Abstract
1. The effects of low temperature on conduction in single myelinated and non-myelinated axons of the feline saphenous nerve were examined and compared. Nerves were cooled by a conventional thermode, but thermal gradients were minimized by an insulating layer of agar-saline gel over the nerve and the face of the thermode.
2. The mean blocking temperature of thirty-one non-myelinated axons, 2·7° C, was significantly lower than that of 111 myelinated axons, 7·2° C. No evidence for a differential block of myelinated axons according to their normal conduction velocity could be demonstrated.
3. Reductions in the proportional conduction velocities of both myelinated and non-myelinated axons were nearly identical between 17 and 37° C. However, below 17° C the rate at which the proportional conduction velocity of the non-myelinated axons fell during cooling was significantly less than for the myelinated axons and was sufficient to account for their lower blocking temperatures. As a result, critical minimum conduction velocities were reached at higher temperatures in myelinated axons than in non-myelinated axons.
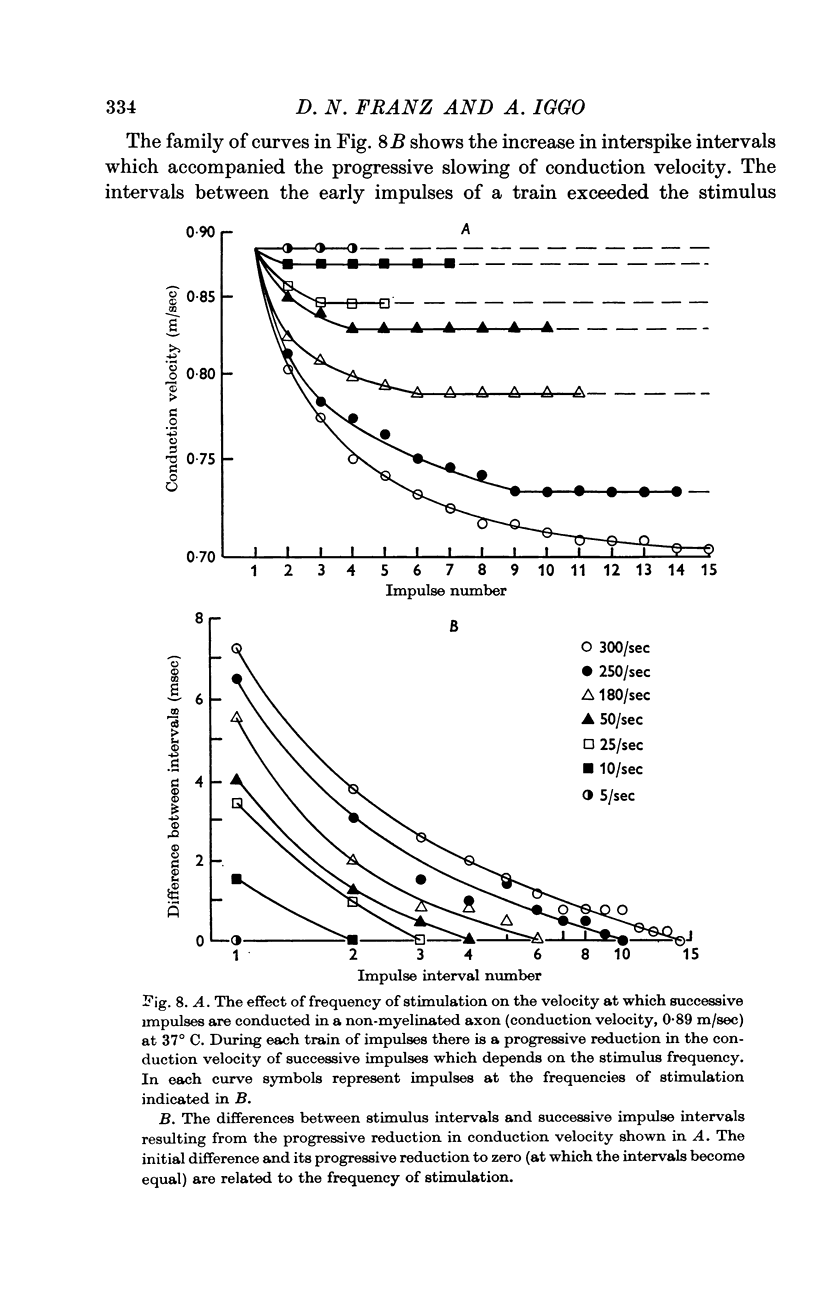
4. The conduction velocity of successive impulses in a train slowed progressively to a constant value which depended on the frequency of stimulation. Consequently, the early impulses were separated by intervals that exceeded those between stimuli and were not affected by temperatures that blocked later impulses. The pattern of block was consistent with an increasing refractoriness of the axons as the temperature fell.
5. The maximal frequency of discharge that myelinated axons could carry at temperatures between normal and 12° C was related directly to fibre size. Non-myelinated axons could conduct low frequency trains of impulses at temperatures that blocked such activity in myelinated axons. In all axons, high frequency trains of impulses could be completely blocked at temperatures which permitted lower frequency trains to pass uninterrupted.
6. Hysteresis in the blocking temperatures of axons was related to the hysteresis in their conduction velocities.
Full text
PDF

























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. LOCATION OF POSTSYNAPTIC INHIBITORY SYNAPSES ON HIPPOCAMPAL PYRAMIDS. J Neurophysiol. 1964 Jul;27:592–607. doi: 10.1152/jn.1964.27.4.592. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODT E. Differential thermosensitivity of mammalian A-fibres. Acta Physiol Scand. 1953 Jun 26;29(1):91–108. doi: 10.1111/j.1748-1716.1953.tb01005.x. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., MALCOLM J. L. The effect of localized cooling on conduction in cat nerves. J Physiol. 1955 Oct 28;130(1):53–71. doi: 10.1113/jphysiol.1955.sp005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASSER H. S. Unmedullated fibers originating in dorsal root ganglia. J Gen Physiol. 1950 Jul 20;33(6):651–690. doi: 10.1085/jgp.33.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY J. A., RITCHIE J. M. Effects of stretch on single myelinated nerve fibres. J Physiol. 1954 Apr 28;124(1):84–99. doi: 10.1113/jphysiol.1954.sp005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M. The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J Physiol. 1968 Feb;194(3):745–793. doi: 10.1113/jphysiol.1968.sp008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. Cutaneous mechanoreceptors with afferent C fibres. J Physiol. 1960 Jul;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. Gastro-intestinal tension receptors with unmyelinated afferent fibres in the vagus of the cat. Q J Exp Physiol Cogn Med Sci. 1957 Jan;42(1):130–143. doi: 10.1113/expphysiol.1957.sp001228. [DOI] [PubMed] [Google Scholar]
- IGGO A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955 Jun 28;128(3):593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. The electrophysiological identification of single nerve fibres, with particular reference to the slowest-conducting vagal afferent fibres in the cat. J Physiol. 1958 Jun 18;142(1):110–126. doi: 10.1113/jphysiol.1958.sp006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. A comparison of the nerve impulses of mammalian non-medullated nerve fibres with those of the smallest diameter medullated fibres. J Physiol. 1967 Dec;193(3):523–533. doi: 10.1113/jphysiol.1967.sp008376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. Block of conduction in mammalian myelinated nerve fibres by low temperatures. J Physiol. 1965 Sep;180(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. Effects of temperature on conduction in single vagal and saphenous myelinated nerve fibres of the cat. J Physiol. 1965 Sep;180(1):20–49. [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. The influence of diameter of medullated nerve fibres of cats on the rising and falling phases of the spike and its recovery. J Physiol. 1966 Jun;184(4):791–811. doi: 10.1113/jphysiol.1966.sp007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge R. C. Respiratory accelerator action of the carotid sinus-cardiac depressor mechanism. J Physiol. 1939 Aug 14;96(3):233–239. doi: 10.1113/jphysiol.1939.sp003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The effect of cooling on the size of the action potential of mammalian non-medullated fibres. J Physiol. 1956 Dec 28;134(3):712–717. doi: 10.1113/jphysiol.1956.sp005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINCLAIR D. C., HINSHAW J. R. Sensory changes in nerve blocks induced by cooling. Brain. 1951 Sep;74(3):318–335. doi: 10.1093/brain/74.3.318. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Receptors in the trachea and bronchi of the cat. J Physiol. 1954 Jan;123(1):71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitteridge D. Afferent nerve fibres from the heart and lungs in the cervical vagus. J Physiol. 1948 Sep 30;107(4):496–512. doi: 10.1113/jphysiol.1948.sp004294. [DOI] [PMC free article] [PubMed] [Google Scholar]


