Abstract
1. Intracellular recordings were made from chromaffin cells isolated from adrenal medullae of gerbils to examine the effects, on membrane potential, of changes in the ionic environment that are known, from other experiments, to influence the rate of catecholamine secretion.
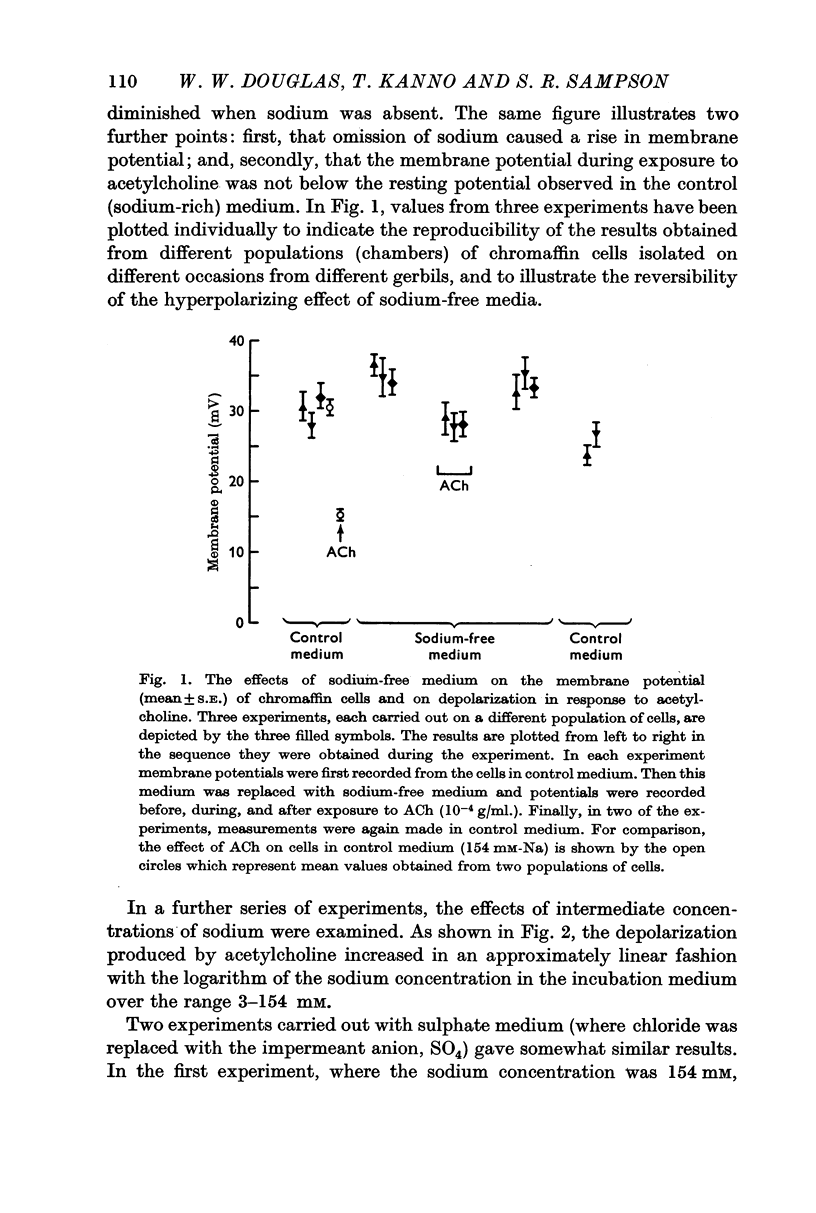
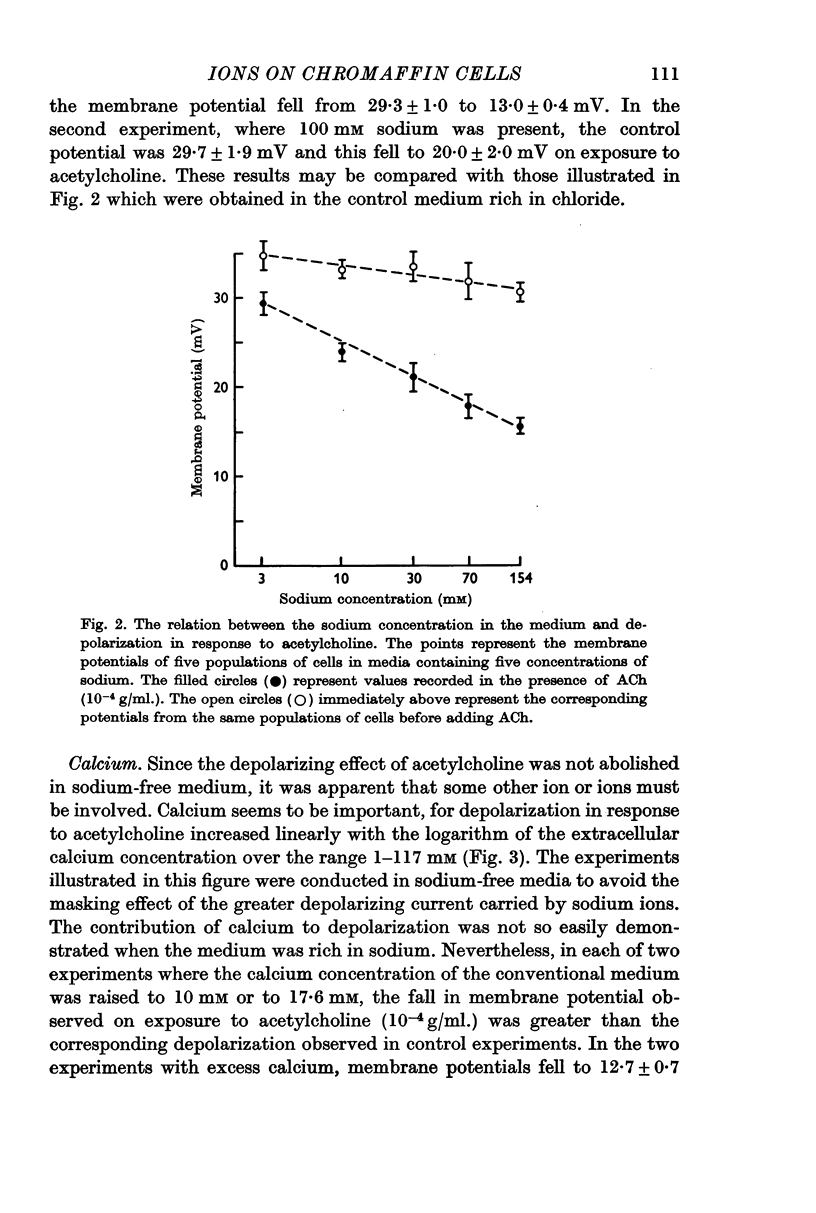
2. Depolarization in response to acetylcholine fell linearly with the logarithm of the extracellular sodium concentration over the range 154-3 mM and reached a value, in sodium-free medium, of about 30% of the control value.
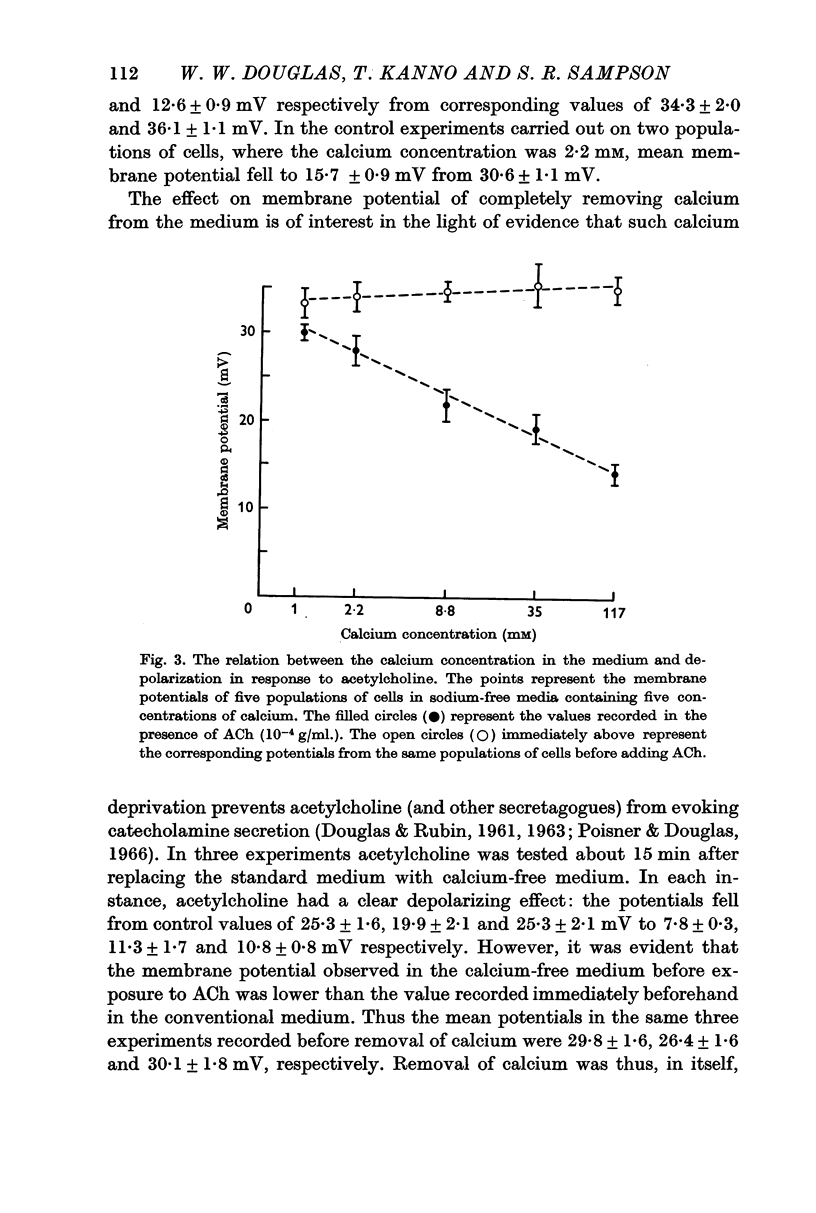
3. The depolarizing effect of acetylcholine in sodium-free media increased linearly with the logarithm of the extracellular calcium concentration over the range 1-117 mM.
It is concluded that depolarization in response to acetylcholine involves inward movement of both sodium and calcium ions.
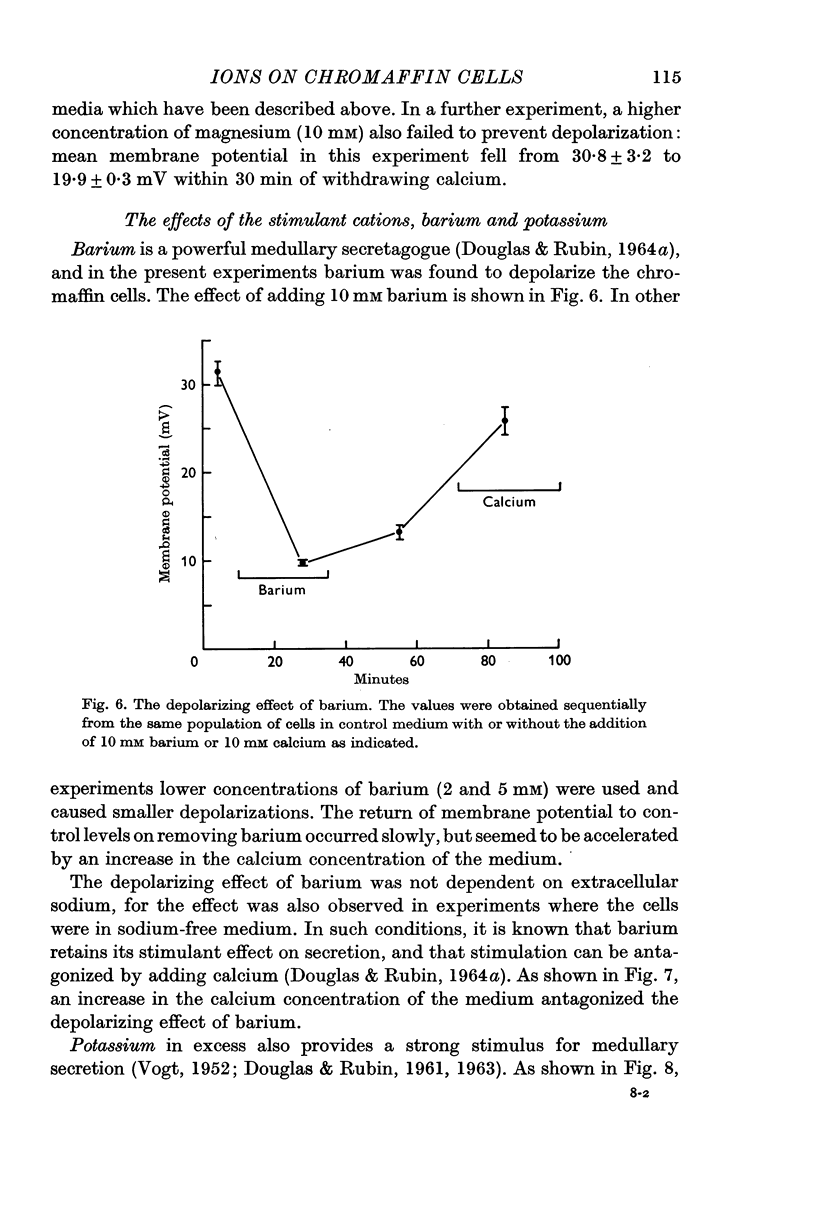
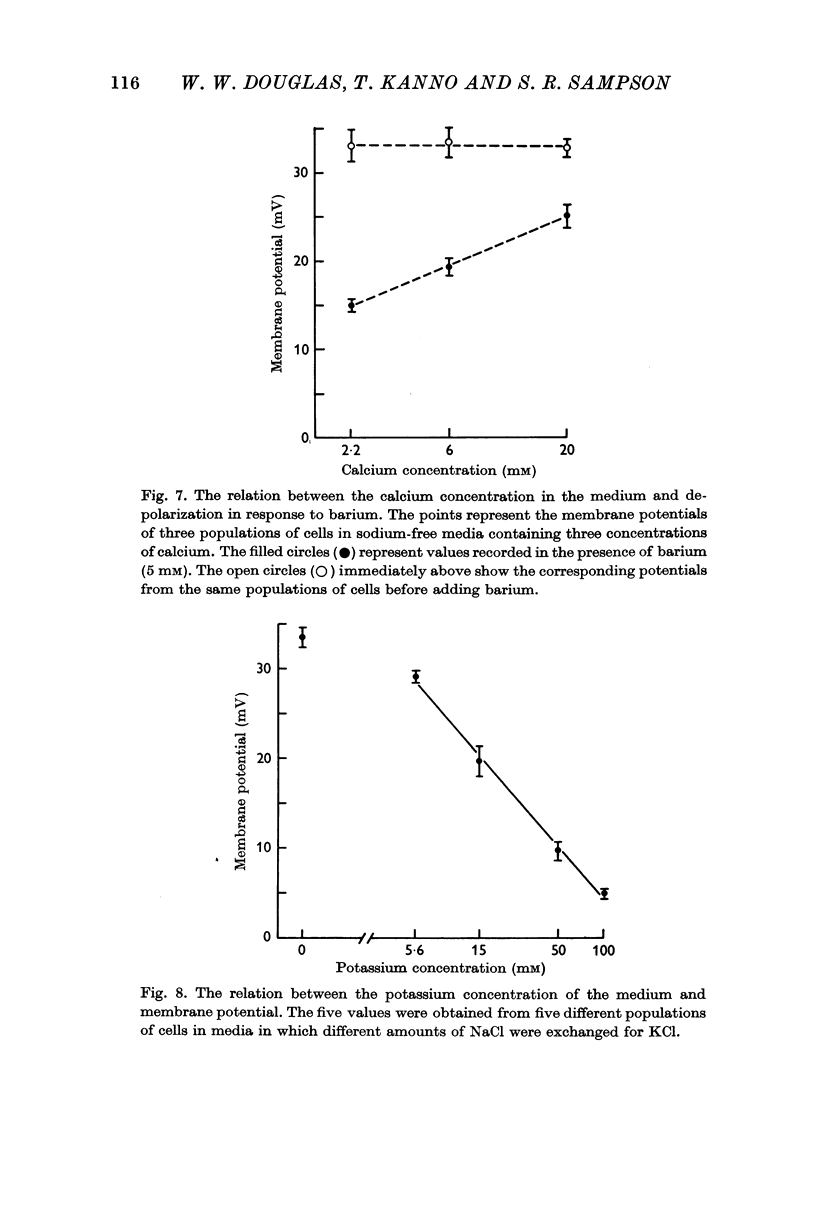
4. Depolarization was also observed in response to the secretagogues, excess potassium and barium, both in sodium-rich and sodium-free media. The effect of barium was antagonized by calcium, and it is suggested that these two cations interact at the level of the plasma membrane.
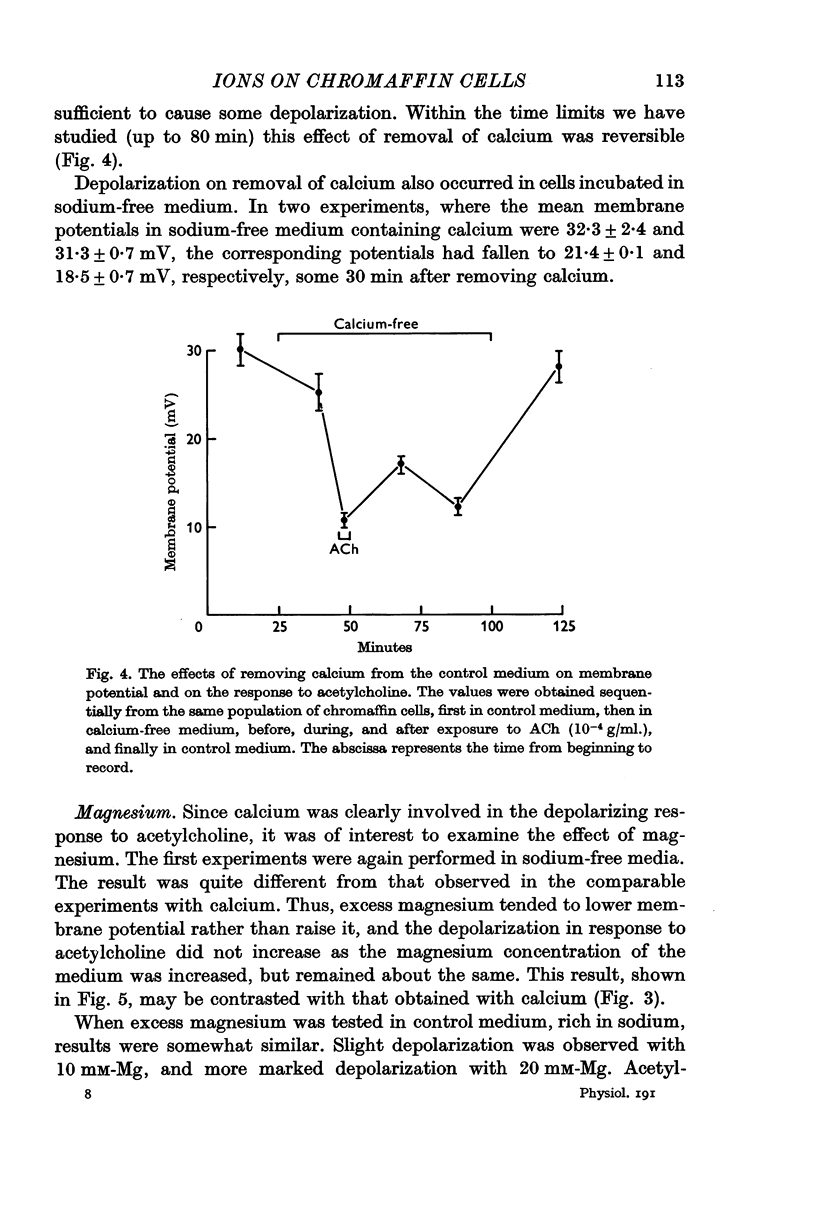
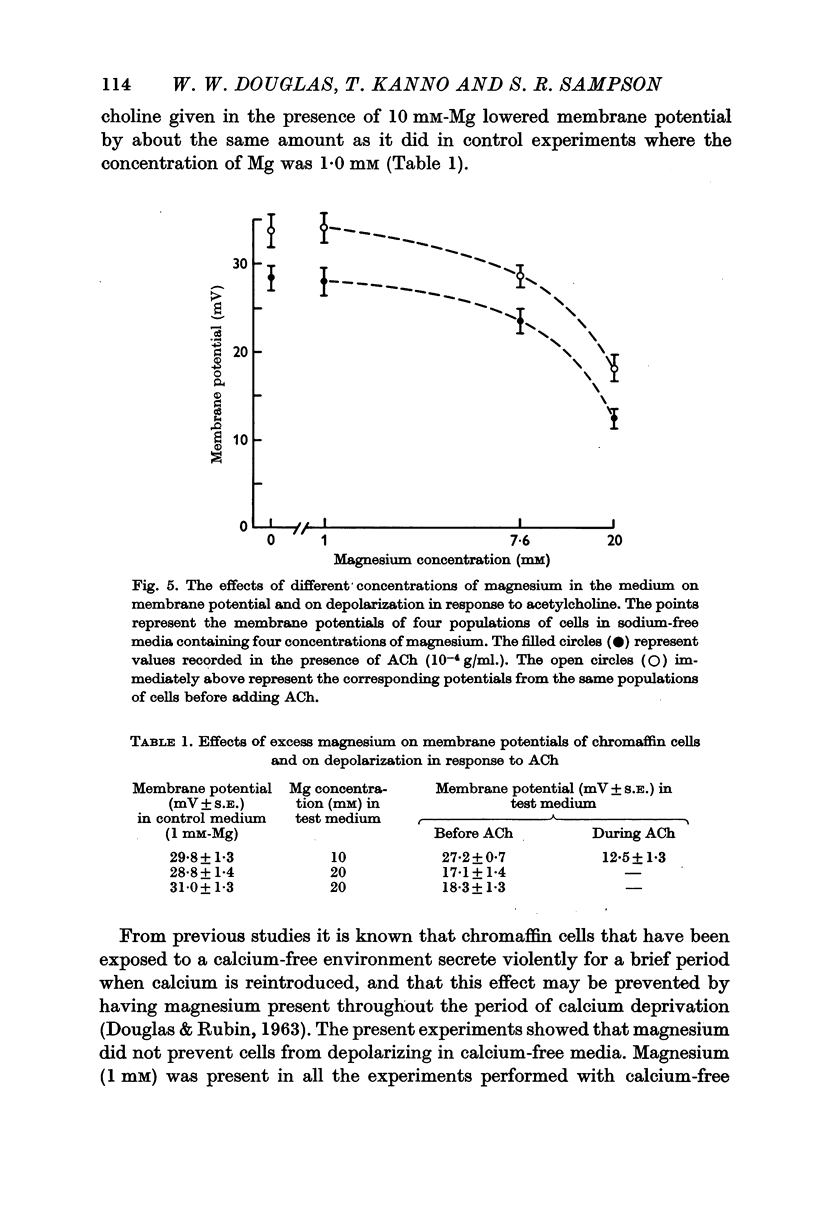
5. Depolarization does not appear to be tightly coupled to secretion, for acetylcholine or excess potassium still depolarized the chromaffin cells when the environment was calcium-free or contained an excess of magnesium, conditions that inhibit secretion. Furthermore, although acetylcholine had some depolarizing effect in sodium-free media, the level to which the membrane potential fell was not below the control `resting' potential since the cells in sodium-free medium were hyperpolarized; yet, secretory responses are augmented in such conditions.
6. It is proposed that depolarization in response to acetylcholine may be no more than the electrical sign of increased permeability to ions such as sodium and calcium, and that depolarization is not, in itself, a key event in stimulus—secretion coupling. The evidence is held to favour the view that movement of calcium into the chromaffin cells on exposure to acetylcholine is responsible for evoking secretion.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armett C. J., Ritchie J. M. On the permeability of mammalian non-myelinated fibres to sodium and to lithium ions. J Physiol. 1963 Jan;165(1):130–140. doi: 10.1113/jphysiol.1963.sp007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armett C. J., Ritchie J. M. The ionic requirements for the action of acetylcholine on mammalian non-myelinated fibres. J Physiol. 1963 Jan;165(1):141–159. doi: 10.1113/jphysiol.1963.sp007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINK F. The role of calcium ions in neural processes. Pharmacol Rev. 1954 Sep;6(3):243–298. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Biophysical aspects of neuro-muscular transmission. Prog Biophys Biophys Chem. 1956;6:121–170. [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. Stimulation of uptake of calcium-45 in the adrenal gland by acetylcholine. Nature. 1961 Dec 30;192:1299–1299. doi: 10.1038/1921299a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. STIMULANT ACTION OF BARIUM ON THE ADRENAL MEDULLA. Nature. 1964 Jul 18;203:305–307. doi: 10.1038/203305a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J Physiol. 1967 Jan;188(1):107–120. doi: 10.1113/jphysiol.1967.sp008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The effect of sodium ions on neuromuscular transmission. J Physiol. 1952 Sep;118(1):73–87. doi: 10.1113/jphysiol.1952.sp004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAM R. G. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963 Feb;29:515–526. doi: 10.1016/s0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- HILL A. V., HOWARTH J. V. The effect of potassium on the resting metabolism of the frog's sartorius. Proc R Soc Lond B Biol Sci. 1957 Aug 24;147(926):21–43. doi: 10.1098/rspb.1957.0034. [DOI] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J. The macromolecular properties of excitable membranes. Ann N Y Acad Sci. 1961 Sep 6;94:390–404. doi: 10.1111/j.1749-6632.1961.tb35553.x. [DOI] [PubMed] [Google Scholar]
- NASTUK W. L. Some ionic factors that influence the action of acetylcholine at the muscle end-plate membrane. Ann N Y Acad Sci. 1959 Aug 28;81:317–327. doi: 10.1111/j.1749-6632.1959.tb49316.x. [DOI] [PubMed] [Google Scholar]
- NISHI S., SOEDA H., KOKETSU K. EFFECT OF ALKALI-EARTH CATIONS ON FROG SPINAL GANGLION CELL. J Neurophysiol. 1965 May;28:457–472. doi: 10.1152/jn.1965.28.3.457. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Douglas W. W. The need for calcium in adrenomedullary secretion evoked by biogenic amines, polypeptides, and muscarinic agents. Proc Soc Exp Biol Med. 1966 Oct;123(1):62–64. doi: 10.3181/00379727-123-31402. [DOI] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. II. The action potential and excitation. Pharmacol Rev. 1958 Jun;10(2):165–273. [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI N. Effects of calcium on the conductance change of the end-plate membrane during the action of transmitter. J Physiol. 1963 Jun;167:141–155. doi: 10.1113/jphysiol.1963.sp007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT M. The secretion of the denervated adrenal medulla of the cat. Br J Pharmacol Chemother. 1952 Jun;7(2):325–330. doi: 10.1111/j.1476-5381.1952.tb01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


