Abstract
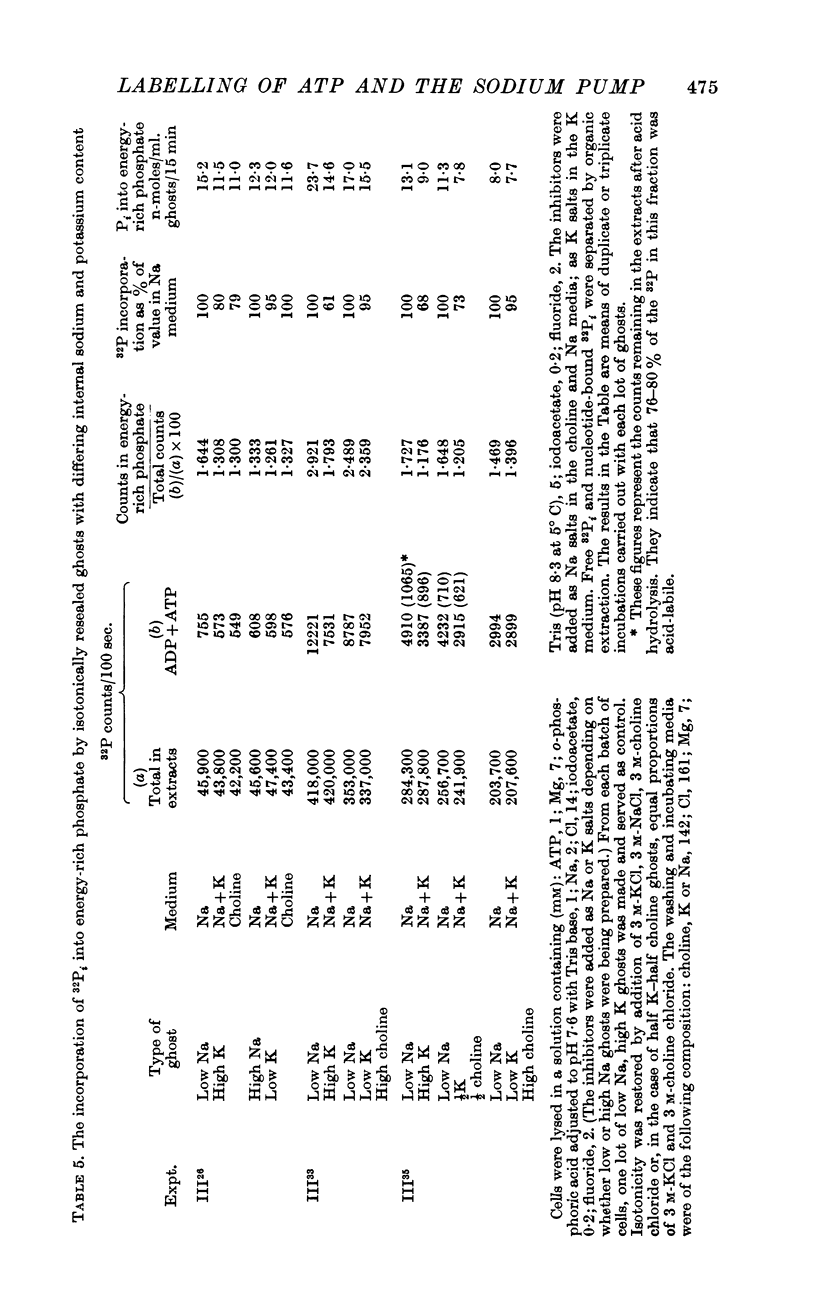
1. The ionic composition of human red cell ghosts and suspending Ringer solutions have been varied independently. Measurements were made of the incorporation of [32P]o-phosphate (32Pi) into ATP associated with different concentration gradients of Na and K across the membrane.
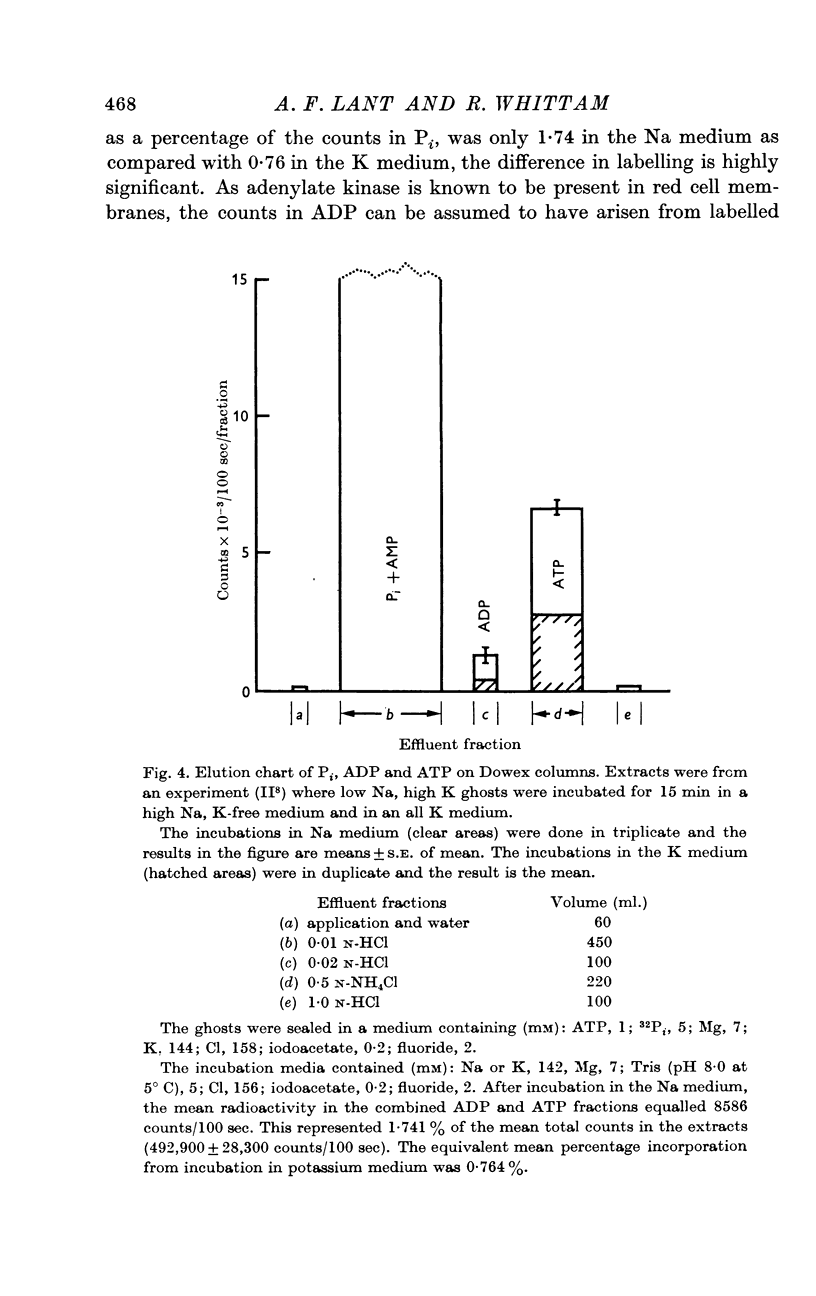
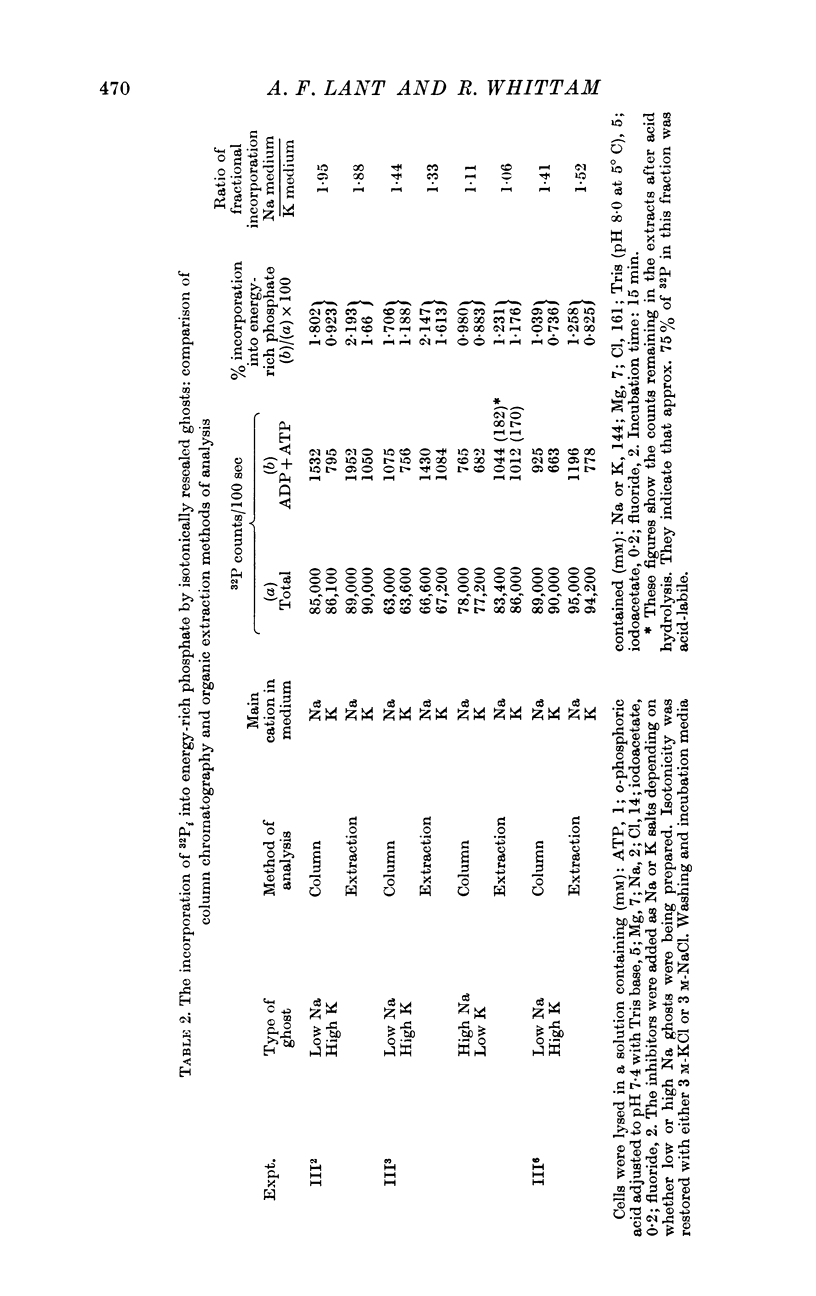
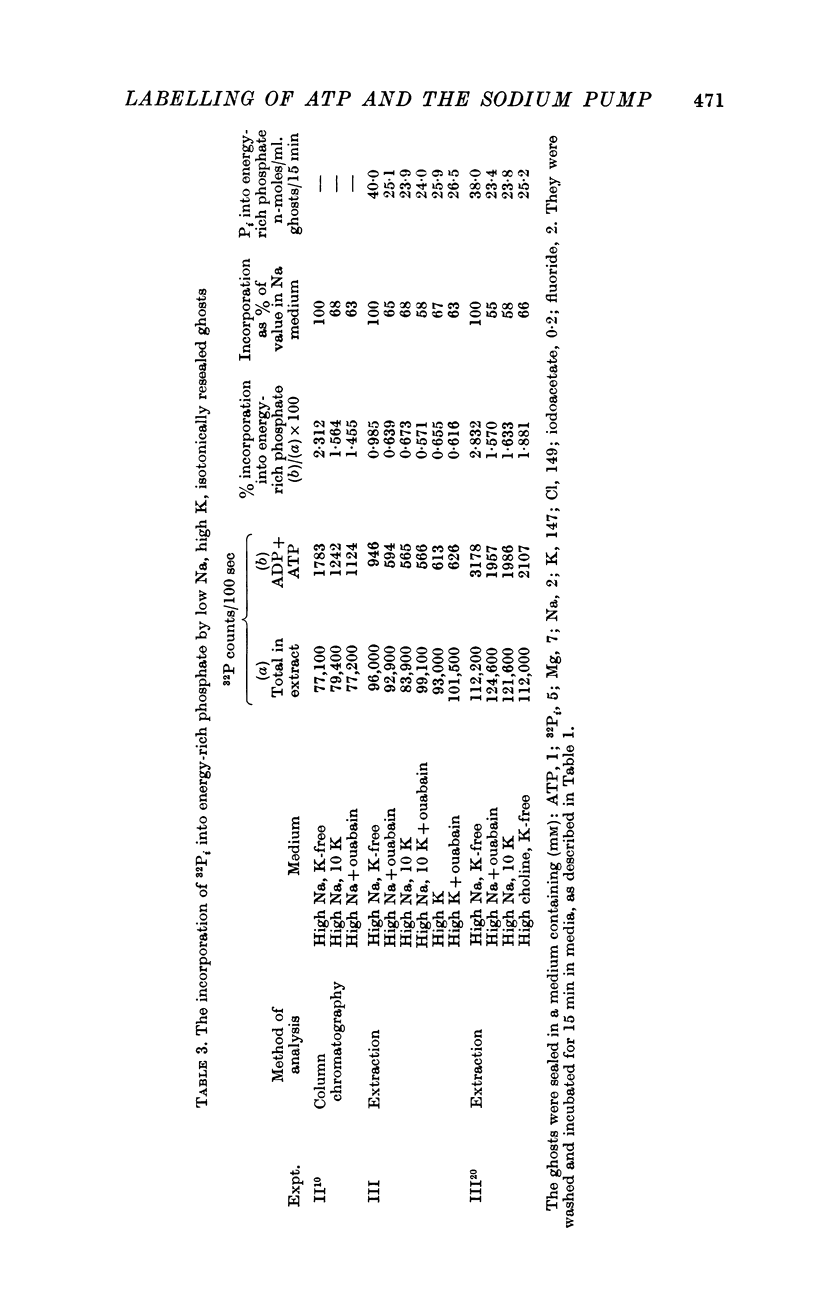
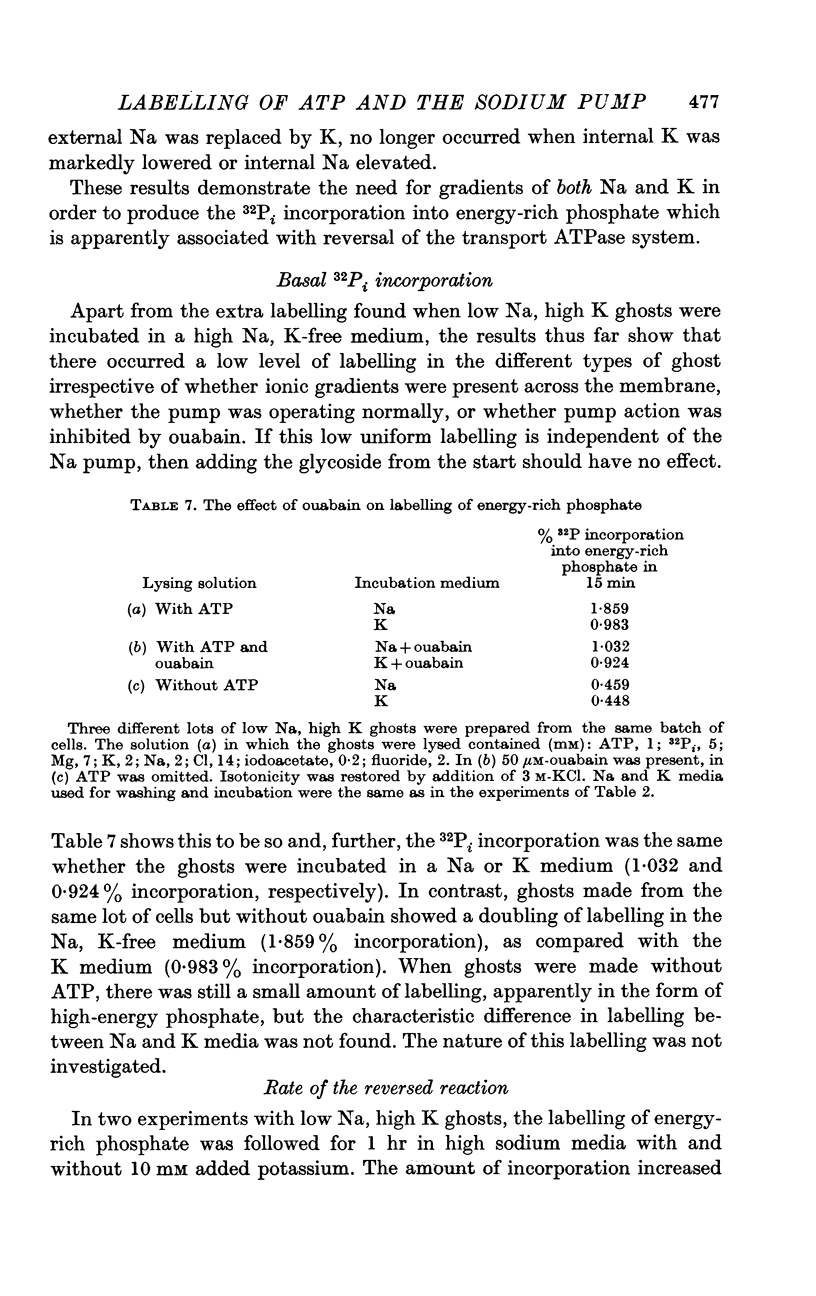
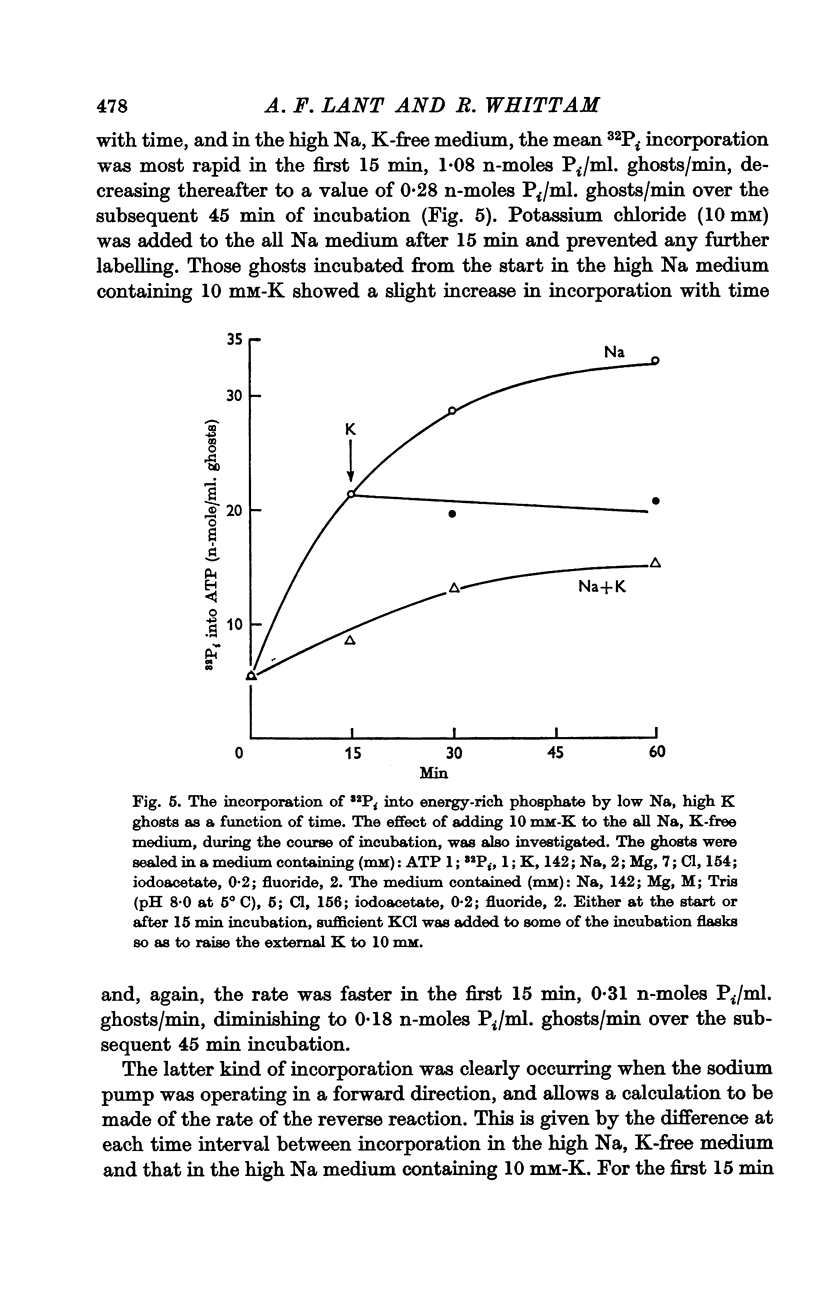
2. Some incorporation of 32Pi was always found irrespective of the ionic composition of ghosts or media. However, additional labelling of energy-rich phosphate occurred when low Na, high K ghosts were incubated in a high Na, K-free medium. This did not occur when there was only a gradient of either Na or K. Downhill movements of both Na into and K out of the ghosts were needed for the extra labelling.
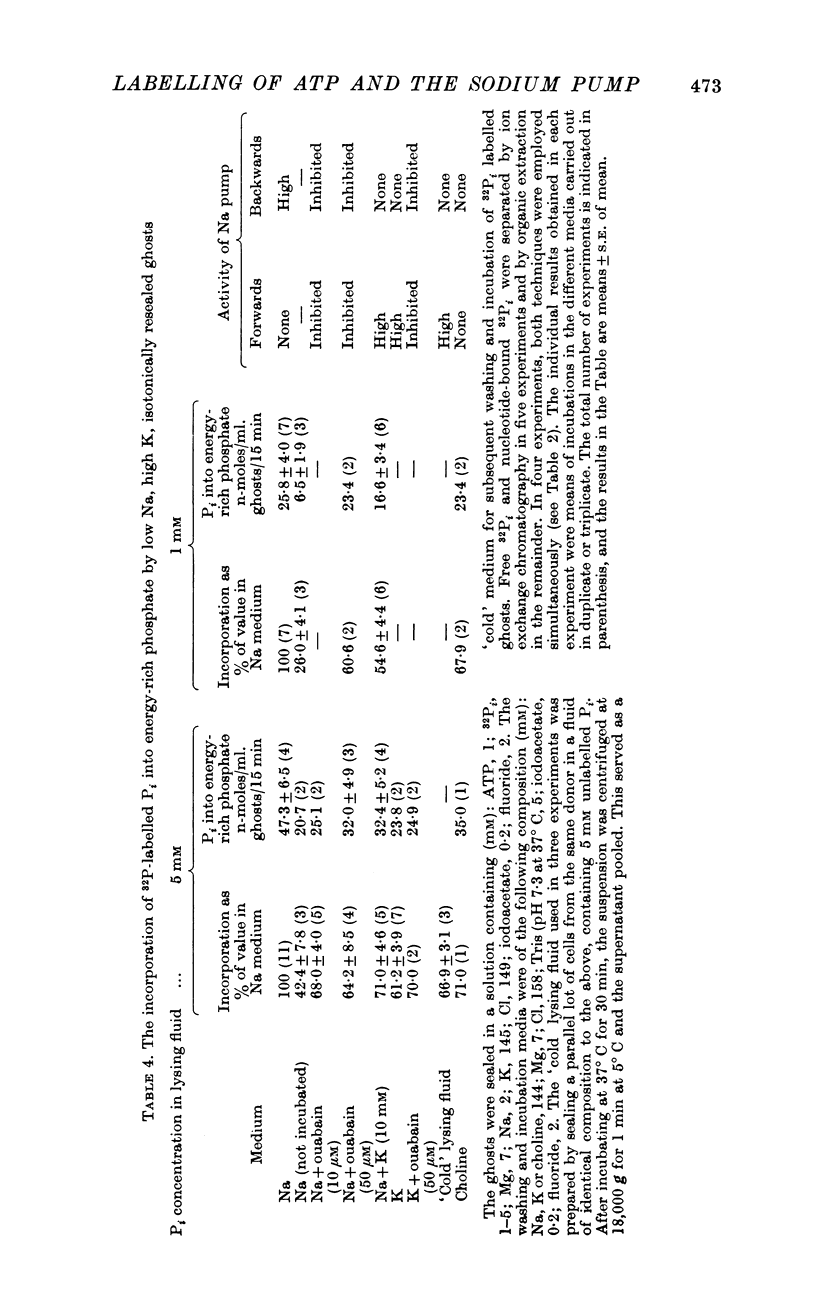
3. Even in the presence of suitable ionic gradients, the extra incorporation was prevented by ouabain or by adding a small amount of external K sufficient to facilitate normal operation of the Na pump.
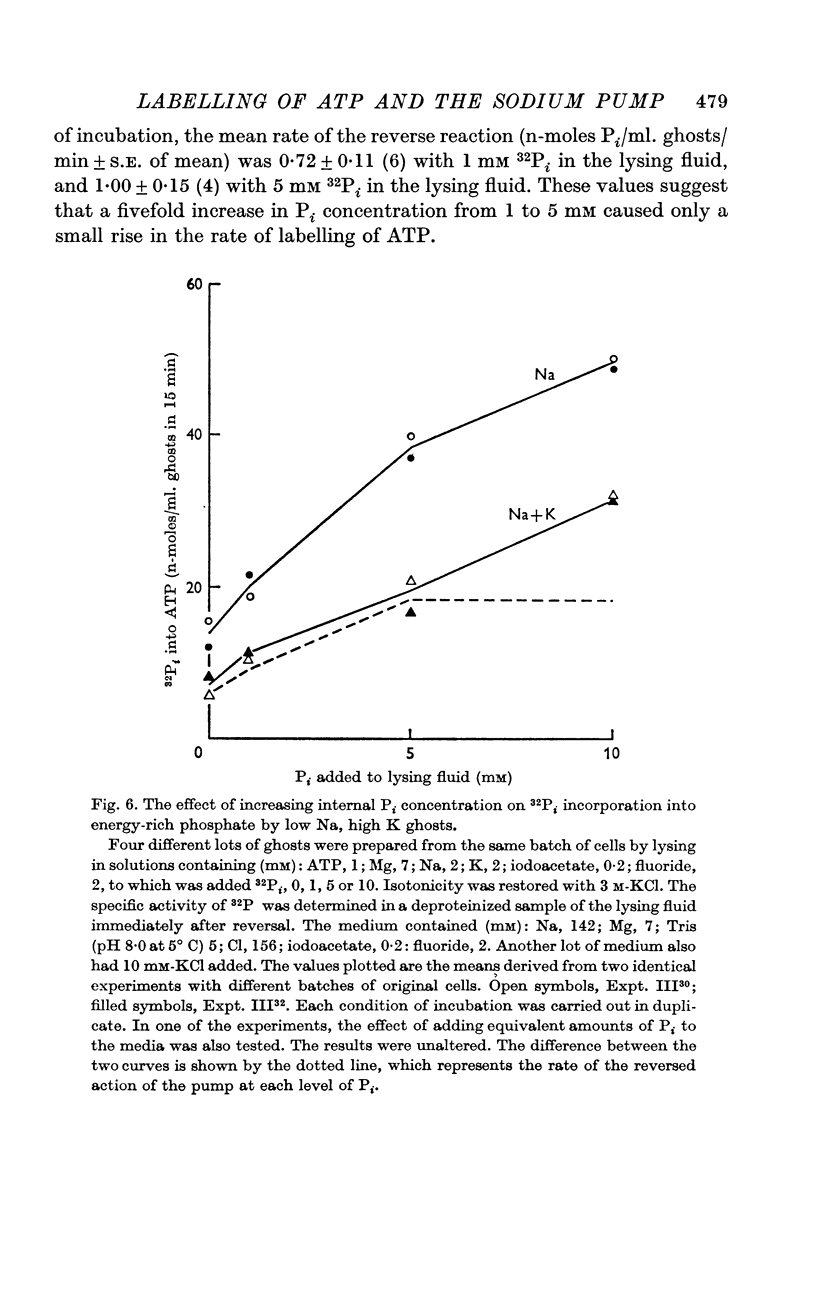
4. Increase in internal Pi stimulated the incorporation.
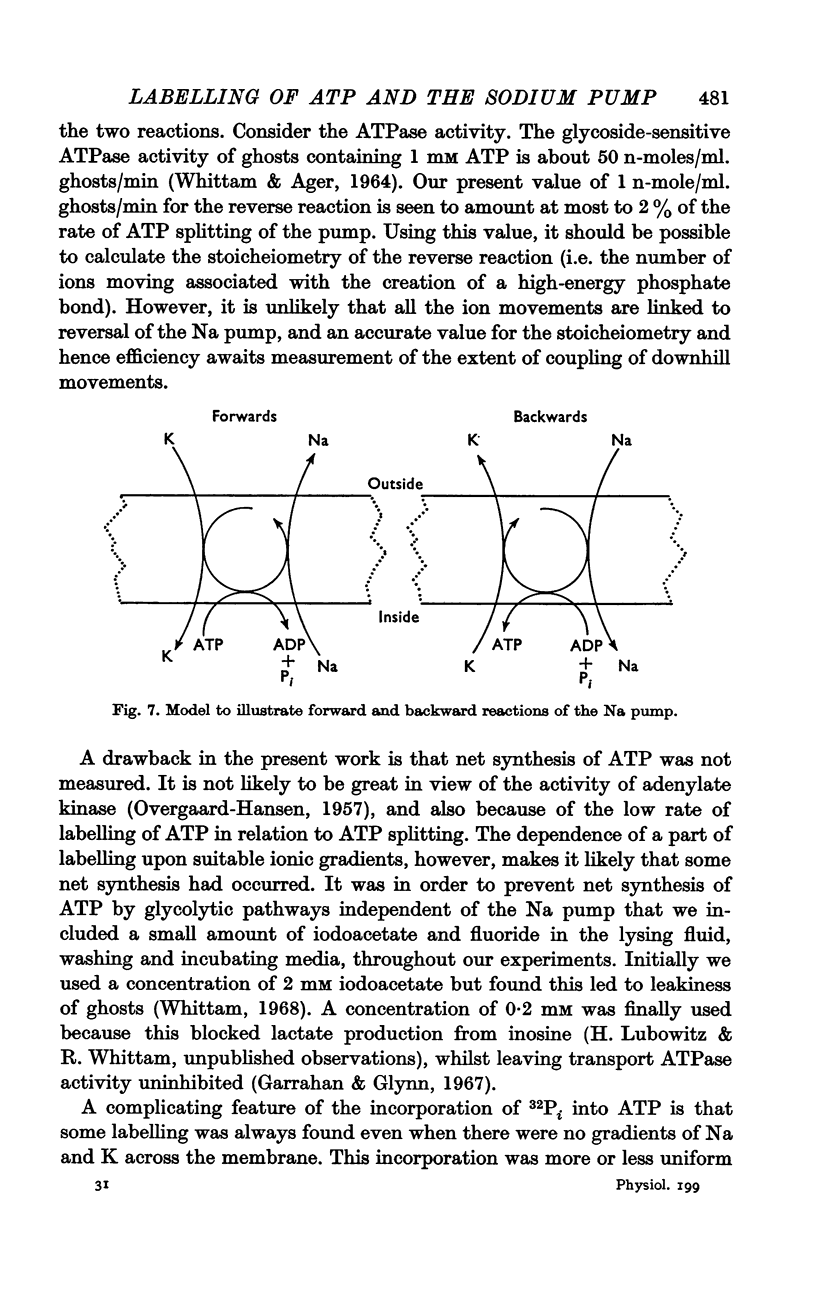
5. The results show that the conditions for forward and backward running of the ATPase system associated with the Na pump are such that both reactions cannot proceed optimally at the same time.
Full text
PDF



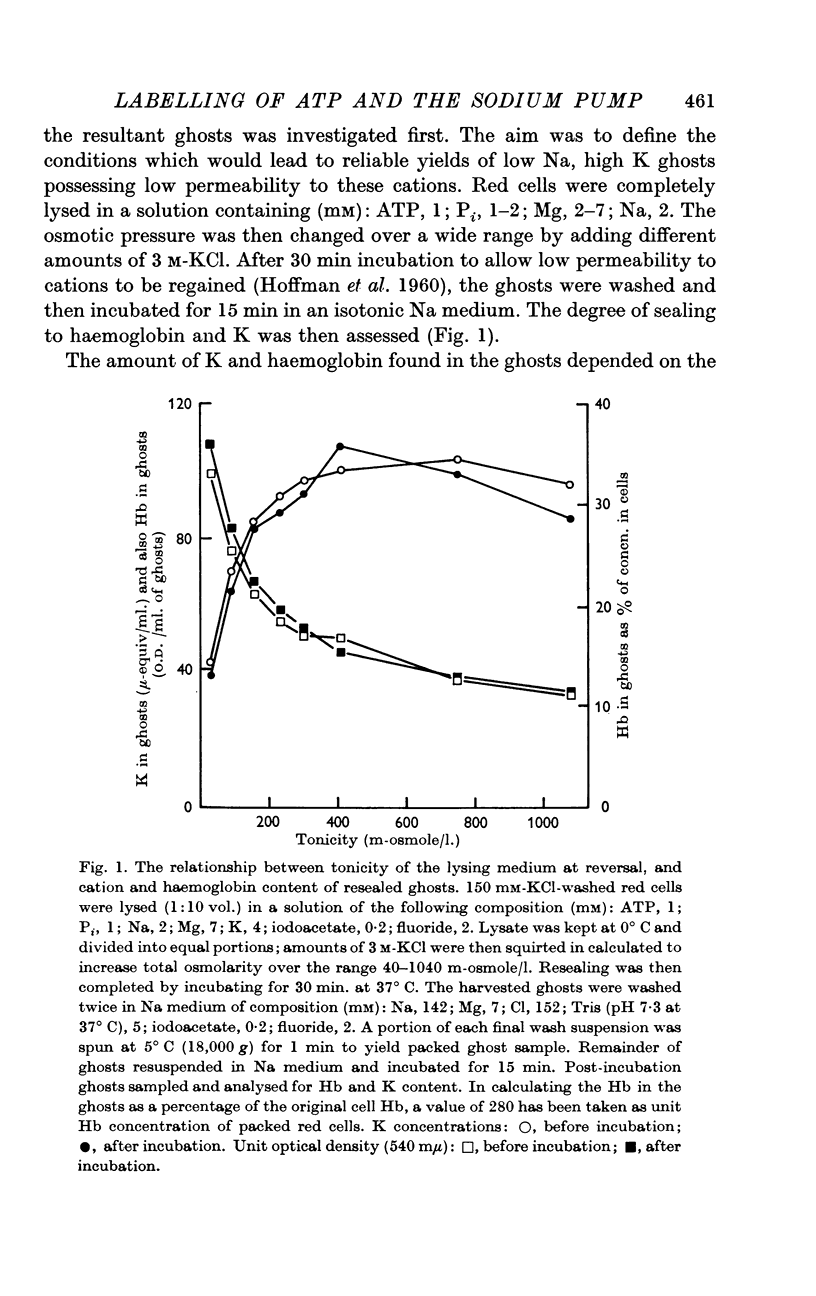


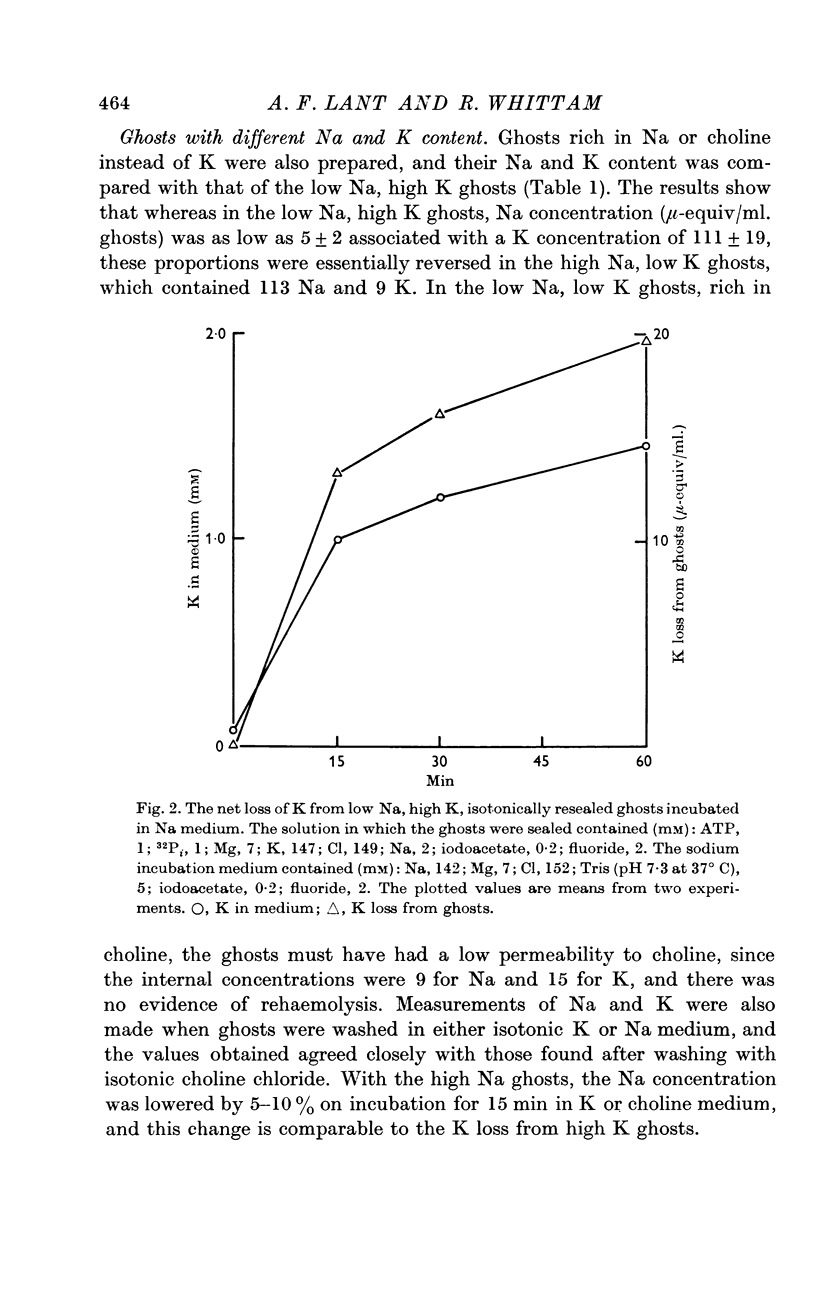
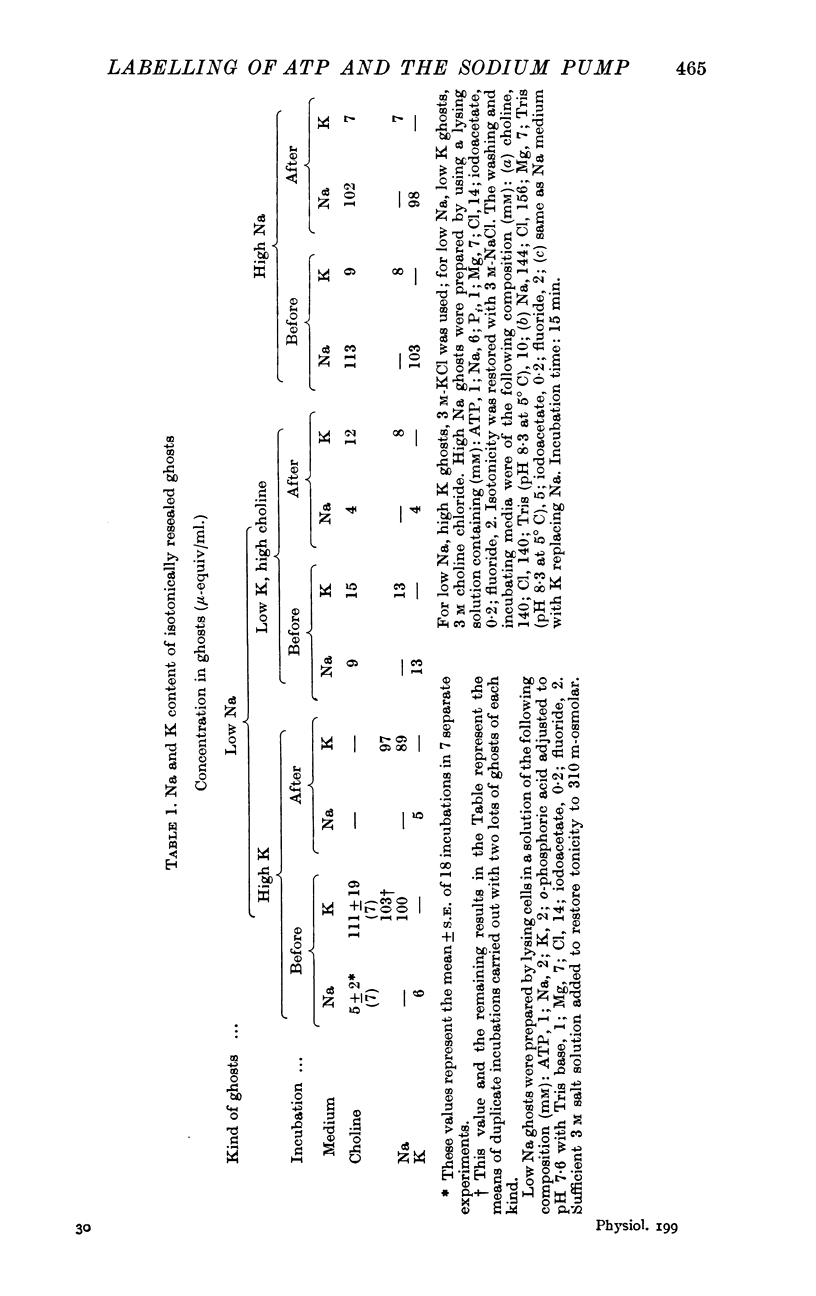
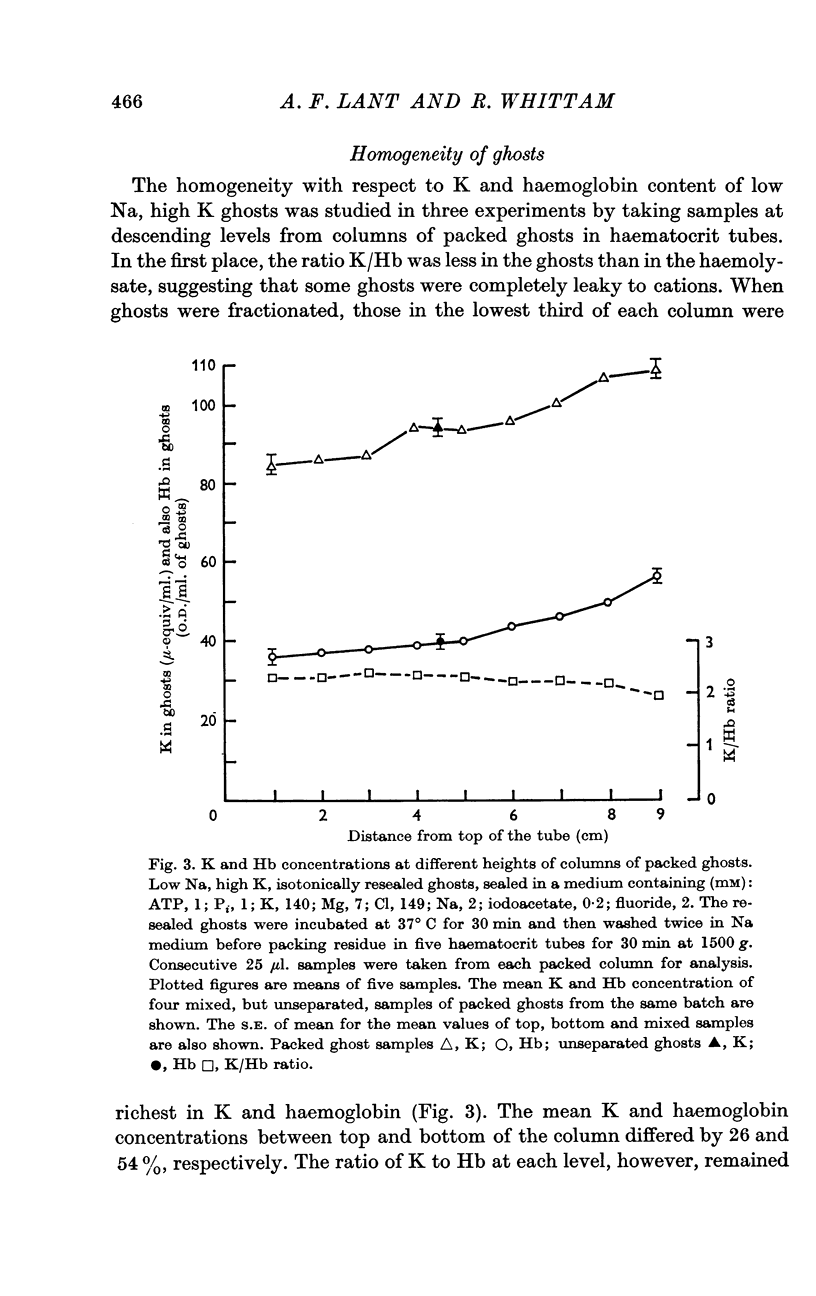









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Methods for the isolation of glycolytic intermediated by column chromatography with ion exchange resins. J Biol Chem. 1959 Mar;234(3):459–465. [PubMed] [Google Scholar]
- BRAUNSBERG H., GUYVER A. AUTOMATIC LIQUID SCINTILLATION COUNTING OF HIGH-ENERGY BETA-EMITTERS IN TISSUE SLICES AND AQUEOUS SOLUTIONS IN THE ABSENCE OF ORGANIC SCINTILLATOR. Anal Biochem. 1965 Jan;10:86–95. doi: 10.1016/0003-2697(65)90241-1. [DOI] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum I., Chain E. Studies on the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):286–294. doi: 10.1042/bj0320286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. I. An associated sodium-activated transphosphorylation. J Biol Chem. 1966 Apr 25;241(8):1882–1889. [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Measurement of 24Na and 42K with a liquid-scintillation counting system without added scintillator. J Physiol. 1966 Oct;186(2):55P–56P. [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The incorporation of inorganic phosphate into adenosine triphosphate by reversal of the sodium pump. J Physiol. 1967 Sep;192(1):237–256. doi: 10.1113/jphysiol.1967.sp008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M. Membrane adenosine triphosphatase and cation transport. Br Med Bull. 1968 May;24(2):165–169. doi: 10.1093/oxfordjournals.bmb.a070620. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. Physiological characteristics of human red blood cell ghosts. J Gen Physiol. 1958 Sep 20;42(1):9–28. doi: 10.1085/jgp.42.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN J. F., TOSTESON D. C., WHITTAM R. Retention of potassium by human erythrocyte ghosts. Nature. 1960 Jan 16;185:186–187. doi: 10.1038/185186a0. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. The active transport of sodium by ghosts of human red blood cells. J Gen Physiol. 1962 May;45:837–859. doi: 10.1085/jgp.45.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy B. A., Williams M. K. Studies on the metabolism of adenosine and adenine in stored and fresh human erythrocytes. Blood. 1966 May;27(5):623–628. [PubMed] [Google Scholar]
- Martin K. Concentrative accumulation of choline by human erythrocytes. J Gen Physiol. 1968 Apr;51(4):497–516. doi: 10.1085/jgp.51.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIELSEN S. O., LEHNINGER A. L. Phosphorylation coupled to the oxidation of ferrocytochrome c. J Biol Chem. 1955 Aug;215(2):555–570. [PubMed] [Google Scholar]
- OVERGAARD-HANSEN K. Rejuvenation of adenosine triphosphate in human erythrocytes by purine nucleosides. Acta Pharmacol Toxicol (Copenh) 1957;14(1):67–76. doi: 10.1111/j.1600-0773.1957.tb01143.x. [DOI] [PubMed] [Google Scholar]
- PRENTICE T. C., BISHOP C. SEPARATION OF RABBIT RED CELLS BY DENSITY METHODS AND CHARACTERISTICS OF SEPARATED LAYERS. J Cell Physiol. 1965 Feb;65:113–125. doi: 10.1002/jcp.1030650114. [DOI] [PubMed] [Google Scholar]
- Piomelli S., Lurinsky G., Wasserman L. R. The mechanism of red cell aging. I. Relationship between cell age and specific gravity evaluated by ultracentrifugation in a discontinuous density gradient. J Lab Clin Med. 1967 Apr;69(4):659–674. [PubMed] [Google Scholar]
- RONQUIST G., AGREN G. 32P-LABELLING OF NUCLEOTIDES FROM A SOLUBLE ERYTHROCYTE-MEMBRANE FRACTION DEVOID OF HAEMOGLOBIN. Nature. 1965 Mar 6;205:1021–1022. doi: 10.1038/2051021a0. [DOI] [PubMed] [Google Scholar]
- SCHRIER S. L. Studies of the metabolism of human erythrocyte membranes. J Clin Invest. 1963 Jun;42:756–766. doi: 10.1172/JCI104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- SNOKE J. E., BLOCH K. Studies on the mechanism of action of glutathione synthetase. J Biol Chem. 1955 Apr;213(2):825–835. [PubMed] [Google Scholar]
- Schrier S. L. ATP synthesis in human erythrocyte membranes. Biochim Biophys Acta. 1967 Sep 9;135(4):591–598. doi: 10.1016/0005-2736(67)90091-0. [DOI] [PubMed] [Google Scholar]
- Schrier S. L. Organization of enzymes in human erythrocyte membranes. Am J Physiol. 1966 Jan;210(1):139–145. doi: 10.1152/ajplegacy.1966.210.1.139. [DOI] [PubMed] [Google Scholar]
- WEBSTER G. C., VARNER J. E. Peptidebond synthesis in higher plants. II. Studies on the mechanism of synthesis of gamma-glutamylcysteine. Arch Biochem Biophys. 1954 Sep;52(1):22–32. doi: 10.1016/0003-9861(54)90085-5. [DOI] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. The catalytic effect of molybdate on the hydrolysis of organic phosphate bonds. Biochem J. 1951 Aug;49(3):286–292. [PMC free article] [PubMed] [Google Scholar]
- WHITTAM R. The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J. 1962 Jul;84:110–118. doi: 10.1042/bj0840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. Vectorial aspects of adenosine-triphosphatase activity in erythrocyte membranes. Biochem J. 1964 Nov;93(2):337–348. doi: 10.1042/bj0930337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R. Control of membrane permeability to potassium in red blood cells. Nature. 1968 Aug 10;219(5154):610–610. doi: 10.1038/219610a0. [DOI] [PubMed] [Google Scholar]
- Whittam R., Wiley J. S. Some aspects of adenosine triphosphate synthesis from adenine and adenosine in human red blood cells. J Physiol. 1968 Dec;199(2):485–494. doi: 10.1113/jphysiol.1968.sp008664. [DOI] [PMC free article] [PubMed] [Google Scholar]


