Abstract
1. Action potentials resulting from direct stimulation can be recorded from the soma of the Aplysia giant neurone (located in the visceral ganglion) in sodium-free and in calcium-free external solutions. The neurones were impaled by internal micro-electrodes throughout the change of external solutions.
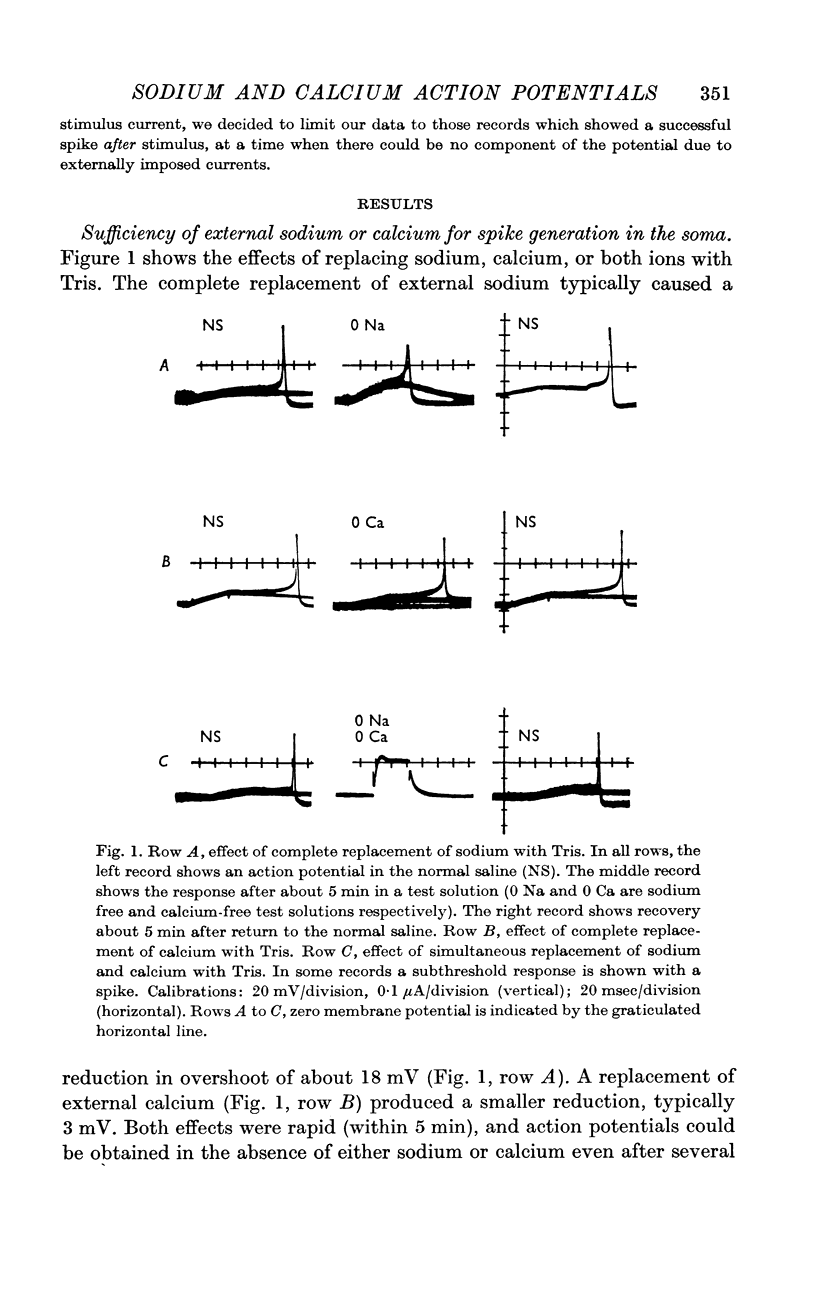
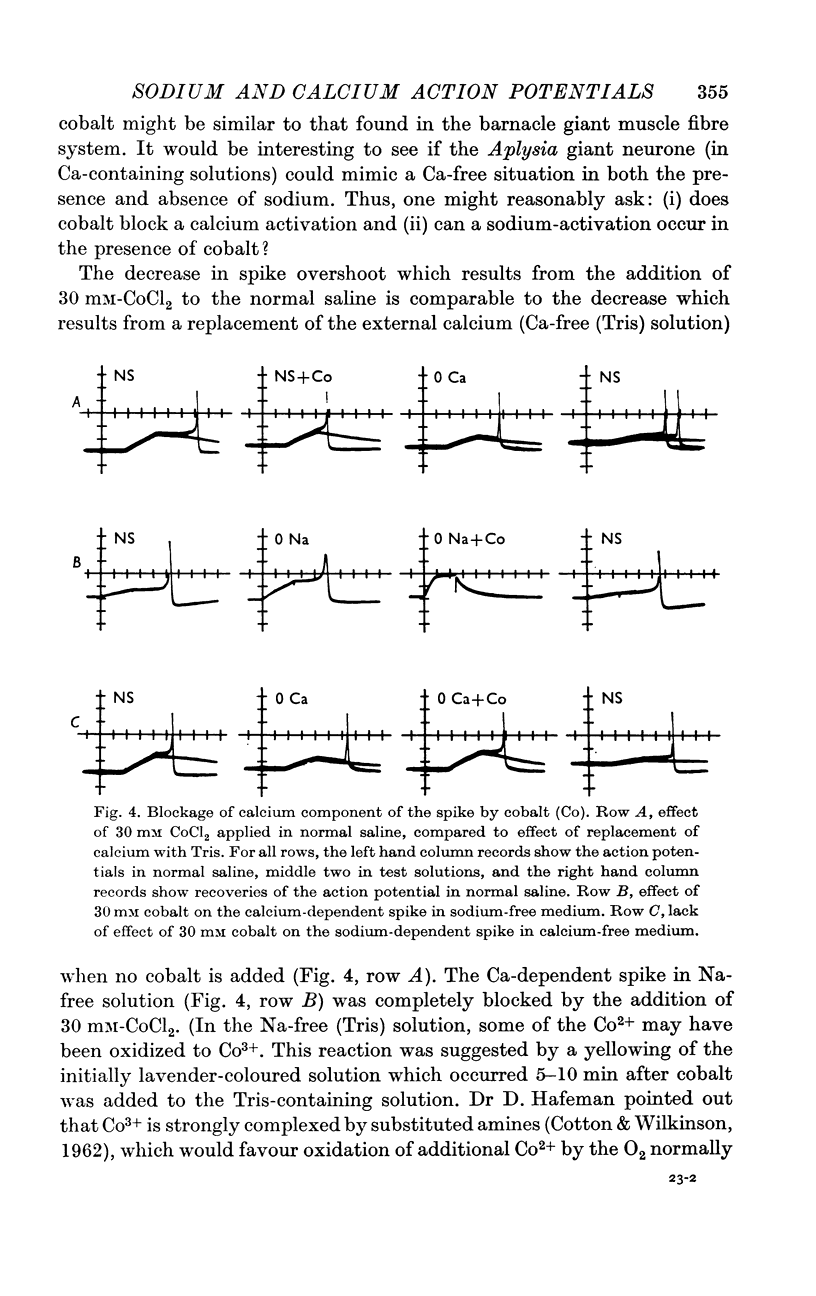
2. Complete replacement of either sodium or calcium in the bathing medium with Tris results in only a partial reduction of spike overshoot. Simultaneous replacement of both sodium and calcium reversibly and quickly abolishes the spike.
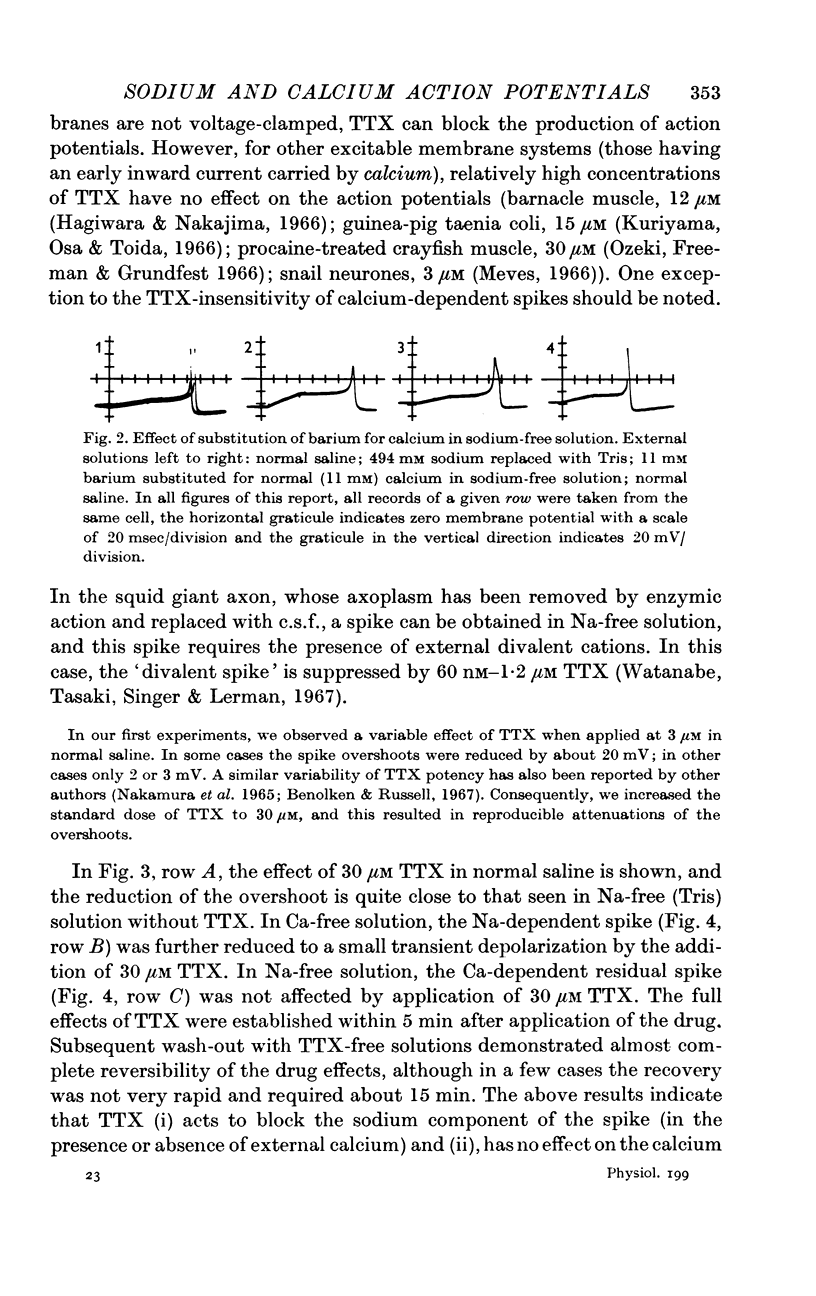
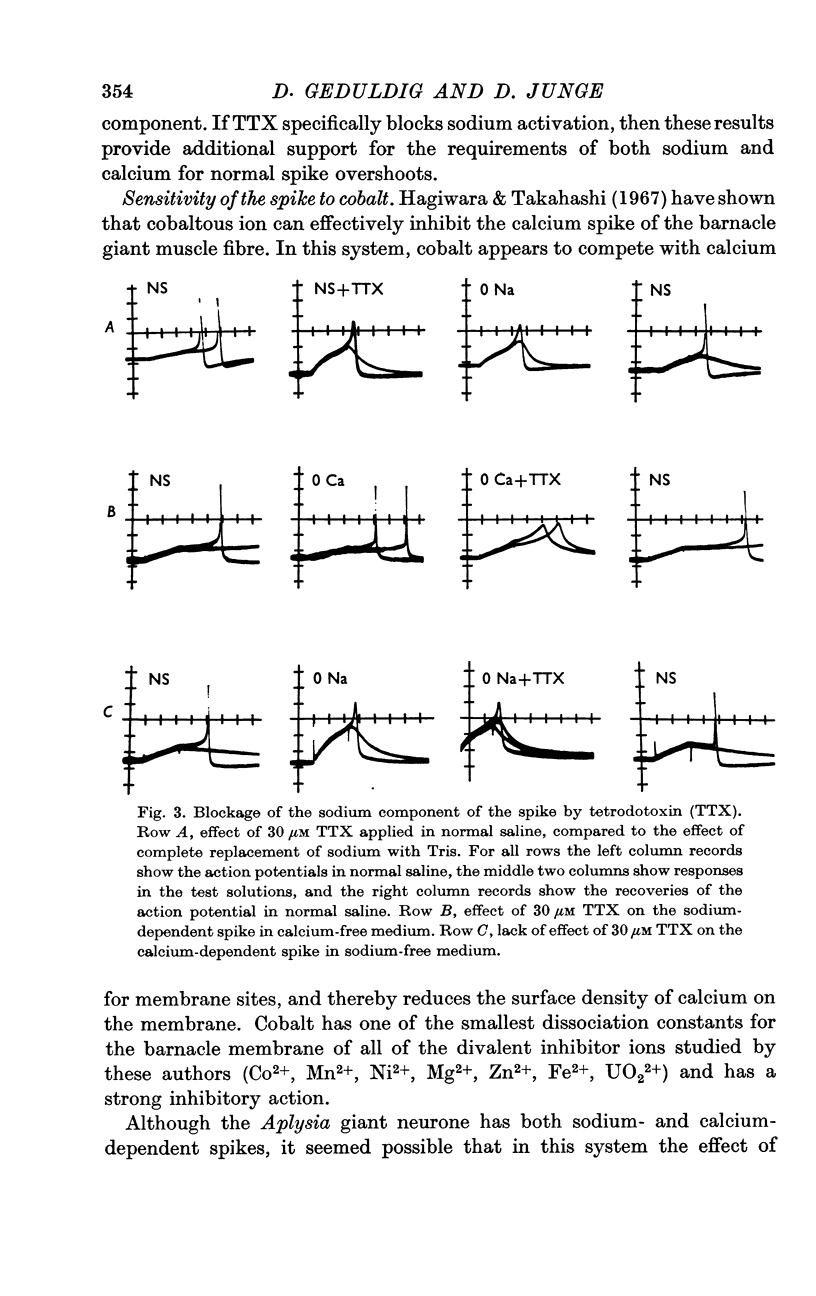
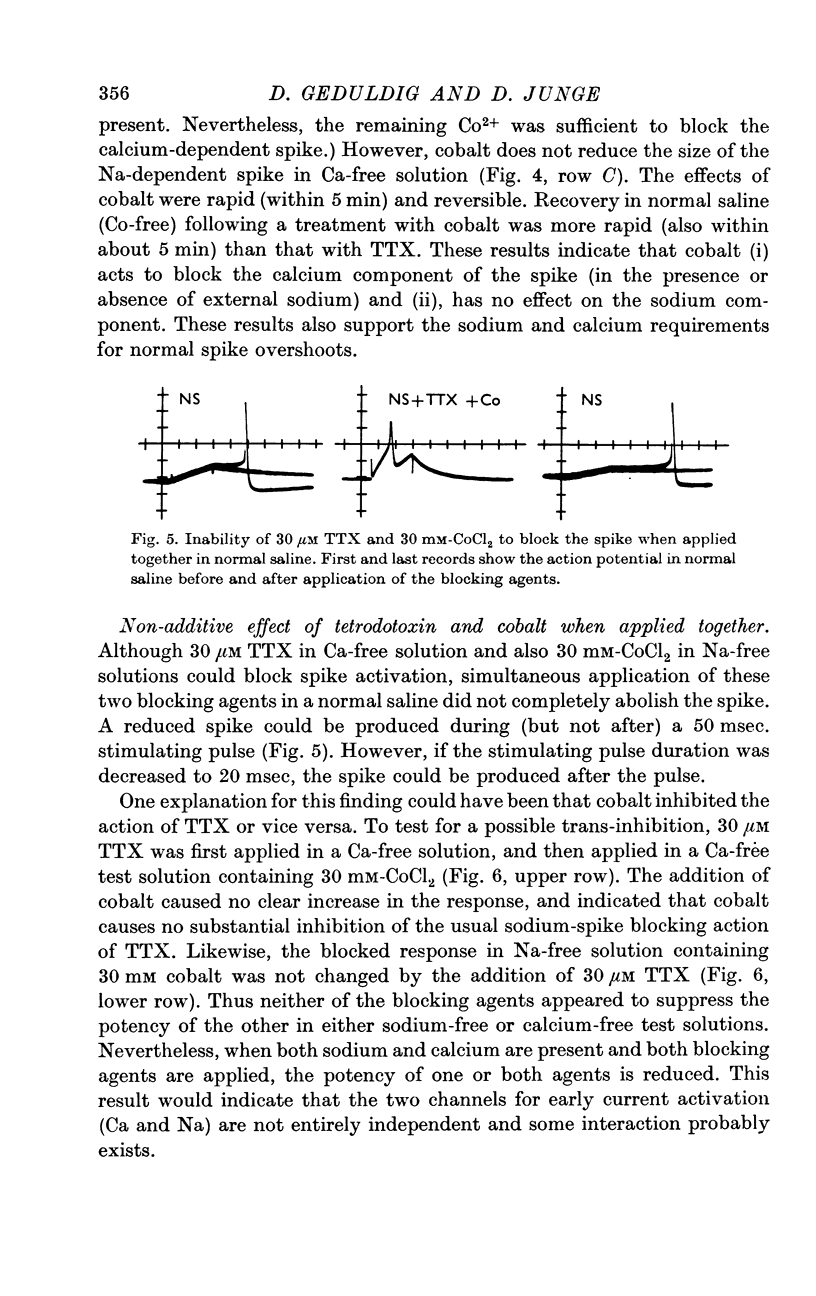
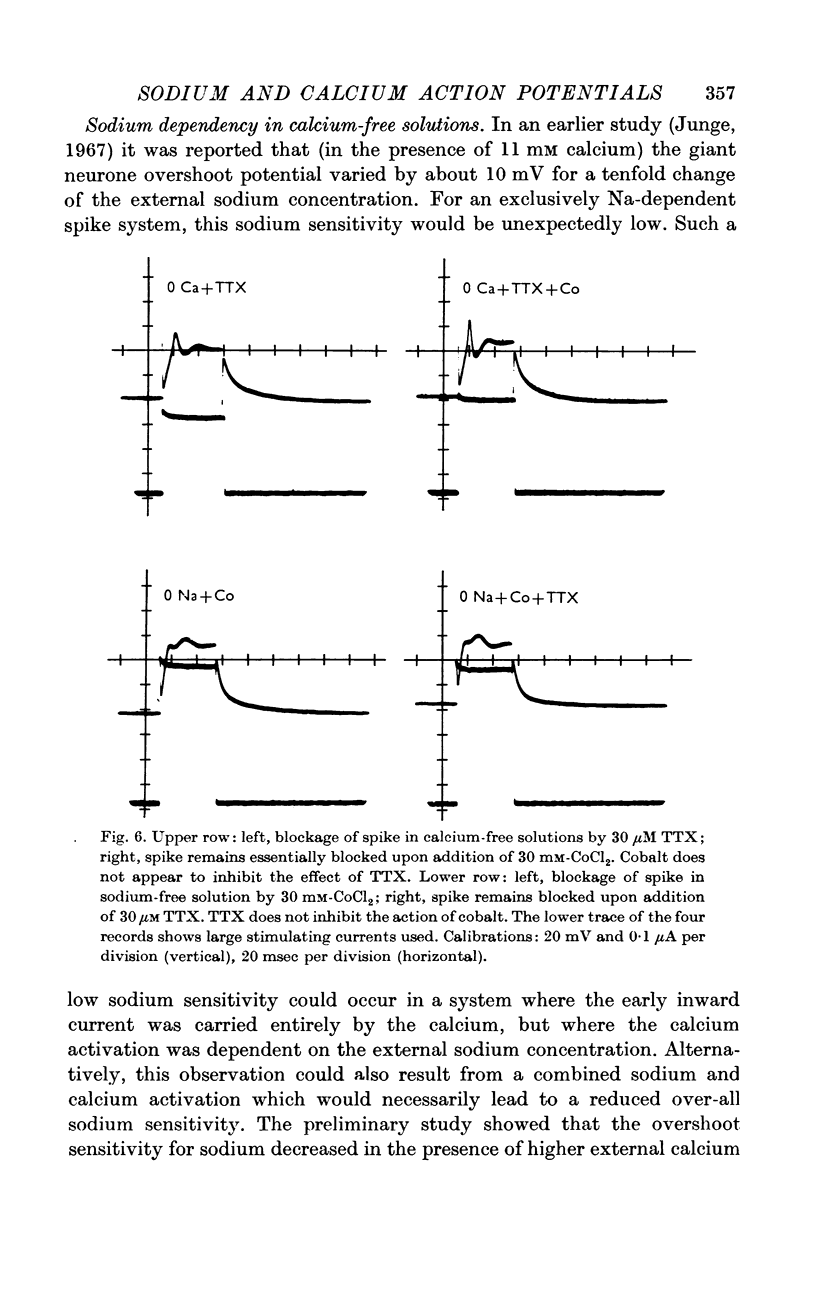
3. The sodium component of the spike in a calcium-free medium is blocked by tetrodotoxin; the drug has no effect on the calcium-dependent spike in sodium-free medium. Externally applied cobalt chloride blocks only the calcium-dependent component.
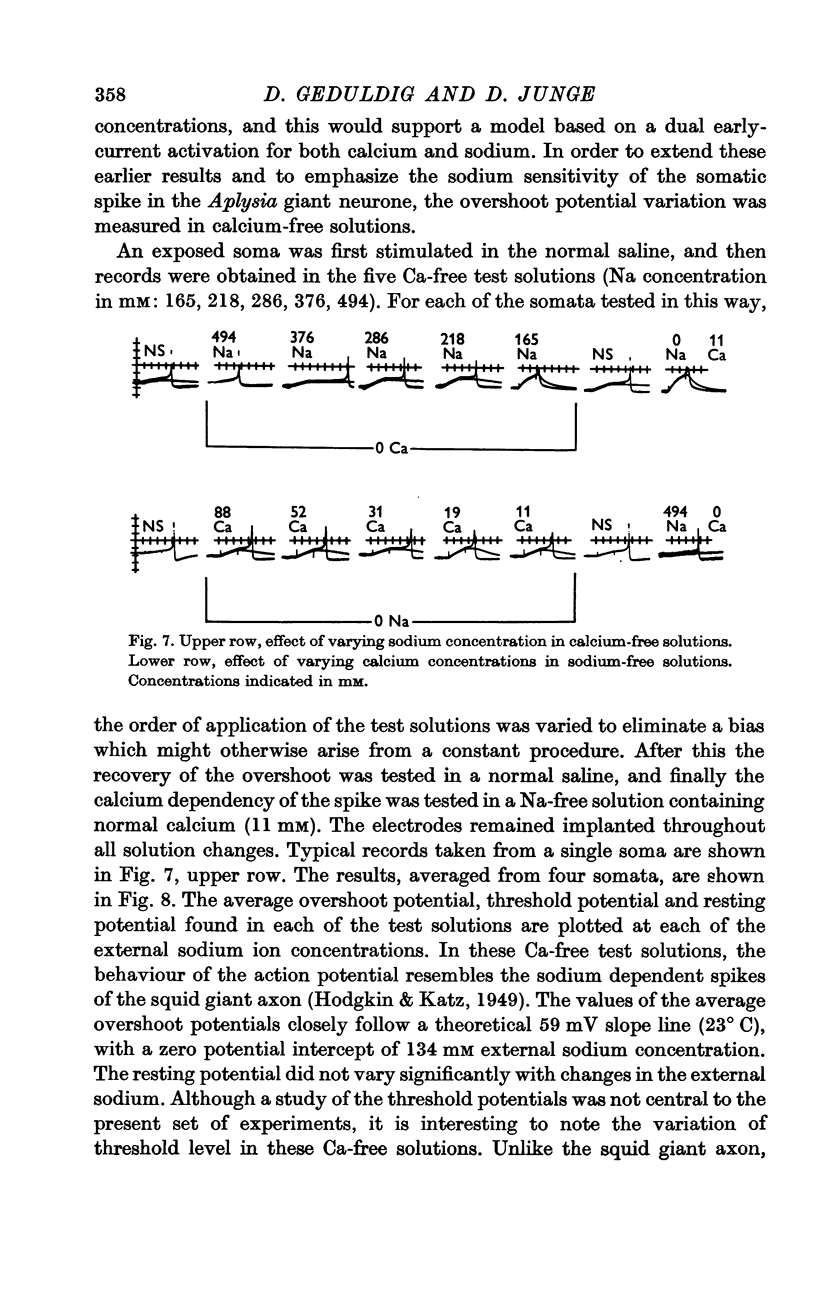
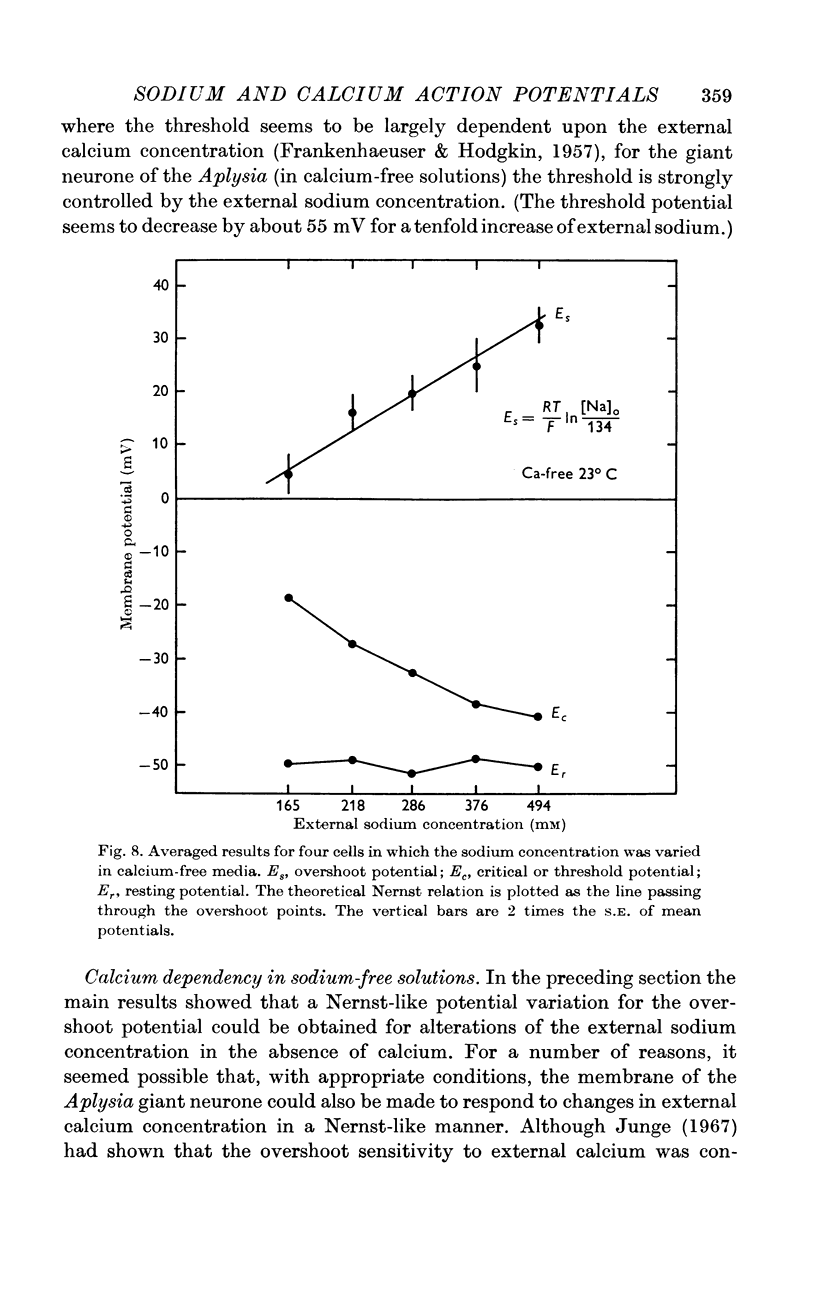
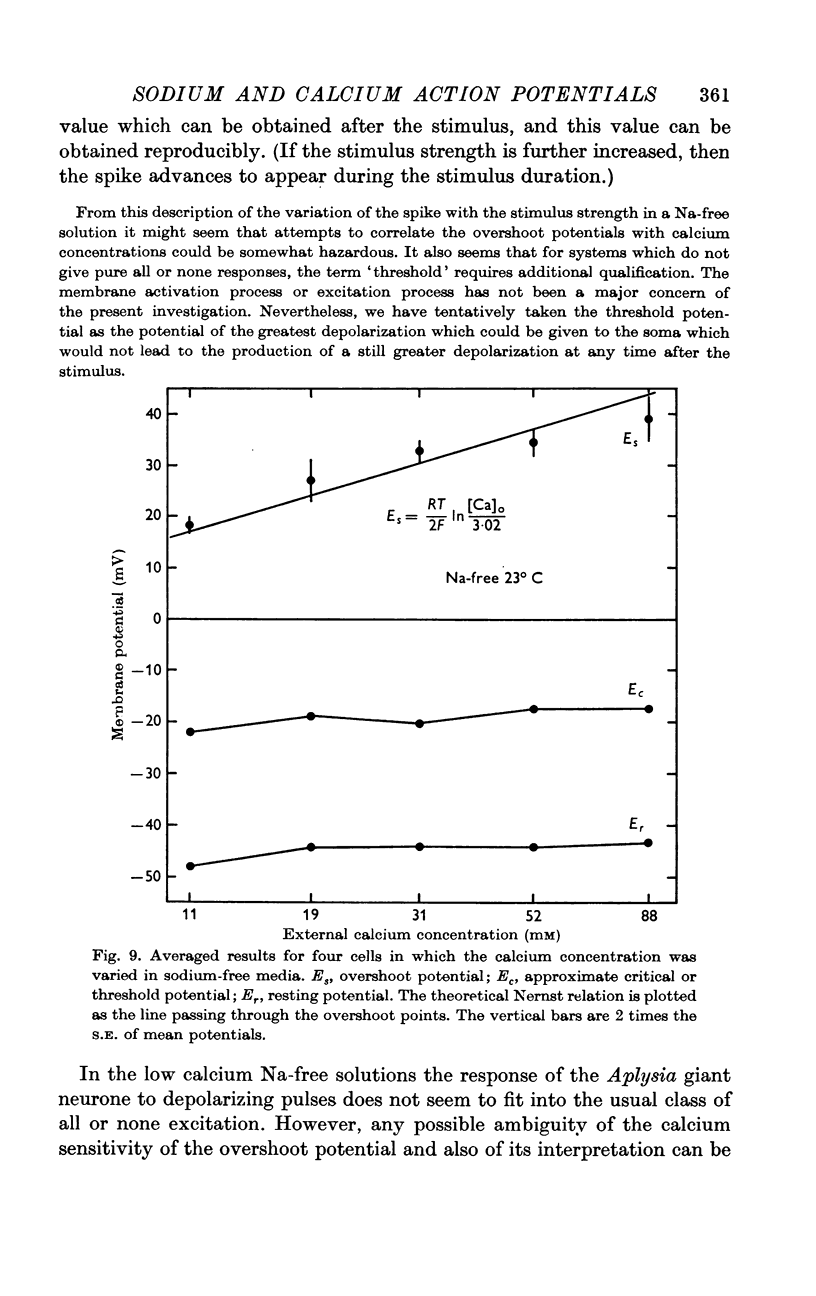
4. In calcium-free media, the overshoot value varies with sodium concentration in the manner predicted for a sodium electrode. In sodium-free media, the membrane behaves like a calcium electrode.
5. These results suggest that, during the normal action potential, both sodium and calcium act as carriers of the inward-directed current.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., PARNAS I. ELECTRICAL AND MECHANICAL RESPONSES IN DEEP ABDOMINAL EXTENSOR MUSCLES OF CRAYFISH AND LOBSTER. J Gen Physiol. 1965 May;48:919–931. doi: 10.1085/jgp.48.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benolken R. M., Russell C. J. Tetrodotoxin blocks a graded sensory response in the eye of Limulus. Science. 1967 Mar 24;155(3769):1576–1577. doi: 10.1126/science.155.3769.1576. [DOI] [PubMed] [Google Scholar]
- Chamberlain S. G., Kerkut G. A. Voltage clamp studies on snail (Helix aspersa) neurons. Nature. 1967 Oct 7;216(5110):89–89. doi: 10.1038/216089a0. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E., Kandel E. R., Kupfermann I., Waziri R. A morphological and functional study on a cluster of identifiable neurosecretory cells in the abdominal ganglion of aplysia californica. J Cell Biol. 1966 Nov 1;31(2):363–368. doi: 10.1083/jcb.31.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. The effect of calcium on the myelinated nerve fibre. J Physiol. 1957 Jul 11;137(2):245–260. doi: 10.1113/jphysiol.1957.sp005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON H. T., WELSH J. H. The role of ions in axon surface reactions to toxic organic compounds. J Cell Physiol. 1948 Jun;31(3):395–419. doi: 10.1002/jcp.1030310311. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., CHICHIBU S., NAKA K. I. THE EFFECTS OF VARIOUS IONS ON RESTING AND SPIKE POTENTIALS OF BARNACLE MUSCLE FIBERS. J Gen Physiol. 1964 Sep;48:163–179. doi: 10.1085/jgp.48.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature. 1966 Jun 18;210(5042):1220–1222. doi: 10.1038/2101220a0. [DOI] [PubMed] [Google Scholar]
- Junge D. Multi-ionic action potentials in molluscan giant neurones. Nature. 1967 Jul 29;215(5100):546–548. doi: 10.1038/215546a0. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Effect of tetrodotoxin on smooth muscle cells of the guinea-pig taenia coli. Br J Pharmacol Chemother. 1966 Aug;27(2):366–376. doi: 10.1111/j.1476-5381.1966.tb01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nakajima S., Grundfest H. The action of tetrodotoxin on electrogenic components of squid giant axons. J Gen Physiol. 1965 Jul;48(6):975–996. [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dependence of the action potential of the frog's heart on the external and intracellular sodium concentration. J Physiol. 1966 May;184(2):312–334. doi: 10.1113/jphysiol.1966.sp007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dual effect of calcium on the action potential of the frog's heart. J Physiol. 1966 May;184(2):291–311. doi: 10.1113/jphysiol.1966.sp007916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki M., Freeman A. R., Grundfest H. The membrane components of crustacean neuromuscular systems. I. Immunity of different electrogenic components to tetrodotoxin and saxitoxin. J Gen Physiol. 1966 Jul;49(6):1319–1334. doi: 10.1085/jgp.0491319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K. Permeability changes associated with the action potential in procaine-treated crayfish abdominal muscle fibers. J Gen Physiol. 1967 Mar;50(4):1049–1074. doi: 10.1085/jgp.50.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Singer I. Membrane macromolecules and nerve excitability: a physico-chemical interpretation of excitation in squid giant axons. Ann N Y Acad Sci. 1966 Jul 14;137(2):792–806. doi: 10.1111/j.1749-6632.1966.tb50200.x. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Tasaki I., Singer I., Lerman L. Effects of tetrodotoxin on excitability of squid giant axons in sodium-free media. Science. 1967 Jan 6;155(3758):95–97. doi: 10.1126/science.155.3758.95. [DOI] [PubMed] [Google Scholar]


