Abstract
Human herpesvirus 6 (HHV-6) has a tropism for T lymphocytes and monocytes/macrophages, suggesting that HHV-6 infection affects the immunosurveillance system. In the present study, we investigated the HHV-6-induced phenotypic and functional alterations of dendritic cells (DCs), which are professional antigen-presenting cells. HHV-6 infection of monocyte-derived immature DCs appeared to induce the up-regulation of CD80, CD83, CD86, and HLA class I and class II molecules, suggesting that HHV-6 infection induces the maturation of DCs. In addition, the antigen capture capacity of DCs was found to decrease following infection with HHV-6. In contrast to up-regulation of mature-DC-associated surface molecules on HHV-6-infected DCs, their capacity for presentation of alloantigens and exogenous virus antigens to T lymphocytes decreased significantly from that of uninfected DCs. In contrast, there appeared to be no reduction in the capacity for presentation of an HLA class II-binding peptide to the peptide-specific CD4+ T lymphocytes. These data indicate that HHV-6 infection induces phenotypic alterations and impairs the antigen presentation capacity of DCs. The present data also suggest that the dysfunction of HHV-6-infected DCs is attributable mainly to impairment of the antigen capture and intracellular antigen-processing pathways.
Human herpesvirus 6 (HHV-6) was first isolated in 1986 from patients with lymphoproliferative disorders and AIDS (34). Subsequent studies have revealed that HHV-6 is a causative agent of exanthem subitum in infants at primary infection (45). Reactivation of HHV-6 occurs frequently in patients who are immune deficient, such as organ transplant recipients and those with AIDS (24), and causes various disorders, including lymphadenitis, pneumonitis, hepatitis, meningoencephalitis, retinitis, infectious mononucleosis-like disease, hemophagocytic syndrome, and hypersensitivity syndrome (2, 6, 41, 42, 44). HHV-6 isolates are divided into two subgroups, HHV-6A and HHV-6B, on the basis of their tropism for certain cell lines, their reactivities with monoclonal antibodies (MAbs) and HHV-6-specific T-lymphocyte clones, and their restriction enzyme cleavage patterns (11, 38, 50).
HHV-6 was initially termed human B-lymphotropic virus because of its in vitro tropism for B lymphocytes (34). However, it is now well known that HHV-6 exhibits tropism mainly for T lymphocytes and monocytes/macrophages and that various kinds of cells, including myeloid precursor cells, megakaryocytes, natural killer cells, fibroblasts, astrocytes, and hepatoma cells, are also susceptible to HHV-6 infection (1, 15, 18, 19, 22). Various immunobiological alterations of T lymphocytes have been observed following infection with HHV-6. HHV-6A infection induces down-regulation of CD3, resulting in impairment of T-lymphocyte activation via CD3/T-cell-receptor complexes (10, 26). Up-regulation of CD4, resulting in susceptibility to human immunodeficiency virus type 1 (HIV-1) infection, has been reported to occur in HHV-6A-infected CD4− T lymphocytes and natural killer cells (23, 25, 27). HHV-6 infection of T lymphocytes reduces both interleukin-2 (IL-2) synthesis and the proliferative response to anti-CD3 MAbs and phytohemagglutinin (17). In addition, it has recently been reported that transcriptional down-regulation of CXCR4 is induced by HHV-6A and HHV-6B infections (14, 46). Although these data suggest that HHV-6 infection causes immunodeficiency due to dysfunction of T lymphocytes, the immunobiological effect of HHV-6 infection on other immunocompetent cells has not been precisely examined.
Dendritic cells (DCs) are considered to be the professional antigen-presenting cells (APCs) on the basis of the finding that they elicit strong proliferative responses of T lymphocytes to alloantigens and to recall antigens. Most importantly, DCs have the ability to activate the immune response by capturing antigens in peripheral tissues and migrating to secondary lymphoid organs, where they sensitize naive T lymphocytes to the antigens. This migration of DCs is concomitant with maturation, during which DCs lose their ability to capture and process the exogenous antigens. Mature DCs express a high level of major histocompatibility complex (MHC) class II and costimulatory molecules on their surfaces, thereby acquiring the ability to prime naive CD4+ T lymphocytes. Several molecules, including CD40, tumor necrosis factor (TNF) receptor, and IL-1 receptor, have been shown to activate DCs and to trigger their transition from immature antigen-capturing cells to mature antigen-presenting DCs.
Numerous other factors have been shown to induce DC maturation, including lipopolysaccharide (LPS), bacterial DNA, double-stranded RNA, and various types of cytokines (5). It has been reported recently that infection with some types of virus affects the maturation of DCs. For example, vaccinia virus inhibits DC maturation, resulting in a reduction in the capacity of DCs to stimulate T lymphocytes (7). A similar phenomenon was also demonstrated for herpes simplex virus (HSV)-infected DCs (35). Although some viruses impair the maturation process of DCs, other viruses have been shown to drive DC maturation. Measles virus infection of immature DCs induces DC maturation and interferes with their capacity to stimulate T lymphocytes through as yet unknown mechanisms (9, 12, 39). It has also been reported that dengue virus infection of immature DCs leads to their maturation (16). These previous data indicate that studies of the interactions between viruses and DCs are essential if we are to clarify the pathogenesis of viral infections and the mechanisms underlying virus-induced immunodeficiency. On the basis of this concept, we investigated the immunobiological effects of HHV-6 on DCs, since HHV-6 exhibits tropism for DCs (4), which play a crucial role in the immune response. The data obtained from the present series of experiments show that HHV-6 infection induces phenotypic alterations of immature DCs and impairs the capacity of HHV-6-infected DCs to present alloantigens and exogenous virus antigens. The mechanisms of functional alteration of HHV-6-infected DCs and its significance in the pathogenesis of HHV-6 infection have been addressed.
MATERIALS AND METHODS
Generation of DCs and HHV-6 infection.
The U1102 strain of HHV-6A and the Z29 strain of HHV-6B were grown in cord blood mononuclear cells that had been stimulated with phytohemagglutinin. HHV-6B was mainly used in the present study, since most of the biological characteristics of HHV-6A and HHV-6B are similar and HHV-6B is more prevalent in the general population than is HHV-6A. Immature DCs were generated from peripheral blood monocytes, as described previously (33). Briefly, monocytes were isolated from peripheral blood mononuclear cells (PBMCs) of healthy individuals by a plastic adherence technique. The plastic-adherent cells were cultured further in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 500 U of recombinant human IL-4 (Genzyme, Boston, Mass.)/ml, and 800 U of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; Kirin Brewery, Tokyo, Japan)/ml. On day 3 or 4 of incubation, half of the medium was replaced by fresh culture medium supplemented with IL-4 and GM-CSF, and culture was continued. On day 7, the cells were harvested and used as monocyte-derived immature DCs. The purity of immature DCs, determined by morphology and flow cytometric analysis using DC-associated MAbs, was more than 90%, and the remaining cells were resting lymphocytes. Immature DCs were washed with RPMI 1640 medium and inoculated with HHV-6 at a multiplicity of infection of approximately 1 50% tissue culture infective dose. HHV-6-inoculated and mock-infected DCs were further cultured in RPMI 1640 medium supplemented with 10% FCS for various periods in a 5% CO2 incubator at 37°C.
HHV-6 replication in DCs.
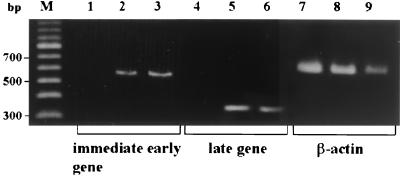
Replication of HHV-6 in DCs was examined by immunofluorescence and detection of mRNA for the HHV-6 immediate-early and late (U83) genes by reverse transcriptase (RT) PCR, as described previously (49, 52). Briefly, total RNAs were extracted from HHV-6-inoculated or mock-infected cells, and cDNA was synthesized by reverse transcription with Moloney murine leukemia virus RT. The cDNAs were amplified by PCR using the following primers: for the immediate-early gene, 5′-TTCTCCAGATGTGCCAGGGAAATCC-3′ and 5′-CATCATTGTTATCGCTTTCACTCTC-3′; for the late gene, 5′-GTCGACCATGTTCATTTGGCTTTTTATTGTT-3′ and 5′-ATGAATTCTCATGATTCTTTGTCTAATTTC-3′. The expected lengths of the amplified cDNA sequences for the HHV-6B immediate-early gene and late gene were 553 and 345 bp, respectively. cDNA for the β-actin gene was also amplified, with primers 5′-TCCTGTGGCATCCACGAAACT-3′ and 5′-GAAGCATTTGCGGTGGACGAT-3′, as a control for RT-PCR.
Flow cytometric analysis of cell surface molecule expression.
Expression of cell surface molecules on DCs was examined by flow cytometric analysis using the following MAbs: anti-CD40 (PharMingen, San Diego, Calif.), anti-CD44 (PharMingen), anti-CD80 (Immunotech, Marseilles, France), anti-CD83 (Immunotech), anti-CD86 (Immunotech), anti-HLA class I (PharMingen), and anti-HLA-DR (PharMingen). To block nonspecific binding of MAbs, DCs were preincubated with human immunoglobulin (Bayer AG, Leverkusen, Germany) at a concentration of 1 mg/ml for 30 min before addition of the MAbs. After a wash, cells were analyzed with a FACScalibur system equipped with CellQuest software (both from Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
Flow cytometric analysis of HHV-6 antigen expression in DCs.
Simultaneous detection of surface CD80 and intracellular HHV-6 antigen expression was performed as follows. HHV-6-infected and mock-infected immature DCs were incubated with a fluorescein isothiocyanate (FITC)-conjugated anti-CD80 MAb (Immunotech). To further detect intracellular HHV-6 antigen expression, cells were then fixed with 3.0% formaldehyde, permeabilized with 0.05% saponin, and incubated with a MAb against the HHV-6 101-kDa virion protein (Chemicon International Inc., Temecula, Calif.), followed by staining with phycoerythrin-conjugated goat anti-mouse immunoglobulin G (Organon Teknika Corp., West Chester, Pa.). After being washed, the cells were analyzed with a FACSCalibur (Becton Dickinson) as described above.
Flow cytometric analysis of endocytosis.
The capacity of the DCs for antigen capture was examined quantitatively by flow cytometric analysis of endocytosis, as described previously (36). Briefly, HHV-6-infected and mock-infected DCs were suspended in RPMI 1640 medium supplemented with 10% FCS at 37°C or 4°C. Lucifer yellow carbohydrazide (CH) (Molecular Probes, Eugene, Oreg.) or lysine-fixable FITC-dextran (Mr, 40,000) (Sigma, St. Louis, Mo.) was added at a final concentration of 1 mg/ml. Cells were incubated for 30 min, washed four times with cold phosphate-buffered saline containing 1% FCS and 0.01% NaN3, and analyzed by using a FACSCalibur. Staining of cells that had been incubated at 4°C was considered to be the control.
Presentation of alloantigens by DCs to T lymphocytes.
The allostimulatory capacities of HHV-6-infected and mock-infected DCs were examined as follows. PBMCs were isolated from donors whose HLA types were not identical to those of the DC donors. PBMCs (105) and various numbers of DCs that had been treated with mitomycin C (MMC; Kyowa Hakko, Tokyo, Japan) were cocultured in flat-bottom microtiter wells containing 0.2 ml of RPMI 1640 medium supplemented with 10% human AB type serum. Cells were then incubated for 6 days. For the final 16 h of incubation, 1 μCi of [3H]TdR (New England Nuclear, Boston, Mass.) was added to each well, and incorporation of [3H]TdR was determined by liquid scintillation counting.
Presentation of exogenous virus antigens by DCs to T lymphocytes.
The exogenous antigen presentation capacity of DCs was examined by using a modification of a previously reported method (47). Briefly, HSV type 1 and human cytomegalovirus (HCMV) antigens were prepared by UV light irradiation of the viruses. PBMCs were isolated from donors who were seropositive for HSV and HCMV. T lymphocytes were purified from PBMCs by passage through nylon-wool columns, and DCs were generated from monocytes of the donors of the T lymphocytes, as described above. T lymphocytes (105) and various numbers of MMC-treated HHV-6-infected or mock-infected DCs in 0.2 ml of RPMI 1640 medium supplemented with 10% human AB type serum were seeded in flat-bottom microtiter wells, to which 0.02 ml of virus antigen or control antigen was added at the optimal dilution. Cells were cultured for 6 days, and incorporation of [3H]TdR was determined as described above.
Presentation of peptides by DCs to peptide-specific T lymphocytes.
The CD4+ T-lymphocyte clones MY-1, specific for the chronic myelogenous leukemia-associated fusion protein BCR-ABL, and HO-1, specific for the acute myelogenous leukemia-associated fusion protein DEK-CAN, were generated as described previously (28, 48). MY-1 induces a BCR-ABL fusion peptide (ATGFKQSSKALQRPVAS)-specific and HLA-DRB1*0901-restricted proliferative response. HO-1 induces a DEK-CAN fusion peptide (TMKQICKKEIRRLHQY)-specific and HLA-DRB4*0103-restricted proliferative response. Proliferative response assays of MY-1 and HO-1 were performed by using a modification of a previously described method (28, 48). Briefly, monocyte-derived DCs were generated from the donors of MY-1 and HO-1. CD4+ T-lymphocyte clone cells (2 × 104) and MMC-treated HHV-6-infected or mock-infected DCs (2 × 104) in 0.2 ml of RPMI 1640 medium supplemented with 10% human AB type serum were seeded into flat-bottom microtiter wells, to which either BCR-ABL or DEK-CAN peptide was added at various concentrations. Cells were cultured for 3 days, and incorporation of [3H]TdR was determined as described above.
RESULTS
Replication of HHV-6 in immature DCs.
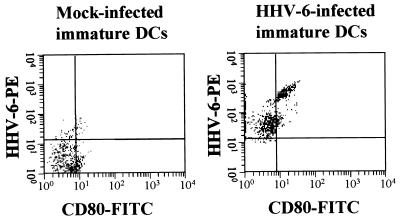
We first addressed the question of whether HHV-6 can replicate in DCs. HHV-6-inoculated DCs did not exhibit any apparent cytopathic effects within 6 days. However, some HHV-6-infected DCs tended to die after 8 days of culture. As shown in Fig. 1, RT-PCR analysis clearly showed the presence of mRNAs for the HHV-6 immediate-early and late genes in immature DCs that had been inoculated with HHV-6B and cultured for 4 days. Similarly, HHV-6 mRNAs were detected in HHV-6A-inoculated immature DCs (data not shown). In addition, as shown in Fig. 2, the HHV-6 antigen was detected in most of the DCs that had been inoculated with HHV-6 and cultured for 4 days by two-color flow cytometry using anti-CD80 and anti-HHV-6 MAbs. Moreover, infectious HHV-6 was recovered from the culture supernatant of HHV-6-inoculated DCs (data not shown). These data confirm the previous report that both HHV-6A and HHV-6B can infect and replicate in immature DCs (4). Since most of the HHV-6-infected DCs were alive and HHV-6 antigen expression was maximal after 6 days of inoculation, DCs were harvested after 6 days of HHV-6 inoculation and used for the experiments described below.
FIG. 1.
Expression of HHV-6 mRNA in DCs. cDNAs synthesized from mock-infected immature DCs (lanes 1, 4, and 7), HHV-6B-infected immature DCs (lanes 2, 5, and 8), and HHV-6B-infected cord blood mononuclear cells (lanes 3, 6, and 9) were amplified by using primers corresponding to the HHV-6 immediate-early and late genes and primers corresponding to the β-actin gene. Lane M, marker DNAs.
FIG. 2.
Flow cytometric analysis of HHV-6 antigen expression in DCs. Mock-infected and HHV-6B-inoculated immature DCs were cultured for 4 days. Expression of cell surface CD80 and intracellular HHV-6 antigen in mock-infected and HHV-6B-infected immature DCs was then examined by two-color flow cytometry. Cell surface CD80 expression, determined by measuring FITC fluorescence intensity, and intracellular HHV-6 antigen expression, determined by measuring phycoerythrin (PE) fluorescence intensity, were examined simultaneously as described in the text.
Flow cytometric analysis of surface molecule expression on mock-infected, HHV-6-infected, and TNF-α-treated immature DCs.
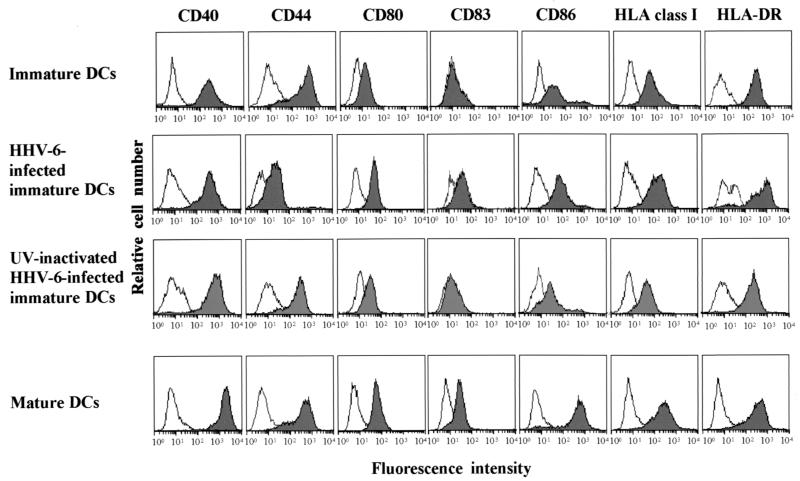
We next examined alterations of surface molecule expression on immature DCs following infection with HHV-6. As shown in Fig. 3, up-regulation of CD80, CD83, CD86, HLA class I, and HLA class II molecules was detected in HHV-6B-infected immature DCs. Inoculation of immature DCs with UV-inactivated HHV-6 did not affect surface molecule expression, suggesting that replication of HHV-6 is necessary to induce up-regulation of these molecules. As described previously, up-regulation of these molecules was also detected in mature DCs that had been generated from immature DCs by treatment with TNF-α (37, 51). A slight up-regulation of CD40 and a marked down-regulation of CD44 were also detected in HHV-6B-infected DCs. Similar alterations of surface molecule expression were also detected in HHV-6A-infected immature DCs (data not shown).
FIG. 3.
Flow cytometric analysis of surface molecule expression. Cell surface expression levels of CD40, CD44, CD80, CD83, CD86, HLA class I, and HLA-DR molecules on immature DCs, mature DCs, HHV-6-infected immature DCs, and immature DCs infected with UV-inactivated HHV-6 were measured by flow cytometry. The stainings with the control antibody, FITC-conjugated mouse IgG, are shown as open histograms.
Flow cytometric analysis of endocytosis by HHV-6-infected and mock-infected DCs.
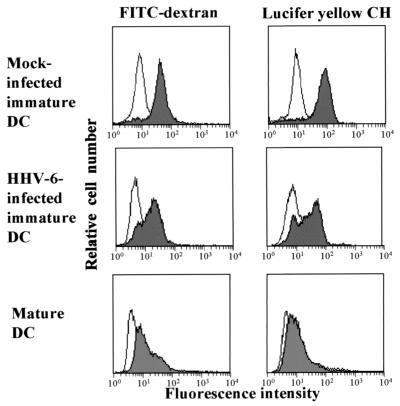
The ability of DCs to capture antigens is an important characteristic of immature DCs and is known to decline during DC maturation. Therefore, we used flow cytometry to address whether HHV-6 infection affects the antigen capture capacity of DCs. Figure 4 shows the endocytosis profiles of mature DCs and HHV-6-infected and mock-infected immature DCs. As described previously, immature DCs showed a strong capacity to induce endocytosis in comparison with mature DCs. However, the level of endocytosis mediated by HHV-6-infected immature DCs was lower than that mediated by mock-infected immature DCs. These data are compatible with those of the flow cytometric analysis of cell surface molecules, suggesting that HHV-6 infection induces DC maturation.
FIG. 4.
Flow cytometric analysis of endocytosis. Mock-infected immature DCs, mature DCs, and HHV-6-infected immature DCs were incubated in a medium containing FITC-dextran or Lucifer yellow CH at 37°C for 30 min (shaded histograms). Controls were measured by incubating cells in a medium containing FITC-dextran or Lucifer yellow CH at 4°C (open histograms).
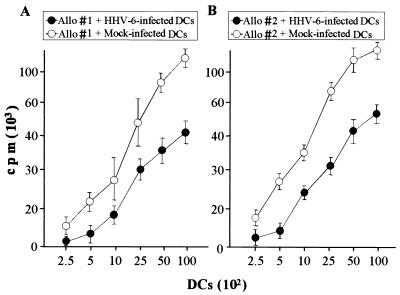
Reduced allostimulatory capacity of HHV-6-infected DCs.
The antigen presentation capacities of HHV-6-infected and mock-infected DCs were first examined by determining the stimulatory capacity of alloantigens. Allogeneic T lymphocytes, which were isolated from donors whose HLA class I and class II types were not identical with those of the DC donors, were cocultured with various numbers of DCs. As shown in Fig. 5, the allostimulatory capacity of HHV-6-infected DCs was significantly decreased from that of mock-infected DCs. The same experiments were performed three times, and similar data were obtained (data not shown). These data indicate that the antigen presentation capacity of DCs, which is their most important function, is impaired by HHV-6 infection.
FIG. 5.
Presentation of alloantigens by mock-infected and HHV-6-infected DCs to T lymphocytes. Incorporation of [3H]TdR into allogeneic T lymphocytes was determined in the presence of mock-infected DCs (open circles) and HHV-6-infected DCs (solid circles). (A) HLA types of donors are as follows. Stimulator: A2/11.1, B51/61, Cw10/w-, and DRB1*08032/*0901. Responder: A26/31, B1/62, Cw3/w-, and DRB1*0901/*1406. (B) HLA types of donors are as follows. Stimulator: A24/-, B51/52, Cw-/w-, and DRB1*1101/*1502. Responder: A26/31, B1/62, Cw3/w-, and DRB1*0901/*1406. Data are means ± standard deviations for quadruplicate wells.
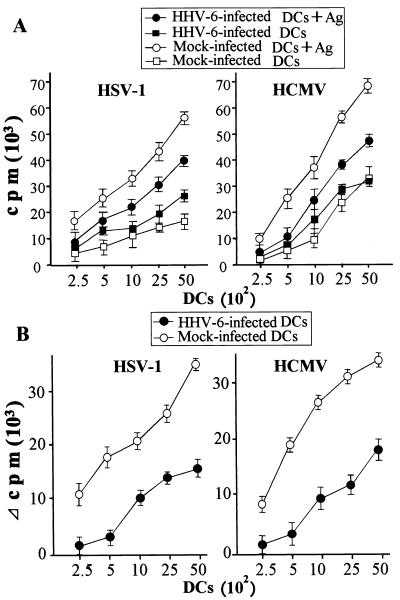
Reduced virus antigen-stimulatory capacity of HHV-6-infected DCs.
We next examined the capacities of HHV-6-infected and mock-infected DCs for presentation of exogenous virus antigens. Exogenous virus antigens were at first captured, processed, and then presented in the context of MHC class II molecules to mainly antigen-specific CD4+ T lymphocytes by APCs. Figure 6 shows the proliferative responses of autologous T lymphocytes to HSV type 1 and HCMV antigens in the presence of HHV-6-infected or mock-infected DCs. Because HHV-6-specific memory T lymphocytes are present in the peripheral blood of most healthy individuals (43), peripheral blood lymphocytes responded to HHV-6-infected DCs even in the absence of exogenous virus antigens (Fig. 6A). In the same manner as the response to alloantigens, the proliferative response of T lymphocytes to exogenous virus antigens was significantly lower when HHV-6-infected DCs were used as APCs than when T lymphocytes were stimulated with antigens in the presence of mock-infected DCs (Fig. 6B). The same experiments were performed three times, and similar data were obtained (data not shown). These data indicate that the presentation pathway of exogenous antigens is also impaired by HHV-6 infection. These data also suggest that the reduced capacity for presenting exogenous antigens is a result of the impairment of antigen capture and/or processing in HHV-6-infected DCs.
FIG. 6.
Presentation of exogenous virus antigens by mock-infected and HHV-6-infected DCs to T lymphocytes. (A) Incorporation of [3H]TdR into autologous T lymphocytes was determined in the presence of mock-infected DCs with (open circles) or without (open squares) the virus antigen and of HHV-6-infected DCs with (solid circles) or without (solid squares) the virus antigen. (B) The antigen-specific proliferative response of T lymphocytes in the presence of mock-infected DCs (open circles) or HHV-6-infected DCs (solid circles) was measured by subtracting the count obtained from preparations with no antigen stimulation from that obtained with virus antigen stimulation. Data are means ± standard deviations for quadruplicate wells.
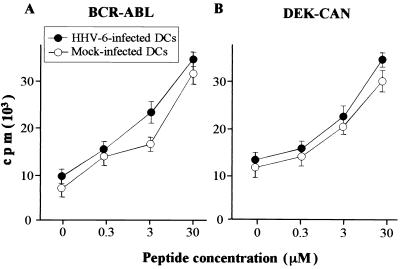
Stimulation of peptide-specific CD4+ T-lymphocyte clones by mock-infected and HHV-6-infected DCs.
In order to examine whether the antigen-processing pathway in DCs is impaired by HHV-6 infection, we examined further the presentation capacity of a 17-mer and a 16-mer peptide, which can be presented to the peptide-specific CD4+ T lymphocytes unnecessarily by being captured and processed by DCs. As reported previously, the BCR-ABL fusion peptide-specific CD4+ T-lymphocyte clone MY-1 and the DEK-CAN fusion peptide-specific CD4+ T-lymphocyte clone HO-1 proliferate in response to stimulation with a BCR-ABL peptide in the presence of HLA-DRB1*0901-positive APCs and with a DEK-CAN peptide in the presence of HLA-DRB4*0103-positive APCs, respectively (28, 48). The proliferative responses of MY-1 and HO-1 to various concentrations of the synthetic peptide in the presence of mock-infected or HHV-6-infected autologous DCs are shown in Fig. 7. In contrast to the proliferative responses of T lymphocytes to alloantigens and exogenous virus antigens, the degrees of proliferative response mediated by MY-1 and HO-1 to the synthetic peptide were almost the same or somewhat higher when HHV-6-infected DCs were used as APCs than when mock-infected DCs were used. Taken together, the data presented in Fig. 3, 4, 5, 6, and 7 suggest strongly that functional alterations of DCs mediated by HHV-6 infection occur at the antigen capture and antigen-processing phases but not at the phase of peptide presentation in the context of HLA molecules.
FIG. 7.
Presentation of peptides by mock-infected and HHV-6-infected DCs to the peptide-specific T-lymphocyte clones. Incorporation of [3H]TdR into BCR-ABL peptide-specific (A) and DEK-CAN peptide-specific (B) autologous T-lymphocyte clones was determined in the presence of mock-infected DCs (open circles) and HHV-6-infected DCs (solid circles) with or without the peptide at various concentrations. Data are means ± standard deviations for quadruplicate wells.
DISCUSSION
Recently, the interaction between viruses and DCs has been of considerable interest in the context of clarifying the pathogenesis of virus infections. DCs are affected by virus infection in several ways. That is, persistent viruses may be sequestered within DCs and may impair their function, as has been shown to occur in the case of HCMV and murine CMV (3, 13, 40). DCs may be susceptible to the cytopathic effects of viruses, as shown with measles virus and HIV (9, 12). Viruses may induce or interfere with DC maturation (7, 8, 16, 35, 39). Moreover, it has recently been reported that the apoptosis-inducing ligand CD95L (FasL) and TNF-related apoptosis-inducing ligand (TRAIL) are up-regulated in HCMV-infected DCs, thereby enabling HCMV-infected DCs to delete activated T lymphocytes (29). In the present study, we investigated the immunobiological effects of HHV-6 infection on DCs, focusing on their surface molecule expression and antigen-presenting function. The findings obtained from the present series of experiments are as follows. First, HHV-6 had a tropism for and could replicate in DCs. Second, HHV-6 infection induced up-regulation of CD80, CD83, CD86, and HLA class I and class II molecules in the same manner as the maturation of DCs. Third, the capacity of DCs for presenting alloantigens and exogenous virus antigens declined following infection with HHV-6. In contrast, the capacity of presenting peptides that can bind to HLA class II molecules unnecessarily captured and processed by DCs to CD4+ T lymphocytes was not affected by HHV-6 infection. These findings indicate that HHV-6 induces up-regulation of mature-DC-associated surface molecules, but that their antigen presentation capacity is impaired.
The maturation of DCs is characterized phenotypically by acquisition of CD83 expression and up-regulation of CD40, CD80, and CD86, which are important costimulatory molecules. Expression levels of HLA class I and class II molecules are also known to be elevated during DC maturation. In the present study, we found that up-regulation of all of the molecules listed above was induced in immature DCs following infection with HHV-6, suggesting that HHV-6 infection of DCs results in their maturation. It has been reported that the maturation of DCs is induced by various stimulatory molecules, including LPS, TNF-α, IL-1, IL-6, IL-10, transforming growth factor-β, and prostaglandins (5). It is also well known that DC maturation is induced by various kinds of microorganisms. Both LPS-positive, gram-negative bacteria and LPS-negative, gram-positive bacteria, as well as some protozoa, are potent inducers of DC maturation (31). Some of the stimulatory factors of these organisms may involve the DNA itself. Invertebrate DNA is not methylated, giving rise to motifs containing CpG dinucleotides that are not present in vertebrates. It has recently been reported that injection of CpG-containing oligonucleotides into mouse skin leads to the maturation of Langerhans cells (20). In addition to bacteria and protozoa, virus infection has also been reported to induce DC maturation. Influenza virus can activate DCs and substitute for CD40 signaling, thus converting DCs into APCs capable of activating CD8+ T lymphocytes directly (32). Measles virus and dengue virus are also known to be potent inducers of DC maturation (16, 39). Although the precise mechanisms of DC maturation promoted by virus infection have not been clarified, the production of cytokines affecting DC maturation, such as gamma interferon, may be involved in the mechanisms of virus-induced DC maturation. We have investigated the possibility that the phenotypic maturation of DCs may be promoted by the soluble factor(s) produced by HHV-6-infected immature DCs; that is, the culture supernatant of HHV-6-infected DCs, which was irradiated with UV light to inactivate any HHV-6 produced in the culture medium, was added repeatedly to immature DCs. Expression levels of surface molecules did not appear to be significantly changed as a consequence, suggesting that the soluble factor(s) produced by HHV-6-infected immature DCs is not the primary inducer of DC maturation-associated molecules. The difference in expression levels of some surface molecules, such as CD40 and CD44, between HHV-6-infected DCs and TNF-α-induced mature DCs suggests that the underlying mechanisms responsible for phenotypic alterations may differ between HHV-6-infected and cytokine-treated DCs. This, taken together with the finding that inactivated HHV-6 exerted no effect on the DC phenotype, indicates that the replication of HHV-6 must be essential for phenotypic alterations of DCs, and an HHV-6 gene product(s) may act as a signal for switching of the DC phenotype from an immature to a mature form.
Another noteworthy finding of the present study was that despite the up-regulation of the maturation-associated phenotype on HHV-6-infected DCs, their antigen presentation capacity was decreased following infection with HHV-6. It has been reported that the phenotypic maturation and decreased allostimulatory capacity of immature DCs are also induced by measles virus infection via as yet undetermined mechanisms (39). Processing of endogenous antigens occurs first in the cytosol through an ATP-dependent proteolytic system, which starts with ubiquitin conjugation. The ubiquitinylated proteins are directed to the proteasome, which cleaves the proteins into peptides. The cleaved peptides are then translocated into the endoplasmic reticulum via transporters associated with antigen processing and are accommodated within the MHC class I-binding groove. On the other hand, exogenous antigens are captured by APCs via receptor-mediated endocytosis, phagocytosis, and macropinocytosis, and are processed and meet with MHC class II molecules in endosomes. Endosomal proteases cleave the invariant chain, and foreign peptide loading takes place. The MHC-class II-peptide complexes are then carried to the cell surface. Although the precise mechanisms of alloantigen presentation by APCs and allorecognition by T lymphocytes remain obscure, a recent study of the crystal structure of a T-cell-receptor molecule, which binds to an allogeneic MHC molecule, has revealed the similarity between alloantigen recognition and the recognition of foreign peptides bound to self-MHC molecules (30).
In the present study, it was found that both the alloantigen and exogenous virus antigen presentation capacities of DCs decreased following infection with HHV-6. In contrast, peptides that can bind to HLA class II molecules by being unnecessarily processed were presented to the peptide-specific CD4+ T lymphocytes as effectively or more effectively by HHV-6-infected DCs than by mock-infected DCs. These findings indicate that the processing pathways of both endogenous and exogenous antigens mentioned above are impaired in HHV-6-infected DCs and that the capacity of HHV-6-infected DCs to present the processed peptide in the context of HLA molecules is intact. The latter conclusion is supported by the evidence that HLA as well as costimulatory molecules remained expressed at high levels in HHV-6-infected DCs. The antigen capture capacity of DCs appeared to decrease following infection with HHV-6. This may be one of the causes of the HHV-6-infection-induced reduction in the capacity of DCs to present exogenous antigens.
In conclusion, we have demonstrated the phenotypic and functional alterations of monocyte-derived DCs induced by HHV-6 infection. Our present findings may shed new light on the pathogenesis of virus-induced immunodeficiency. Although the status of HHV-6 infection in DCs in vivo is unknown, HHV-6 is known to infect monocytes/macrophages latently (21), suggesting that reactivation of HHV-6 may induce functional alteration of monocyte-derived DCs in vivo. Reactivation of HHV-6 occurs frequently in immunodeficient patients, such as recipients of bone marrow and organ transplants and those with AIDS. Functional alterations of DCs, which play crucial roles in resistance to virus infection, may exacerbate the immunodeficient status of immunocompromised hosts. Further studies focusing on the interaction between DCs and various viruses are essential to clarify the pathogenesis of virus-induced immunodeficiency and to develop novel ways of improving the immunodeficient status of patients with virus infections.
Acknowledgments
We thank Mikiko Tohyama, Kirin Brewery Co. Ltd., Dainippon Pharmaceutical Co. Ltd., and Kyowa Hakko Co. Ltd. for providing the anti-HHV-6 MAb, GM-CSF, TNF-α, and MMC, respectively.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Sagawa Cancer Research Foundation, the Cancer Research Foundation, the Yamanouchi Foundation for Research on Metabolic Disorders, and the Uehara Memorial Foundation.
REFERENCES
- 1.Ablashi, D. V., P. Lusso, C.-L. Hung, S. Z. Salahuddin, S. F. Josephs, T. Llana, B. Kramarsky, P. Biberfeld, P. D. Markham, and R. C. Gallo. 1988. Utilization of human hematopoietic cell lines for the propagation and characterization of HBLV (human herpesvirus 6). Int. J. Cancer 42:787-791. [DOI] [PubMed] [Google Scholar]
- 2.Akashi, K., Y. Eizuru, Y. Sumiyoshi, T. Minematsu, S. Hara, M. Harada, M. Kikuchi, Y. Niho, and Y. Minamishima. 1993. Severe infectious mononucleosis-like syndrome and primary human herpesvirus 6 infection in an adult. N. Engl. J. Med. 329:168-171. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. [DOI] [PubMed] [Google Scholar]
- 4.Asada, H., V. Klaus-Kovtun, H. Golding, S. I. Katz, and A. Blauvelt. 1999. Human herpesvirus 6 infects dendritic cells and suppresses human immunodeficiency virus type 1 replication in coinfected cultures. J. Virol. 73:4019-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.-J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 6.Braun, D. K., G. Dominguez, and P. E. Pellett. 1997. Human herpesvirus 6. Clin. Microbiol. Rev. 10:521-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 8.Frankel, S. S., B. M. Wenig, A. P. Burke, P. Mannan, L. D. Thompson, S. L. Abbondanzo, A. M. Nelson, M. Pope, and R. M. Steinman. 1996. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science 272:115-117. [DOI] [PubMed] [Google Scholar]
- 9.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M.-C. Rissoan, Y.-J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa, M., M. Yasukawa, Y. Yakushijin, and S. Fujita. 1994. Distinct effects of human herpesvirus 6 and human herpesvirus 7 on surface molecule expression and function of CD4+ T cells. J. Immunol. 152:5768-5775. [PubMed] [Google Scholar]
- 11.Gompels, U. A., J. Nicholas, G. Lawrence, M. Jones, B. J. Thomson, M. E. Martin, S. Efstathiou, M. Craxton, and H. A. Macaulay. 1995. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29-51. [DOI] [PubMed] [Google Scholar]
- 12.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn, G., R. Jores, and E. S. Mocarski. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 95:3937-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasegawa, A., M. Yasukawa, I. Sakai, and S. Fujita. 2001. Transcriptional down-regulation of CXCR4 induced by impaired association of transcription regulator YY1 with c-Myc in human herpesvirus 6-infected cells. J. Immunol. 166:1125-1131. [DOI] [PubMed] [Google Scholar]
- 15.He, J., M. McCarthy, Y. Zhou, B. Chandran, and C. Wood. 1996. Infection of primary human astrocytes by human herpesvirus 6. J. Virol. 70:1296-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho, L.-J., J.-J. Wang, M.-F. Shaio, C.-L. Kao, D.-M. Chang, S.-W. Han, and J.-H. Lai. 2001. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 166:1499-1506. [DOI] [PubMed] [Google Scholar]
- 17.Horvat, R. T., M. J. Parmely, and B. Chandran. 1993. Human herpesvirus 6 inhibits the proliferative responses of human peripheral blood mononuclear cells. J. Infect. Dis. 167:1274-1280. [DOI] [PubMed] [Google Scholar]
- 18.Inagi, R., R. Guntapong, M. Nakao, Y. Ishino, K. Kawanishi, Y. Isegawa, and K. Yamanishi. 1996. Human herpesvirus 6 induces IL-8 gene expression in human hepatoma cell line, Hep G2. J. Med. Virol. 49:34-40. [DOI] [PubMed] [Google Scholar]
- 19.Isomura, H., M. Yamada, M. Yoshida, H. Tanaka, T. Kitamura, M. Oda, S. Nii, and Y. Seino. 1997. Suppressive effects of human herpesvirus 6 on in vitro colony formation of hematopoietic progenitor cells. J. Med. Virol. 52:406-412. [DOI] [PubMed] [Google Scholar]
- 20.Jakob, T., P. S. Walker, A. M. Krieg, M. C. Udey, and J. C. Vogel. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J. Immunol. 161:3042-3049. [PubMed] [Google Scholar]
- 21.Kondo, K., T. Kondo, T. Okuno, M. Takahashi, and K. Yamanishi. 1991. Latent human herpesvirus 6 infection of human monocytes/macrophages. J. Gen. Virol. 72:1401-1408. [DOI] [PubMed] [Google Scholar]
- 22.Luka, J., M. Okano, and G. Thiele. 1990. Isolation of human herpesvirus-6 from clinical specimens using human fibroblast cultures. J. Clin. Lab. Anal. 4:483-486. [DOI] [PubMed] [Google Scholar]
- 23.Lusso, P., A. De Maria, M. Malnati, F. Lori, S. E. DeRocco, M. Baseler, and R. C. Gallo. 1991. Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature 349:533-535. [DOI] [PubMed] [Google Scholar]
- 24.Lusso, P., and R. C. Gallo. 1995. Human herpesvirus 6 in AIDS. Immunol. Today 16:67-71. [DOI] [PubMed] [Google Scholar]
- 25.Lusso, P., A. Garzino-Demo, R. W. Crowley, and M. S. Malnati. 1995. Infection of γ/δ T lymphocytes by human herpesvirus 6: transcriptional induction of CD4 and susceptibility to HIV infection. J. Exp. Med. 181:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lusso, P., M. Malnati, A. De Maria, C. Balotta, S. E. DeRocco, P. D. Markham, and R. C. Gallo. 1991. Productive infection of CD4+ and CD8+ mature human T cell populations and clones by human herpesvirus 6. Transcriptional down-regulation of CD3. J. Immunol. 147:685-691. [PubMed] [Google Scholar]
- 27.Lusso, P., M. S. Malnati, A. Garzino-Demo, R. W. Crowley, E. O. Long, and R. C. Gallo. 1993. Infection of natural killer cells by human herpesvirus 6. Nature 362:458-462. [DOI] [PubMed] [Google Scholar]
- 28.Ohminami, H., M. Yasukawa, S. Kaneko, Y. Yakushijin, Y. Abe, Y. Kasahara, Y. Ishida, and S. Fujita. 1999. Fas-independent and nonapoptotic cytotoxicity mediated by a human CD4+ T-cell clone directed against acute myelogenous leukemia-associated DEK-CAN fusion peptide. Blood 93:925-935. [PubMed] [Google Scholar]
- 29.Raftery, M. J., M. Schwab, S. M. Eibert, Y. Samstag, H. Walczak, and G. Schonrich. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus. A multilayered viral defense strategy. Immunity 15:997-1009. [DOI] [PubMed] [Google Scholar]
- 30.Reiser, J.-B., C. Darnault, A. Guimezanes, C. Grégoire, T. Mosser, A.-M. Schmitt-Verhulst, J. C. Fontecilla-Camps, B. Malissen, D. Housset, and G. Mazza. 2000. Crystal structure of a T cell receptor bound to an allogeneic MHC molecule. Nat. Immunol. 4:291-297. [DOI] [PubMed] [Google Scholar]
- 31.Reis e Sousa, C., A. Sher, and P. Kaye. 1999. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr. Opin. Immunol. 11:392-399. [DOI] [PubMed] [Google Scholar]
- 32.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 33.Romani, N., D. Reider, M. Heuer, S. Ebner, E. Kampgen, B. Eibl, D. Niederwieser, and G. Schuler. 1996. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods 196:137-151. [DOI] [PubMed] [Google Scholar]
- 34.Salahuddin, S. Z., D. V. Ablashi, P. D. Markham, S. F. Josephs, S. Sturzenegger, M. Kaplan, G. Halligan, P. Biberfeld, F. Wong-Staal, B. Kramarsky, and R. C. Gallo. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596-601. [DOI] [PubMed] [Google Scholar]
- 35.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schirmer, E. C., L. S. Wyatt, K. Yamanishi, W. J. Rodriguez, and N. Frenkel. 1991. Differentiation between two distinct classes of viruses now classified as human herpesvirus 6. Proc. Natl. Acad. Sci. USA 88:5922-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnorr, J.-J., S. Xanthakos, P. Keikavoussi, E. Kämpgen, V. ter Meulen, and S. Schneider-Schaulies. 1997. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc. Natl. Acad. Sci. USA 94:5326-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 41.Sugita, K., H. Kurumada, M. Eguchi, and T. Furukawa. 1995. Human herpesvirus 6 infection associated with hemophagocytic syndrome. Acta Haematol. 93:108-109. [DOI] [PubMed] [Google Scholar]
- 42.Tohyama, M., Y. Yahata, M. Yasukawa, R. Inagi, Y. Urano, K. Yamanishi, and K. Hashimoto. 1998. Severe hypersensitivity syndrome due to sulfasalazine associated with reactivation of human herpesvirus 6. Arch. Dermatol. 134:1113-1117. [DOI] [PubMed] [Google Scholar]
- 43.Yakushijin, Y., M. Yasukawa, and Y. Kobayashi. 1991. T-cell immune response to human herpesvirus-6 in healthy adults. Microbiol. Immunol. 35:655-660. [DOI] [PubMed] [Google Scholar]
- 44.Yamanishi, K. 1992. Human herpesvirus 6. Microbiol. Immunol. 36:551-561. [DOI] [PubMed] [Google Scholar]
- 45.Yamanishi, K., T. Okuno, K. Shiraki, M. Takahashi, T. Kondo, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causative agent for exanthem subitum. Lancet i:1065-1067. [DOI] [PubMed]
- 46.Yasukawa, M., A. Hasegawa, I. Sakai, H. Ohminami, J. Arai, S. Kaneko, Y. Yakushijin, K. Maeyama, H. Nakashima, R. Arakaki, and S. Fujita. 1999. Down-regulation of CXCR4 by human herpesvirus 6 (HHV-6) and HHV-7. J. Immunol. 162:5417-5422. [PubMed] [Google Scholar]
- 47.Yasukawa, M., A. Inatsuki, T. Horiuchi, and Y. Kobayashi. 1991. Functional heterogeneity among herpes simplex virus-specific CD4+ T cells. J. Immunol. 146:1341-1347. [PubMed] [Google Scholar]
- 48.Yasukawa, M., H. Ohminami, S. Kaneko, Y. Yakushijin, Y. Nishimura, T. Miyakuni, K. Inokuchi, S. Nakao, K. Kishi, I. Kubonishi, K. Dan, and S. Fujita. 1998. CD4+ cytotoxic T-cell clones specific for bcr-abl b3a2 fusion peptide augment colony formation by chronic myelogenous leukemia cells in a b3a2-specific and HLA-DR-restricted manner. Blood 92:3355-3361. [PubMed] [Google Scholar]
- 49.Yasukawa, M., H. Ohminami, E. Sada, Y. Yakushijin, M. Kaneko, K. Yanagisawa, H. Kohno, S. Bando, and S. Fujita. 1999. Latent infection and reactivation of human herpesvirus 6 in two novel myeloid cell lines. Blood 93:991-999. [PubMed] [Google Scholar]
- 50.Yasukawa, M., Y. Yakushijin, M. Furukawa, and S. Fujita. 1993. Specificity analysis of human CD4+ T-cell clones directed against human herpesvirus 6 (HHV 6), HHV-7, and human cytomegalovirus. J. Virol. 67:6259-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou, L. J., and T. F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA 93:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou, P., Y. Isegawa, K. Nakano, M. Haque, Y. Horiguchi, and K. Yamanishi. 1999. Human herpesvirus 6 open reading frame U83 encodes a functional chemokine. J. Virol. 73:5926-5933. [DOI] [PMC free article] [PubMed] [Google Scholar]