been shown to efficiently initiate HDV III genome replication (4). Cells were treated with carrier alone or increasing concentrations of FTI-277, and intracellular HDV RNA replication as well as the release of genome-containing virions were measured (Fig. 5A). Similar to the results of Fig. 3, replication of HDV III appeared unaffected at all tested concentrations of FTI-277 (Fig. 5A, lanes 2 through 6). Release of genome-containing particles into the medium, however, was strongly inhibited by 100 nM of FTI-277 (lane 9) and became undetectable at higher concentrations (lanes 10 to 12). Quantitation of these results (Fig. 5B) indicated that less than 5% of HDV III RNA detectable at 0 μM was released in the medium of cells treated with 0.1 μM FTI-277. Once again, measures of nonspecific toxicity of the drug (total RNA extraction yields and HBV surface antigen secretion) were similar at all concentrations of FTI-277 used (Fig. 5B). We conclude that, like HDV I, HDV III particle production is also sensitive to prenylation inhibition. These results underscore the therapeutic potential of large delta antigen farnesylation as a target for drug treatment.
FIG. 5.
FTI-277 inhibits production of HDV genotype III genome-containing particles. (A) Following transfection with both HDV genotype III and HBV genome-encoding constructs, as described in Fig. 1 and in Materials and Methods, Huh7 cells were maintained in a daily changed medium containing carrier (0.22% DMSO and 400 μM DTT) alone (lanes 2 and 8) or carrier plus 0.1 μM (lanes 3 and 9), 0.5 μM (lanes 4 and 10), 1 μM (lanes 5 and 11), or 5 μM (lanes 6 and 12) FTI-277. On day 10 after transfection, cells (lanes 1 to 6) and supernatants (lanes 7 to 12) were processed for Northern analysis of HDV RNA, as described in Materials and Methods. Lanes 1 and 7 correspond to nontransfected cells subjected to carrier-containing medium. MW markers are indicated at the left of the figure. (B) HDV RNA in the culture medium of cells treated with the indicated amounts of FTI-277 was quantitated using a phosphorimager and plotted as a percentage of the no-drug control (0 μM) (black bars). Also shown are the results from total RNA quantitation (Total RNA) (grey bars) and HBV surface antigen synthesis and secretion (HBsAg) (empty bars).
DISCUSSION
In previous studies, our initial strategy to evaluate the potential of prenylation inhibition-based anti-HDV therapy was to first create a cell line which produces HDV VLPs, then identify a compound capable of pharmacologically inhibiting HDV prenylation, and finally test the effect of the inhibitor on the production of HDV VLPs (16). To this end, we isolated an NIH 3T3 cell line stably transfected with the genes encoding HDV large delta antigen and HBV surface antigen. This cell line produces both free surface antigen and HDV VLPs composed of large delta antigen and HBV envelope protein. To identify an appropriate inhibitor, we relied on the fact that prenylation of HDV consists of the addition of the prenyl lipid farnesyl to large delta antigen, a reaction catalyzed by farnesyltransferase (37). We therefore evaluated the effect of a known FTI on delta antigen prenylation. For this we chose BZA-5B, a FTI originally shown to prevent prenylation of ras (33). When tested in vitro using rabbit reticulocyte lysates, BZA-5B was shown to be a potent inhibitor of large delta antigen prenylation at micromolar concentrations. When tested in our cell culture model, BZA-5B was also shown to specifically inhibit the prenylation-dependent production of HDV VLPs in a dose-dependent manner. At 50 μM BZA-5B, no particles could be detected. Controls for nonspecific inhibition of protein synthesis and secretion showed essentially no effect of BZA-5B (16).
To further extend these findings toward our aim of developing a practical clinical therapy, we wished to determine if complete infectious HDV virions were as sensitive to prenylation inhibition as are the VLPs in the above-described BZA-5B experiments. We also wished to try another prenylation inhibitor to validate the hypothesis that HDV's sensitivity to BZA-5B reflects a general property of FTIs and is not restricted to BZA-5B.
We therefore first set up a system based on a previously validated methodology (45, 48) to produce complete infectious HDV particles containing an intact genome (Fig. 1). Cotransfection of Huh7 cells with plasmids encoding the complete HDV and HBV genomes yielded HDV virions released into the media supernatant. As hoped for, such virions contained an intact HDV genome. Furthermore, they were produced only in cells transfected with the HDV plasmid and a source of envelope proteins provided by the HBV plasmid.
We next sought to prove that these in vitro-produced virions are infectious by inoculating them onto primary human hepatocytes. As shown in Fig. 2, 1 week postinfection, about 5% of the latter displayed the nuclear staining pattern characteristic of HDV infection when analyzed by immunofluorescence with an antibody against delta antigen. Thus, not only do the in vitro-produced HDV virions contain an intact RNA genome, but they are also infectious. Moreover, to our knowledge, this represents the first use of cultured human primary hepatocytes as a target for HDV infection.
Next, we tested the effect of FTI-277, another FTI, on the production of the HDV virions. As shown in Fig. 3, while in the absence of drug HDV virions are readily produced, they are dramatically inhibited at mid-nanomolar concentrations of FTI-277 and are below our limit of detection at micromolar concentrations of compound. Control assays for nonspecific toxicity (free HBV surface antigen which assesses effects on general protein synthesis and secretion, and XTT assays which measure overall cell metabolism) are essentially unaffected over these concentrations of FTI-277 and suggest the existence of an encouraging therapeutic index. As shown in Fig. 4, FTI-277 inhibited not only the production of physical HDV particles but also infectious HDV virions as well.
Last, we tested the hypothesis that genotype III virion production is also sensitive to prenylation inhibition. Remarkably, as shown in Fig. 5, in spite of almost no amino acid homology between genotypes I and III in the region known to be critical for HDV assembly including the CXXX box, HDV III virion production appeared to be as sensitive to FTI-277 as was HDV I assembly. These results represent the first study of genotype III assembly. They reveal that at least one critical assembly determinant of genotype I is shared by genotype III. Indeed, in spite of potential greater efficiency of HDV virion production by genotype III than genotype I (unpublished observations), HDV III virion production is still sensitive to prenylation inhibition. Moreover, comparison of Fig. 3 and 5 suggests that HDV III assembly may even be inhibited at somewhat lower concentrations of FTI-277. This might reflect a different affinity of farnesyltransferase for the C terminus of the respective isoforms of large delta antigen. It could also be related to secondary features of particle formation such as differences in the number of prenylated large delta antigen proteins necessary to make a mature viral particle, or the interaction with HBV surface antigen.
Contrary to more-classical approaches to antiviral therapy, in which agents are designed to target a virus-specific activity, the use of prenylation inhibitors to target HDV is an example of a different paradigm. Specifically, the strategy we propose actually seeks to deprive the virus of access to a host function. One attractive consequence is the potential for making the evolutionary task of developing resistance more difficult as the targeted locus does not lie within the viral genome.
The remarkable tolerance of host cells for FTIs (10, 22) suggests the cells are able to benefit from a variety of redundancies when encountering an inhibition of farnesyltransferase. At present, it appears that HDV, however, is not able to benefit from these back-up mechanisms.
Taken together, our results provide further validation of the hypothesis that pharmacological inhibition of prenylation can interfere with HDV particle production. Furthermore, compounds like BZA-5B and FTI-277 are thus representatives of a novel class of potential antiviral agents for use against HDV and other viruses similarly dependent on prenylation (15). Finally, these results should provide added impetus for the in vivo evaluation of prenylation inhibitors in animal models of HDV (14, 35, 36).
Acknowledgments
This work was supported by a Veterans Administration Merit Review Award (to J.S.G.). J.S.G. is also supported by an Amgen/AASLD American Liver Foundation Award and a Burroughs Wellcome Fund Career Award. M.A.K. was supported by the Anna Ng Charitable Trust and grant AI41320.
REFERENCES
- 1.Bonino, F., B. Hoyer, J. W. Shih, M. Rizzetto, R. H. Purcell, and J. L. Gerin. 1984. Delta hepatitis agent: structural and antigenic properties of the delta-associated particle. Infect. Immun. 43:1000-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey, J. L. 1998. Hepatitis delta virus: molecular biology, pathogenesis and immunology. Antivir. Therapy 3:37-42. [PubMed] [Google Scholar]
- 3.Casey, J. L., T. L. Brown, E. J. Colan, F. S. Wignall, and J. L. Gerin. 1993. A genotype of hepatitis D virus that occurs in northern South America. Proc. Natl. Acad. Sci. USA 90:9016-9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey, J. L., and J. L. Gerin. 1998. Genotype-specific complementation of hepatitis delta virus RNA replication by hepatitis delta antigen. J. Virol. 72:2806-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey, J. L., and J. L. Gerin. 1995. Hepatitis D virus RNA editing: specific modification of adenosine in the antigenomic RNA. J. Virol. 69:7593-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey, J. L., G. A. Niro, R. E. Engle, A. Vega, H. Gomez, M. McCarthy, D. M. Watts, K. C. Hyams, and J. L. Gerin. 1996. Hepatitis B virus (HBV)/hepatitis D virus (HDV) coinfection in outbreaks of acute hepatitis in the Peruvian Amazon basin: the roles of HDV genotype III and HBV genotype F. J. Infect. Dis. 174:920-926. [DOI] [PubMed] [Google Scholar]
- 7.Casey, P. J., P. A. Solski, C. J. Der, and J. E. Buss. 1989. p21ras is modified by a farnesyl isoprenoid. Proc. Natl. Acad. Sci. USA 86:8323-8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, F. L., P. J. Chen, S. J. Tu, C. J. Wang, and D. S. Chen. 1991. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc. Natl. Acad. Sci. USA. 88:8490-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Y., and P. L. Marion. 1996. Amino acids essential for RNase H activity of hepadnaviruses are also required for efficient elongation of minus-strand viral DNA. J. Virol. 70:6151-6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalton, M. B., K. S. Fantle, H. A. Bechtold, L. DeMaio, R. M. Evans, A. Krystosek, and M. Sinensky. 1995. The farnesyl protein transferase inhibitor BZA-5B blocks farnesylation of nuclear lamins and p21ras but does not affect their function or localization. Cancer Res. 55:3295-3304. [PubMed] [Google Scholar]
- 11.Der, C. J., and A. D. Cox. 1991. Isoprenoid modification and plasma membrane association: critical factors for ras oncogenicity. Cancer Cells 3:331-340. [PubMed] [Google Scholar]
- 12.Galibert, F., E. Mandart, F. Fitoussi, P. Tiollais, and P. Charnay. 1979. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature 281:646-650. [DOI] [PubMed] [Google Scholar]
- 13.Ganem, D. 1991. Assembly of hepadnaviral virions and subviral particles. Curr. Top. Microbiol. Immunol. 168:61-83. [DOI] [PubMed] [Google Scholar]
- 14.Gerin, J. L. 2001. Animal models of hepatitis delta virus infection and disease. Ilar J. 42:103-106. [DOI] [PubMed] [Google Scholar]
- 15.Glenn, J. S. 1999. Shutting the door on hepatitis delta virus (HDV): Sensitivity to prenylation inhibition prompts new therapeutic strategy. Viral Hepatitis Rev. 5:13-26. [Google Scholar]
- 16.Glenn, J. S., J. C. Marsters, Jr., and H. B. Greenberg. 1998. Use of a prenylation inhibitor as a novel antiviral agent. J. Virol. 72:9303-9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenn, J. S., J. M. Taylor, and J. M. White. 1990. In vitro-synthesized hepatitis delta virus RNA initiates genome replication in cultured cells. J. Virol. 64:3104-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenn, J. S., J. A. Watson, C. M. Havel, and J. M. White. 1992. Identification of a prenylation site in delta virus large antigen. Science 256:1331-1333. [DOI] [PubMed] [Google Scholar]
- 19.Hancock, J. F., A. I. Magee, J. E. Childs, and C. J. Marshall. 1989. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57:1167-1177. [DOI] [PubMed] [Google Scholar]
- 20.Hoofnagle, J. H. 1989. Type D (delta) hepatitis. JAMA 261:1321-1325. [PubMed] [Google Scholar]
- 21.Hourioux, C., C. Sureau, F. Poisson, D. Brand, A. Goudeau, and P. Roingeard. 1998. Interaction between hepatitis delta virus-encoded proteins and hepatitis B virus envelope protein domains. J. Gen. Virol. 79:1115-1119. [DOI] [PubMed] [Google Scholar]
- 22.James, G. L., M. S. Brown, M. H. Cobb, and J. L. Goldstein. 1994. Benzodiazepine peptidomimetic BZA-5B interrupts the MAP kinase activation pathway in H-Ras-transformed Rat-1 cells, but not in untransformed cells. J. Biol. Chem. 269:27705-27714. [PubMed] [Google Scholar]
- 23.Kuo, M. Y., M. Chao, and J. Taylor. 1989. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J. Virol. 63:1945-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai, M. M. 1995. The molecular biology of hepatitis delta virus. Annu. Rev. Biochem. 64:259-286. [DOI] [PubMed] [Google Scholar]
- 25.Lazinski, D. W., and J. M. Taylor. 1994. Recent developments in hepatitis delta virus research. Adv. Virus Res. 43:187-231. [DOI] [PubMed] [Google Scholar]
- 26.Lee, C. Z., P. J. Chen, and D. S. Chen. 1995. Large hepatitis delta antigen in packaging and replication inhibition: role of the carboxyl-terminal 19 amino acids and amino-terminal sequences. J. Virol. 69:5332-5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, C. Z., P. J. Chen, M. M. Lai, and D. S. Chen. 1994. Isoprenylation of large hepatitis delta antigen is necessary but not sufficient for hepatitis delta virus assembly. Virology 199:169-175. [DOI] [PubMed] [Google Scholar]
- 28.Lerner, E. C., Y. Qian, M. A. Blaskovich, R. D. Fossum, A. Vogt, J. Sun, A. D. Cox, C. J. Der, A. D. Hamilton, and S. M. Sebti. 1995. Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. J. Biol. Chem. 270:26802-26806. [DOI] [PubMed] [Google Scholar]
- 29.Lieber, A., C. Y. He, S. J. Polyak, D. R. Gretch, D. Barr, and M. A. Kay. 1996. Elimination of hepatitis C virus RNA in infected human hepatocytes by adenovirus-mediated expression of ribozymes. J. Virol. 70:8782-8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo, G. X., M. Chao, S. Y. Hsieh, C. Sureau, K. Nishikura, and J. Taylor. 1990. A specific base transition occurs on replicating hepatitis delta virus RNA. J. Virol. 64:1021-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maltese, W. A. 1990. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 4:3319-3328. [DOI] [PubMed] [Google Scholar]
- 32.Manock, S. R., P. M. Kelley, K. C. Hyams, R. Douce, R. D. Smalligan, D. M. Watts, T. W. Sharp, J. L. Casey, J. L. Gerin, R. Engle, A. Alava-Alprecht, C. M. Martínez, N. B. Bravo, A. G. Guevara, K. L. Russell, W. Mendoza, and C. Vimos. 2000. An outbreak of fulminant hepatitis delta in the Waorani, an indigenous people of the Amazon basin of Ecuador. Am. J. Trop. Med. Hyg. 63:209-213. [DOI] [PubMed] [Google Scholar]
- 33.Marsters, J. C., Jr., R. S. McDowell, M. E. Reynolds, D. A. Oare, T. C. Somers, M. S. Stanley, T. E. Rawson, M. E. Struble, D. J. Burdick, K. S. Chan, et al. 1994. Benzodiazepine peptidomimetic inhibitors of farnesyltransferase. Bioorganic Med. Chem. 2:949-957. [DOI] [PubMed] [Google Scholar]
- 34.Nakano, T., C. N. Shapiro, S. C. Hadler, J. L. Casey, M. Mizokami, E. Orito, and B. H. Robertson. 2001. Characterization of hepatitis D virus genotype III among Yucpa Indians in Venezuela. J. Gen. Virol. 82:2183-2189. [DOI] [PubMed] [Google Scholar]
- 35.Negro, F. 1996. Animal models of hepatitis delta virus infection. Viral Hepatitis Rev. 2:175-185. [Google Scholar]
- 36.Ohashi, K., P. L. Marion, H. Nakai, L. Meuse, J. M. Cullen, B. B. Bordier, R. Schwall, H. B. Greenberg, J. S. Glenn, and M. A. Kay. 2000. Sustained survival of human hepatocytes in mice: A model for in vivo infection with human hepatitis B and hepatitis delta viruses. Nat. Med. 6:327-331. [DOI] [PubMed] [Google Scholar]
- 37.Otto, J. C., and P. J. Casey. 1996. The hepatitis delta virus large antigen is farnesylated both in vitro and in animal cells. J. Biol. Chem. 271:4569-4572. [DOI] [PubMed] [Google Scholar]
- 38.Reiser, J. 2000. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 7:910-913. [DOI] [PubMed] [Google Scholar]
- 39.Rizzetto, M. 1983. The delta agent. Hepatology 3:729-737. [DOI] [PubMed] [Google Scholar]
- 40.Rizzetto, M., M. G. Canese, S. Aricò, O. Crivelli, C. Trepo, F. Bonino, and G. Verme. 1977. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 18:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowinsky, E. K., J. J. Windle, and D. D. Von Hoff. 1999. Ras protein farnesyltransferase: A strategic target for anticancer therapeutic development. J. Clin. Oncol. 17:3631-3652. [DOI] [PubMed] [Google Scholar]
- 42.Schafer, W. R., and J. Rine. 1992. Protein prenylation: Genes, enzymes, targets, and functions. Annu. Rev. Genet. 30:209-237. [DOI] [PubMed] [Google Scholar]
- 43.Scudiero, D. A., R. H. Shoemaker, K. D. Paull, A. Monks, S. Tierney, T. H. Nofziger, M. J. Currens, D. Seniff, and M. R. Boyd. 1988. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 48:4827-4833. [PubMed] [Google Scholar]
- 44.Sureau, C., J. R. Jacob, J. W. Eichberg, and R. E. Lanford. 1991. Tissue culture system for infection with human hepatitis delta virus. J. Virol. 65:3443-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sureau, C., A. M. Moriarty, G. B. Thornton, and R. E. Lanford. 1992. Production of infectious hepatitis delta virus in vitro and neutralization with antibodies directed against hepatitis B virus pre-S antigens. J. Virol. 66:1241-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor, J. M. 1992. The structure and replication of hepatitis delta virus. Annu. Rev. Microbiol. 46:253-276. [DOI] [PubMed] [Google Scholar]
- 47.Wang, K. S., Q. L. Choo, A. J. Weiner, J. H. Ou, R. C. Najarian, R. M. Thayer, G. T. Mullenbach, K. J. Denniston, J. L. Gerin, and M. Houghton. 1986. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature 323:508-514. Erratum: 328:456, 1987. [DOI] [PubMed] [Google Scholar]
- 48.Wu, J. C., P. J. Chen, M. Y. Kuo, S. D. Lee, D. S. Chen, and L. P. Ting. 1991. Production of hepatitis delta virus and suppression of helper hepatitis B virus in a human hepatoma cell line. J. Virol. 65:1099-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeh, T. S., and Y. H. Lee. 1998. Assembly of hepatitis delta virus particles: package of multimeric hepatitis delta virus genomic RNA and role of phosphorylation. Virology 249:12-20. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, F. L., and P. J. Casey. 1996. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65:241-269. [DOI] [PubMed] [Google Scholar]