Abstract
1. A study has been made of the effects of Na+ and Ca2+ on the responses generated by intracellular current pulses in smooth muscle cells of the guinea-pig vas deferens and upon the propagated action potential.
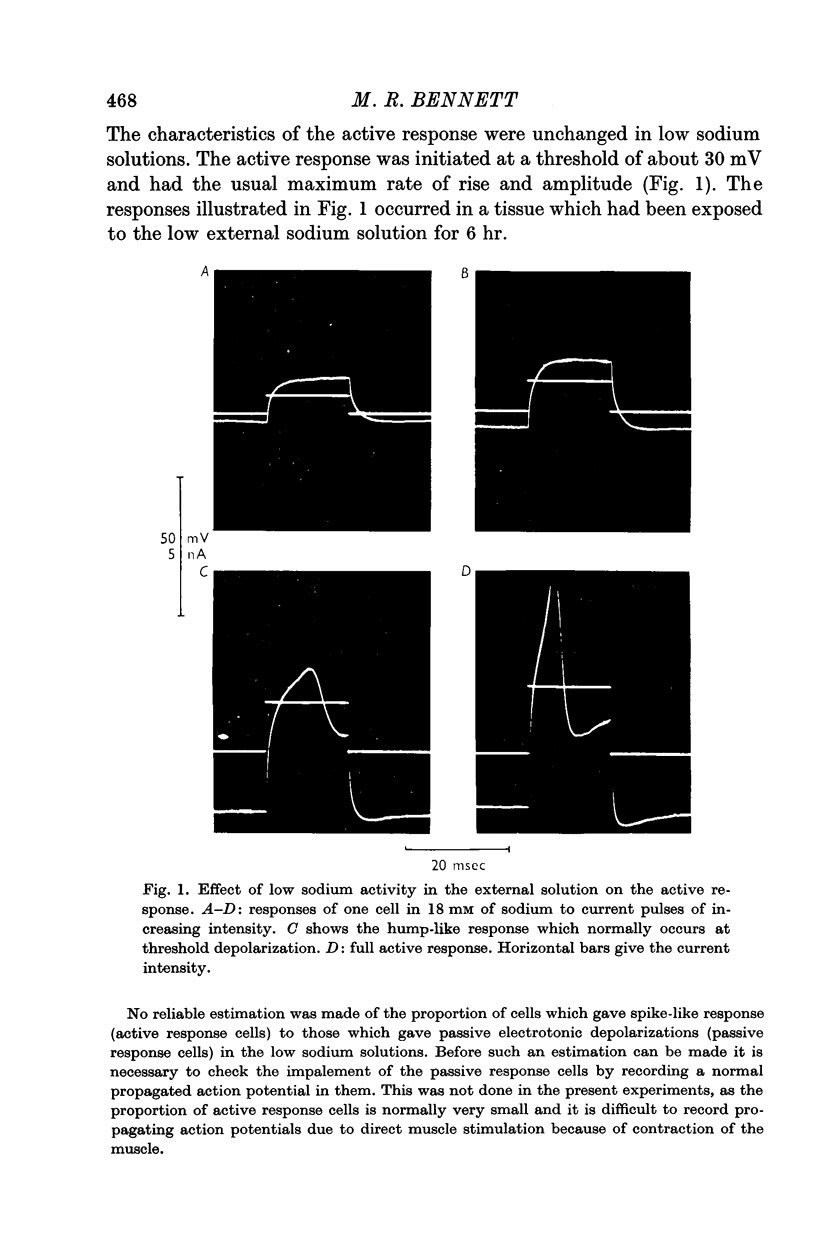
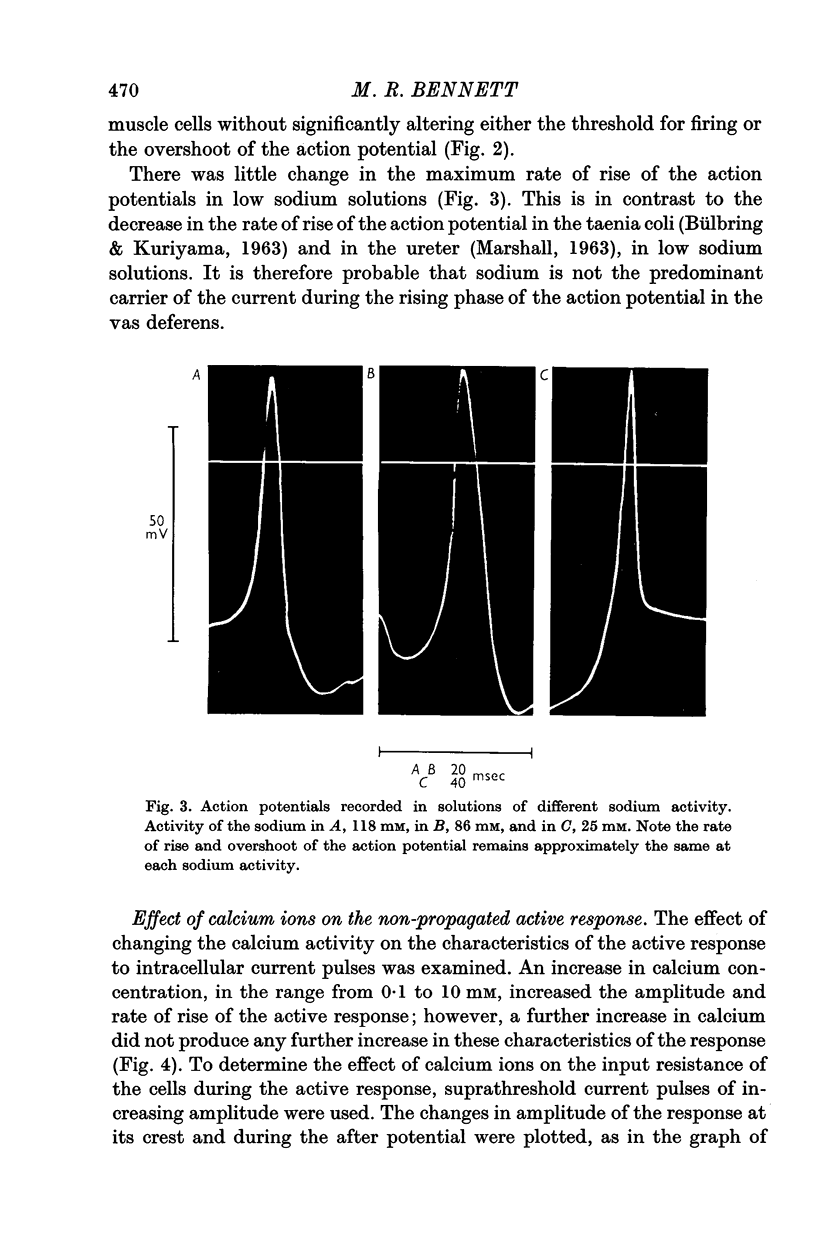
2. Reduction of the extracellular Na+ activity (aNa) to less than 30 mM did not alter the characteristics of the spike-like response to intracellular current pulses (the active response) or of the propagated action potential.
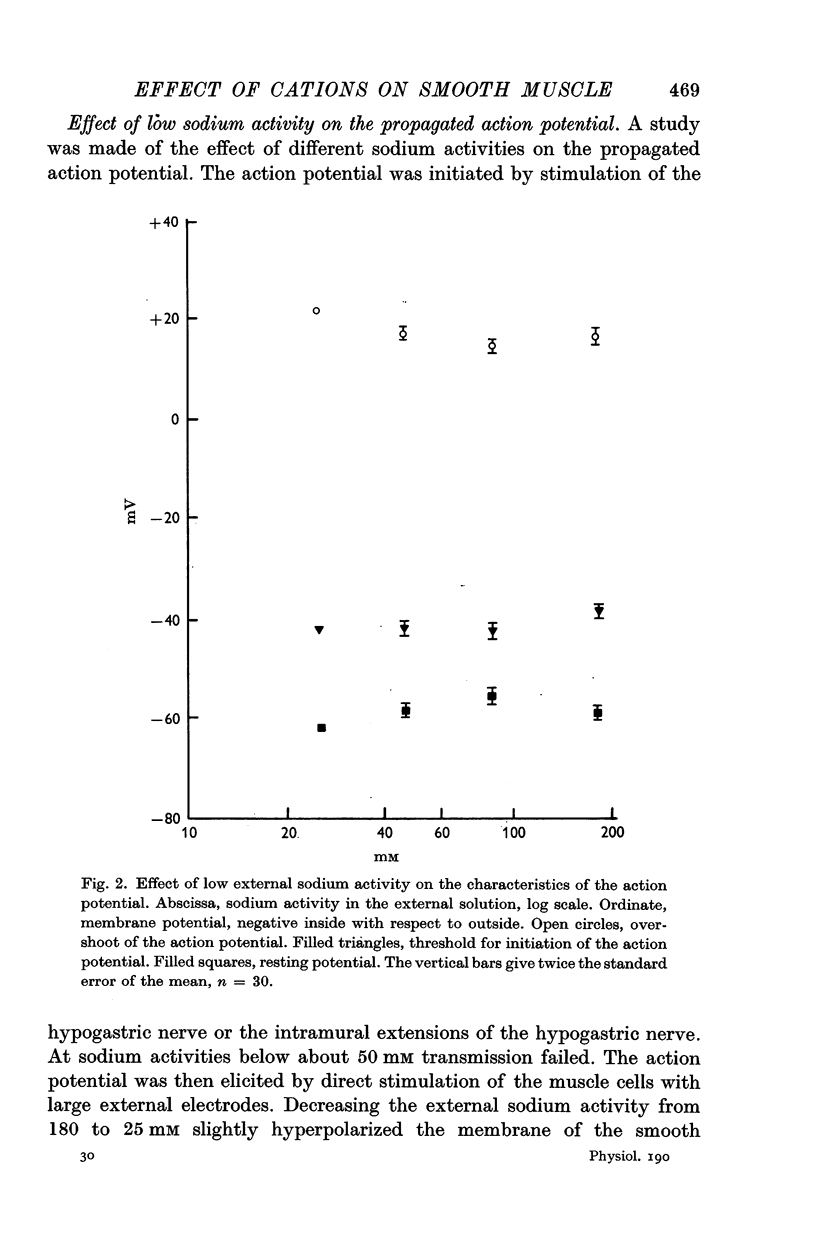
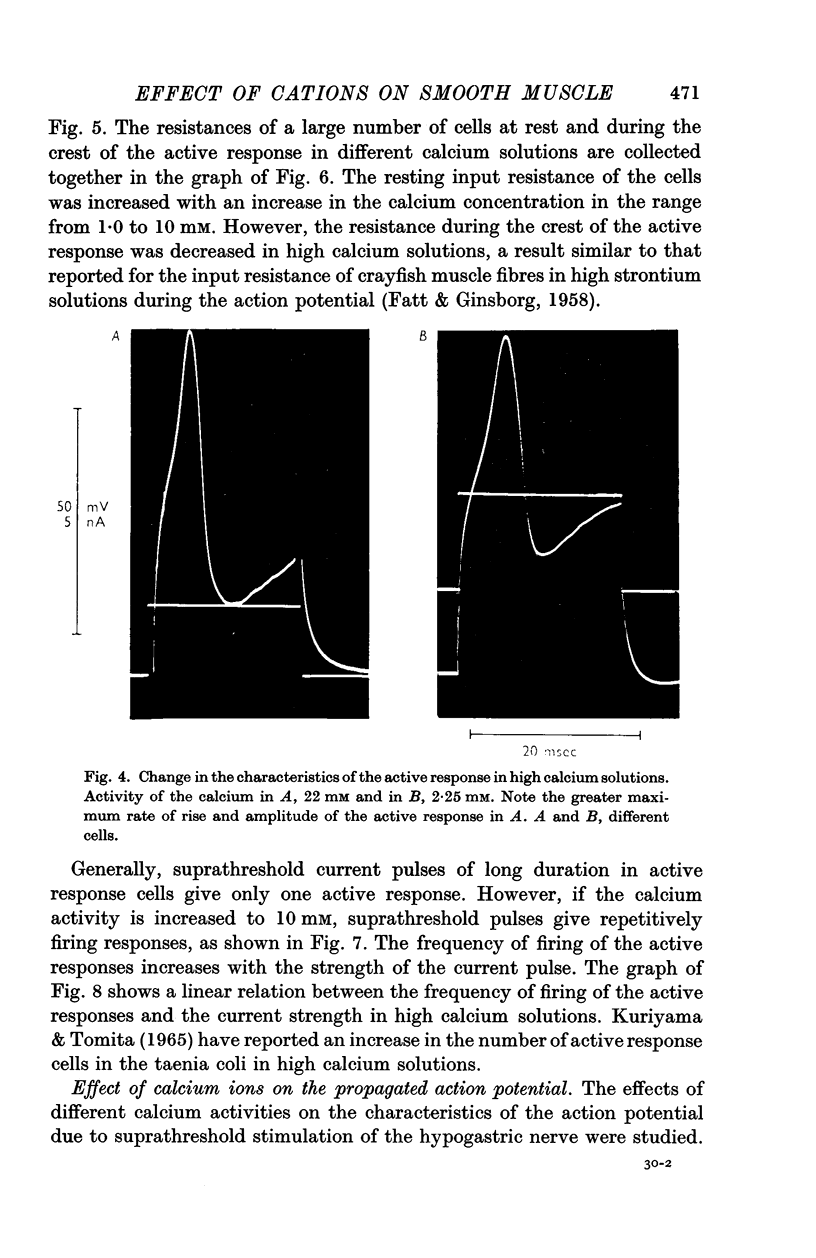
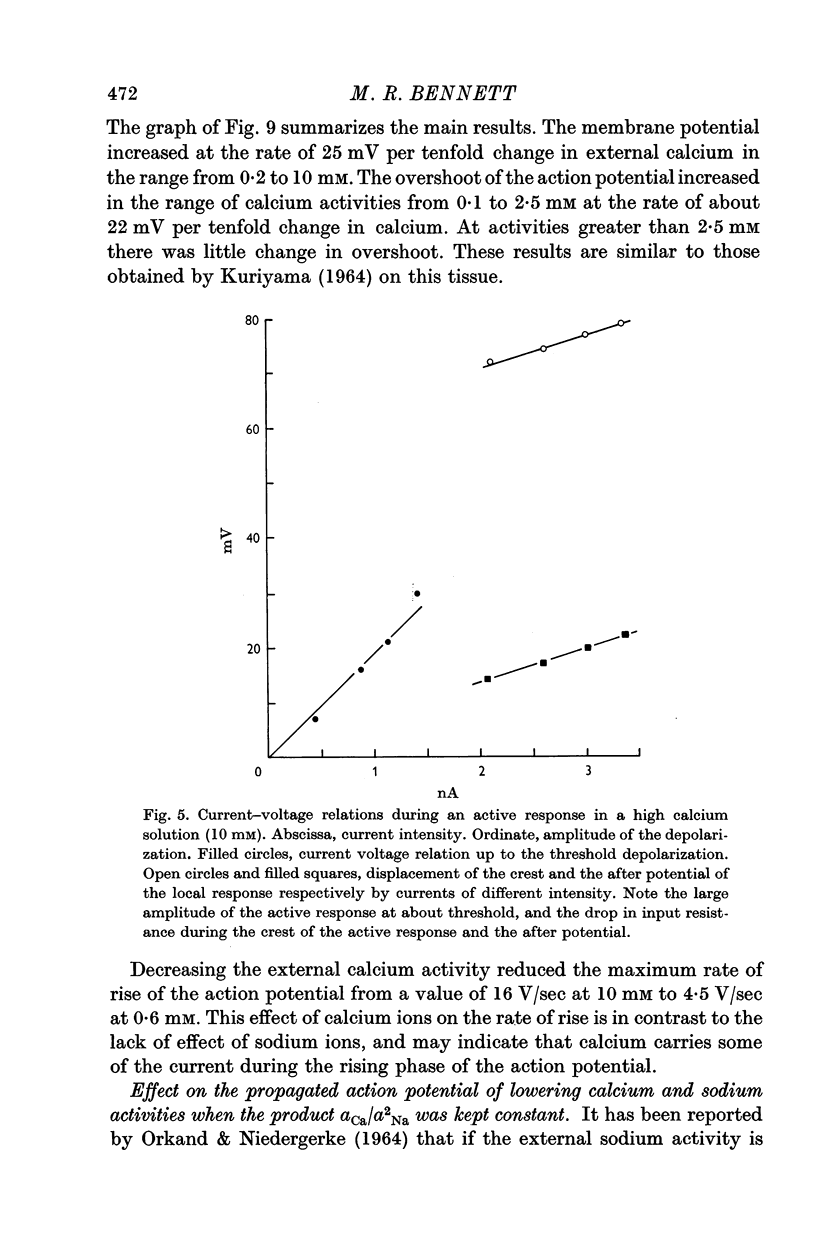
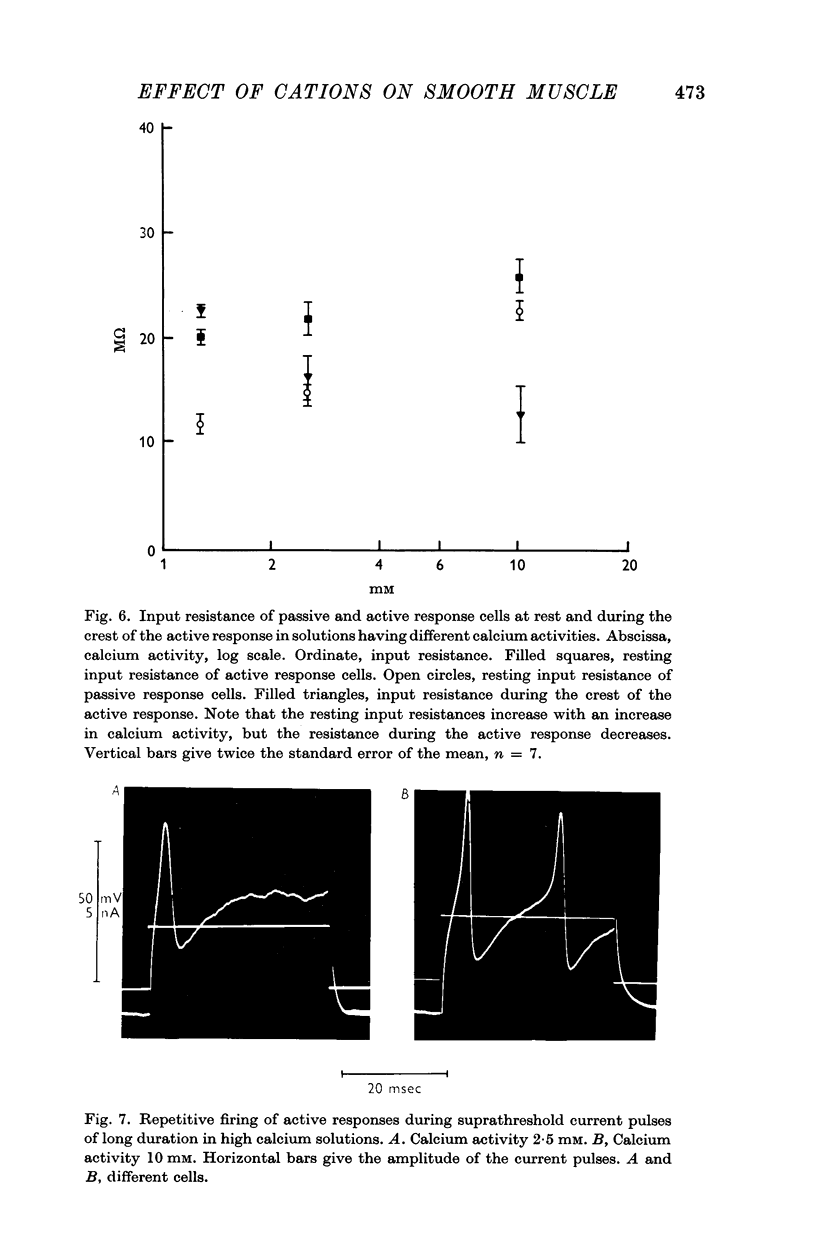
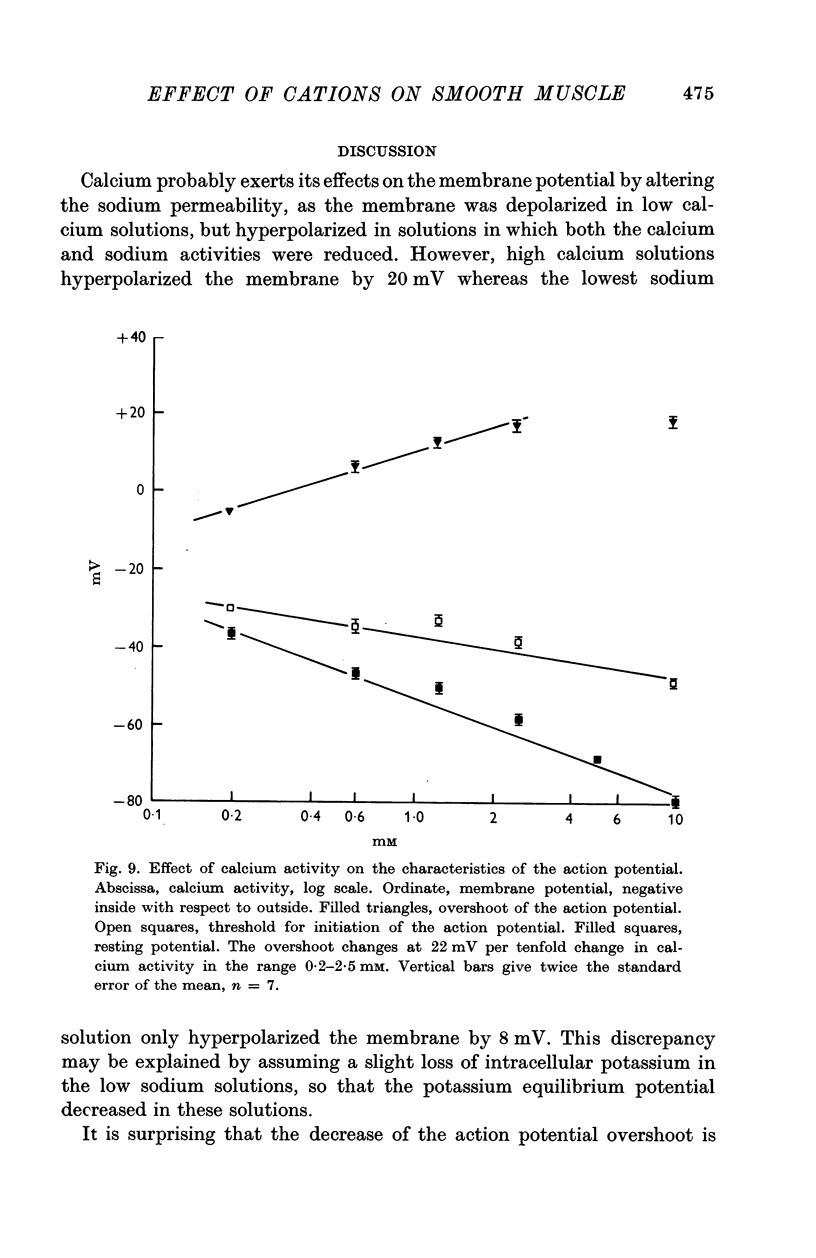
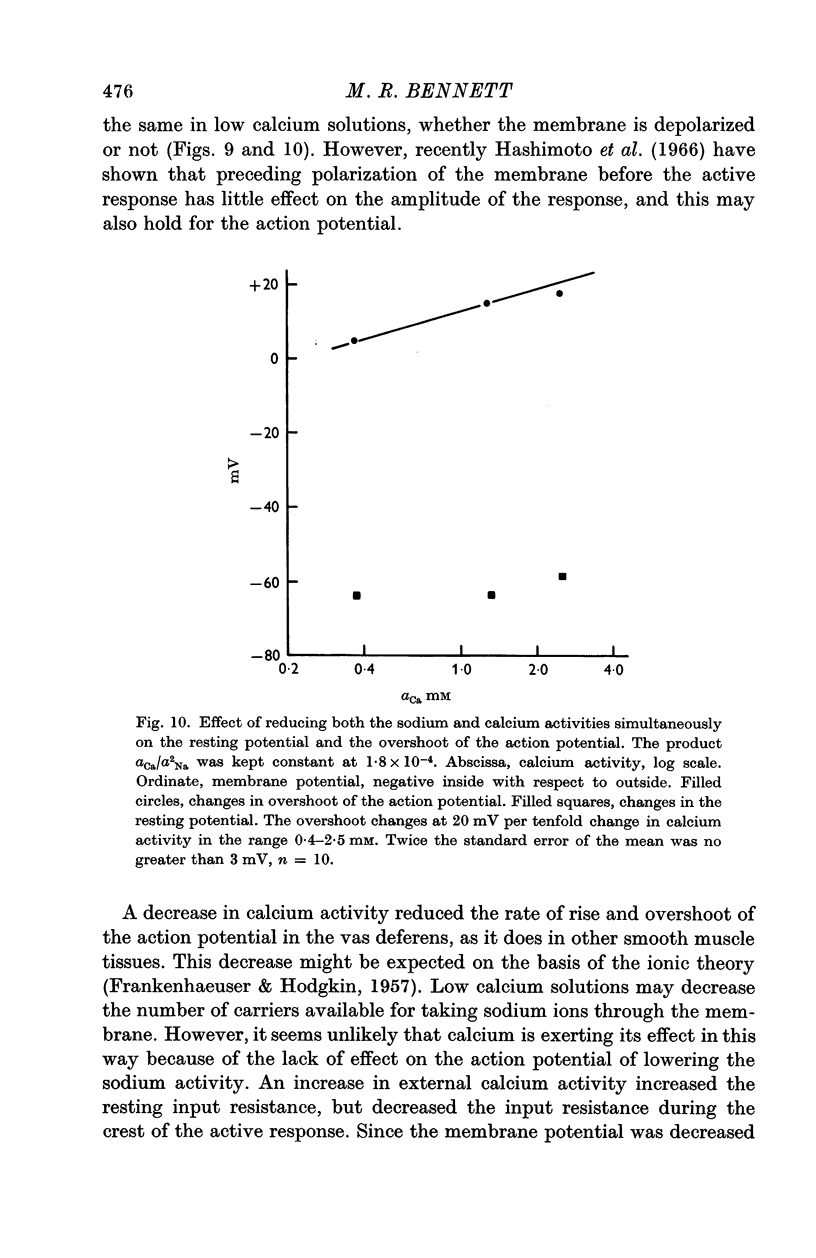
3. Reduction of the extracellular Ca2+ activity (aCa) to 0·1 mM decreased the resting input resistance of the cells but increased the input resistance during the crest of the active response. Reduction of Ca2+ decreased the overshoot of the action potential by 22 mV per tenfold change in Ca2+ and the resting potential by 25 mV per tenfold change.
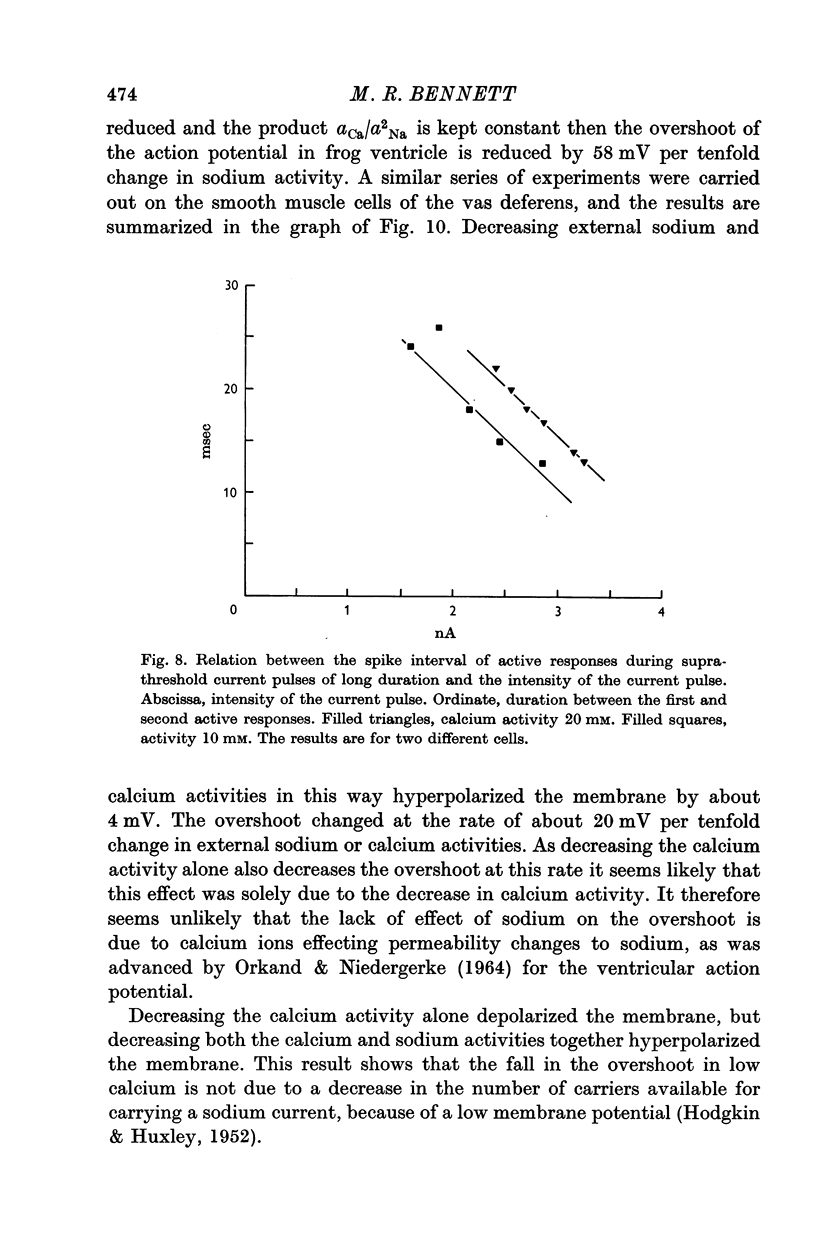
4. Reduction of the extracellular Ca2+, keeping the product aCa/a2Na constant, did not change the resting potential, but decreased the action potential overshoot by 20 mV per tenfold change.
5. It is suggested that part of the current responsible for the rising phase of the action potential is carried by Ca2+.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., STRAUB R. W. A method for studying the effects of ions and drugs on the resting and action potentials in smooth muscle with external electrodes. J Physiol. 1958 Jan 23;140(1):156–167. doi: 10.1113/jphysiol.1958.sp005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G. Application of the sucrose-gap method to determine the ionic basis of the membrane potential of smooth muscle. J Physiol. 1966 Apr;183(3):637–648. doi: 10.1113/jphysiol.1966.sp007889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Merrillees N. C. An analysis of the transmission of excitation from autonomic nerves to smooth muscle. J Physiol. 1966 Aug;185(3):520–535. doi: 10.1113/jphysiol.1966.sp008000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. Model of the membrane of smooth muscle cells of the guinea-pig taenia coli muscle during transmission from inhibitory and excitatory nerves. Nature. 1966 Sep 10;211(5054):1149–1152. doi: 10.1038/2111149a0. [DOI] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady A. J., Woodbury J. W. The sodium-potassium hypothesis as the basis of electrical activity in frog ventricle. J Physiol. 1960 Dec;154(2):385–407. doi: 10.1113/jphysiol.1960.sp006586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECK K. A., TRAUTWEIN W. IONIC CURRENTS IN CARDIAC EXCITATION. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:63–80. doi: 10.1007/BF00412616. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J. S. The electrical and mechanical responses of intestinal smooth muscle cells to stimulation of their extrinsic parasympathetic nerves. J Physiol. 1962 Jun;162:76–92. doi: 10.1113/jphysiol.1962.sp006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFORD P. J., HERMANSEN K. Sodium and potassium movements in the unstriated muscle of the guinea-pig taenia coli. J Physiol. 1961 Oct;158:426–448. doi: 10.1113/jphysiol.1961.sp006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., CHICHIBU S., NAKA K. I. THE EFFECTS OF VARIOUS IONS ON RESTING AND SPIKE POTENTIALS OF BARNACLE MUSCLE FIBERS. J Gen Physiol. 1964 Sep;48:163–179. doi: 10.1085/jgp.48.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Holman M. E., Tille J. Electrical properties of the smooth muscle membrane of the guinea-pig vas deferens. J Physiol. 1966 Sep;186(1):27–41. doi: 10.1113/jphysiol.1966.sp008018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI M., IRISAWA H. EFFECT OF SODIUM DEFICIENCY ON THE ACTION POTENTIAL OF THE SMOOTH MUSCLE OF URETER. Am J Physiol. 1964 Jan;206:205–210. doi: 10.1152/ajplegacy.1964.206.1.205. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H. EFFECT OF CALCIUM AND MAGNESIUM ON NEUROMUSCULAR TRANSMISSION IN THE HYPOGASTRIC NERVE-VAS DEFERENS PREPARATION OF THE GUINEA-PIG. J Physiol. 1964 Dec;175:211–230. doi: 10.1113/jphysiol.1964.sp007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H., TOMITA T. THE RESPONSES OF SINGLE SMOOTH MUSCLE CELLS OF GUINEA-PIG TAENIA COLI TO INTRACELLULARLY APPLIED CURRENTS, AND THEIR EFFECT ON THE SPONTANEOUS ELECTRICAL ACTIVITY. J Physiol. 1965 May;178:270–289. doi: 10.1113/jphysiol.1965.sp007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dependence of the action potential of the frog's heart on the external and intracellular sodium concentration. J Physiol. 1966 May;184(2):312–334. doi: 10.1113/jphysiol.1966.sp007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura Y., Hotta Y., Ohashi H. Tetrodotoxin and manganese ions: effects on electrical activity and tension in taenia coli of guinea pig. Science. 1966 Apr 1;152(3718):97–98. doi: 10.1126/science.152.3718.97. [DOI] [PubMed] [Google Scholar]
- ORKAND R. K., NIEDERGERKE R. HEART ACTION POTENTIAL: DEPENDENCE ON EXTERNAL CALCIUM AND SODIUM IONS. Science. 1964 Nov 27;146(3648):1176–1177. doi: 10.1126/science.146.3648.1176. [DOI] [PubMed] [Google Scholar]


