Abstract
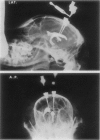
1. An operation on dogs for the implantation of guide tubes to the lateral ventricle and cisterna magna and a method whereby the ventricular space can be repeatedly perfused in conscious and unrestrained animals are described.
2. The characteristics of a recirculatory perfusion system were examined and the bulk formation and absorption of cerebrospinal fluid and the volume of the ventricular space perfused were derived from the concentrations achieved during the infusion of inulin into the system.
3. 5-hydroxyindol-3-ylacetic acid (5-HIAA), the acid metabolite of 5-hydroxytryptamine, and 3-methoxy-4-hydroxyphenylacetic acid (HVA), the main acid metabolite of dopamine, were demonstrated to be mainly removed from cerebrospinal fluid (c.s.f.) by an active transport system localized in the region of the fourth ventricle.
4. It was possible to inhibit the active transport of these acids from cerebrospinal fluid by pre-treating the dogs with probenecid.
Full text
PDF






























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft G. W., Crawford T. B., Dow R. C., Guldberg H. C. Homovanillic acid, 3,4-dihydroxyphenylacetic acid and 5-hydroxyindol-3-ylacetic acid in serial samples of cerebrospinal fluid from the lateral ventricle of the dog. Br J Pharmacol Chemother. 1968 Jul;33(3):441–456. doi: 10.1111/j.1476-5381.1968.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERING E. A., Jr, SATO O. HYDROCEPHALUS: CHANGES IN FORMATION AND ABSORPTION OF CEREBROSPINAL FLUID WITHIN THE CEREBRAL VENTRICLES. J Neurosurg. 1963 Dec;20:1050–1063. doi: 10.3171/jns.1963.20.12.1050. [DOI] [PubMed] [Google Scholar]
- BRADBURY M. W., DAVSON H. THE TRANSPORT OF UREA, CREATININE AND CERTAIN MONOSACCHARIDES BETWEEN BLOOD AND FLUID PERFUSING THE CEREBRAL VENTRICULAR SYSTEM OF RABBITS. J Physiol. 1964 Jan;170:195–211. doi: 10.1113/jphysiol.1964.sp007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W., Davson H. The transport of potassium between blood, cerebrospinal fluid and brain. J Physiol. 1965 Nov;181(1):151–174. doi: 10.1113/jphysiol.1965.sp007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMERON G. Secretory activity of the chorioid plexus in tissue culture. Anat Rec. 1953 Sep;117(1):115–125. doi: 10.1002/ar.1091170109. [DOI] [PubMed] [Google Scholar]
- Cserr H. Potassium exchange between cerebrospinal fluid, plasma, and brain. Am J Physiol. 1965 Dec;209(6):1219–1226. doi: 10.1152/ajplegacy.1965.209.6.1219. [DOI] [PubMed] [Google Scholar]
- DAVSON H., KLEEMAN C. R., LEVIN E. Quantitative studies of the passage of different substances out of the cerebrospinal fluid. J Physiol. 1962 Apr;161:126–142. doi: 10.1113/jphysiol.1962.sp006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVSON H., POLLAY M. Influence of various drugs on the transport of 131-I and PAH across the cerebrospinal-fluid-blood barrier. J Physiol. 1963 Jul;167:239–246. doi: 10.1113/jphysiol.1963.sp007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVSON H., POLLAY M. The turnover of 24Na in the cerebrospinal fluid and its bearing on the blood-brain barrier. J Physiol. 1963 Jul;167:247–255. doi: 10.1113/jphysiol.1963.sp007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESPOPOULOS A., WEISSBACH H. Renal metabolism of 5-hydroxyindolacetic acid. Am J Physiol. 1957 Jun;189(3):548–550. doi: 10.1152/ajplegacy.1957.189.3.548. [DOI] [PubMed] [Google Scholar]
- FELDBERG W., FLEISCHHAUER K. Penetration of bromophenol blue from the perfused cerebral ventricles into the brain tissue. J Physiol. 1960 Feb;150:451–462. doi: 10.1113/jphysiol.1960.sp006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDBERG W., FLEISCHHAUER K. THE HIPPOCAMPUS AS THE SITE OF ORIGIN OF THE SEIZURE DISCHARGE PRODUCED BY TUBOCURARINE ACTING FROM THE CEREBRAL VENTRICLES. J Physiol. 1963 Sep;168:435–442. doi: 10.1113/jphysiol.1963.sp007201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Fleischhauer K. Site of origin of the abnormal discharge in the electrocorticogram produced by tubocurarine perfused through the anterior horn of a lateral ventricle. J Physiol. 1967 Aug;191(3):487–500. doi: 10.1113/jphysiol.1967.sp008263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldberg H. C., Ashcroft G. W., Crawford T. B. Concentrations of 5-hydroxyindolylacetic acid and homovanillic acid in the cerebrospinal fluid of the dog before and during treatment with probenecid. Life Sci. 1966 Sep;5(17):1571–1575. doi: 10.1016/0024-3205(66)91026-5. [DOI] [PubMed] [Google Scholar]
- HEISEY S. R., HELD D., PAPPENHEIMER J. R. Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am J Physiol. 1962 Nov;203:775–781. doi: 10.1152/ajplegacy.1962.203.5.775. [DOI] [PubMed] [Google Scholar]
- HEYROVSKY A. A new method for the determination of inulin in plasma and urine. Clin Chim Acta. 1956 Sep-Oct;1(5):470–474. doi: 10.1016/0009-8981(56)90020-1. [DOI] [PubMed] [Google Scholar]
- LEUSEN I. R. Chemosensitivity of the respiratory center influence of changes in the H+ and total buffer concentrations in the cerebral ventricles on respiration. Am J Physiol. 1954 Jan;176(1):45–51. doi: 10.1152/ajplegacy.1953.176.1.45. [DOI] [PubMed] [Google Scholar]
- LEUSEN I. R. Chemosensitivity of the respiratory center; influence of CO2 in the cerebral ventricles on respiration. Am J Physiol. 1954 Jan;176(1):39–44. doi: 10.1152/ajplegacy.1953.176.1.39. [DOI] [PubMed] [Google Scholar]
- LEUSEN I. The influence of calcium, potassium and magnesium ions in cerebrospinal fluid on vasomotor system. J Physiol. 1949 Dec;110(3-4):319–329. doi: 10.1113/jphysiol.1949.sp004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPPELT W. W., MAREN T. H., OWENS E. S., RALL D. P. EFFECTS OF ACID-BASE ALTERATIONS ON CEREBROSPINAL FLUID PRODUCTION. Proc Soc Exp Biol Med. 1963 Oct;114:86–89. doi: 10.3181/00379727-114-28593. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., FENCL V., HEISEY S. R., HELD D. ROLE OF CEREBRAL FLUIDS IN CONTROL OF RESPIRATION AS STUDIED IN UNANESTHETIZED GOATS. Am J Physiol. 1965 Mar;208:436–450. doi: 10.1152/ajplegacy.1965.208.3.436. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., HEISEY S. R., JORDAN E. F. Active transport of Diodrast and phenolsulfonphthalein from cerebrospinal fluid to blood. Am J Physiol. 1961 Jan;200:1–10. doi: 10.1152/ajplegacy.1961.200.1.1. [DOI] [PubMed] [Google Scholar]
- POLLAY M., DAVSON H. The passage of certain substances out of the cerebrosphinal fluid. Brain. 1963 Mar;86:137–150. doi: 10.1093/brain/86.1.137. [DOI] [PubMed] [Google Scholar]
- Palaić D., Page I. H., Khairallah P. A. Uptake and metabolism of [14C] serotonin in rat brain. J Neurochem. 1967 Jan;14(1):63–69. doi: 10.1111/j.1471-4159.1967.tb09494.x. [DOI] [PubMed] [Google Scholar]
- RALL D. P., SHELDON W. Transport of organic acid dyes by the isolated choroid plexus of the spiny dogfish S. acanthias. Biochem Pharmacol. 1962 Feb;11:169–170. doi: 10.1016/0006-2952(62)90107-7. [DOI] [PubMed] [Google Scholar]
- SMITH D. E., STREICHER E., MILKOVIC K., KLATZO I. OBSERVATIONS ON THE TRANSPORT OF PROTEINS BY THE ISOLATED CHOROID PLEXUS. Acta Neuropathol. 1964 Mar 4;3:372–386. doi: 10.1007/BF00691845. [DOI] [PubMed] [Google Scholar]
- WELCH K. Active transport of iodide by choroid plexus of the rabbit in vitro. Am J Physiol. 1962 Apr;202:757–760. doi: 10.1152/ajplegacy.1962.202.4.757. [DOI] [PubMed] [Google Scholar]
- WELCH K. Concentration of thiocyanate by the choroid plexus of the rabbit in vitro. Proc Soc Exp Biol Med. 1962 Apr;109:953–954. doi: 10.3181/00379727-109-27389. [DOI] [PubMed] [Google Scholar]