Abstract
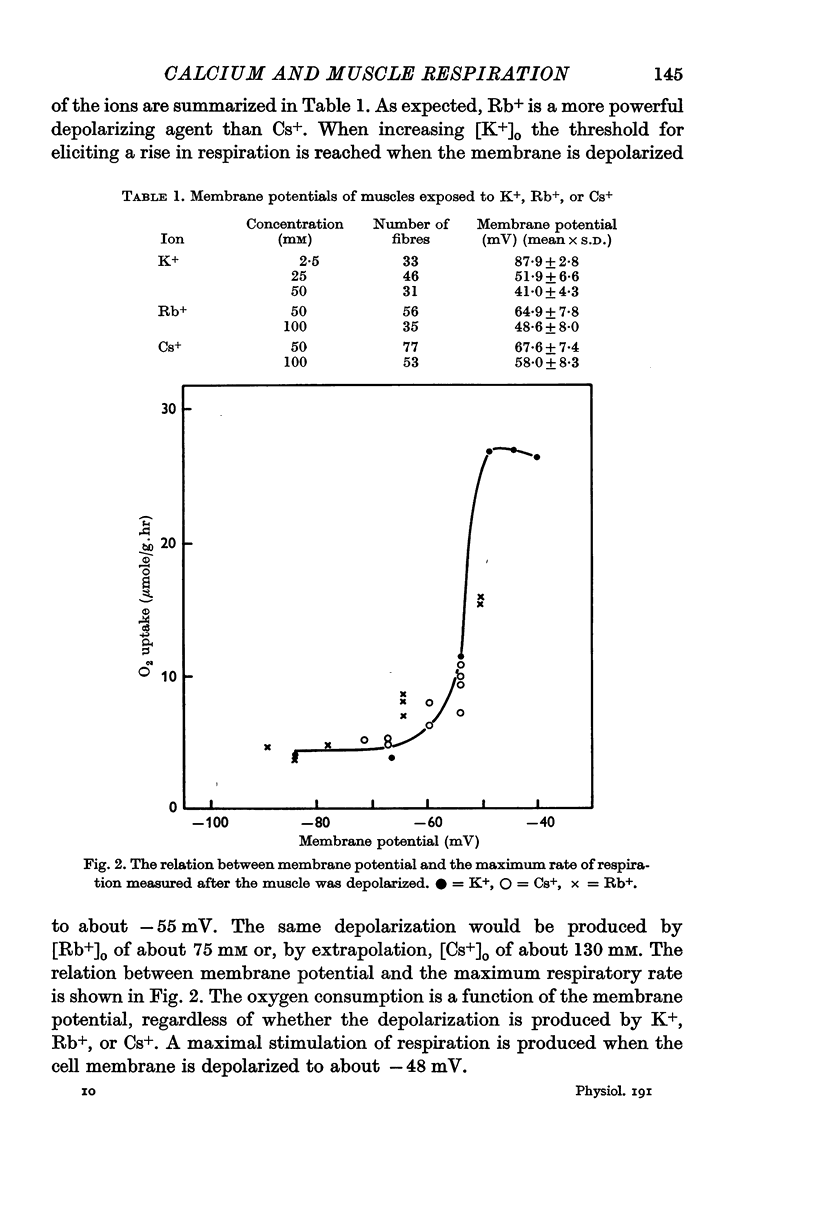
1. In 1931 Fenn showed that the respiration of frog twitch muscles increases when [K+]o is raised. The present paper is a further study of potassium-stimulated respiration. Stimulation depends on membrane potential, since respiration is also stimulated by elevated [Rb+]o or [Cs+]o in direct relation to their ability to depolarize.
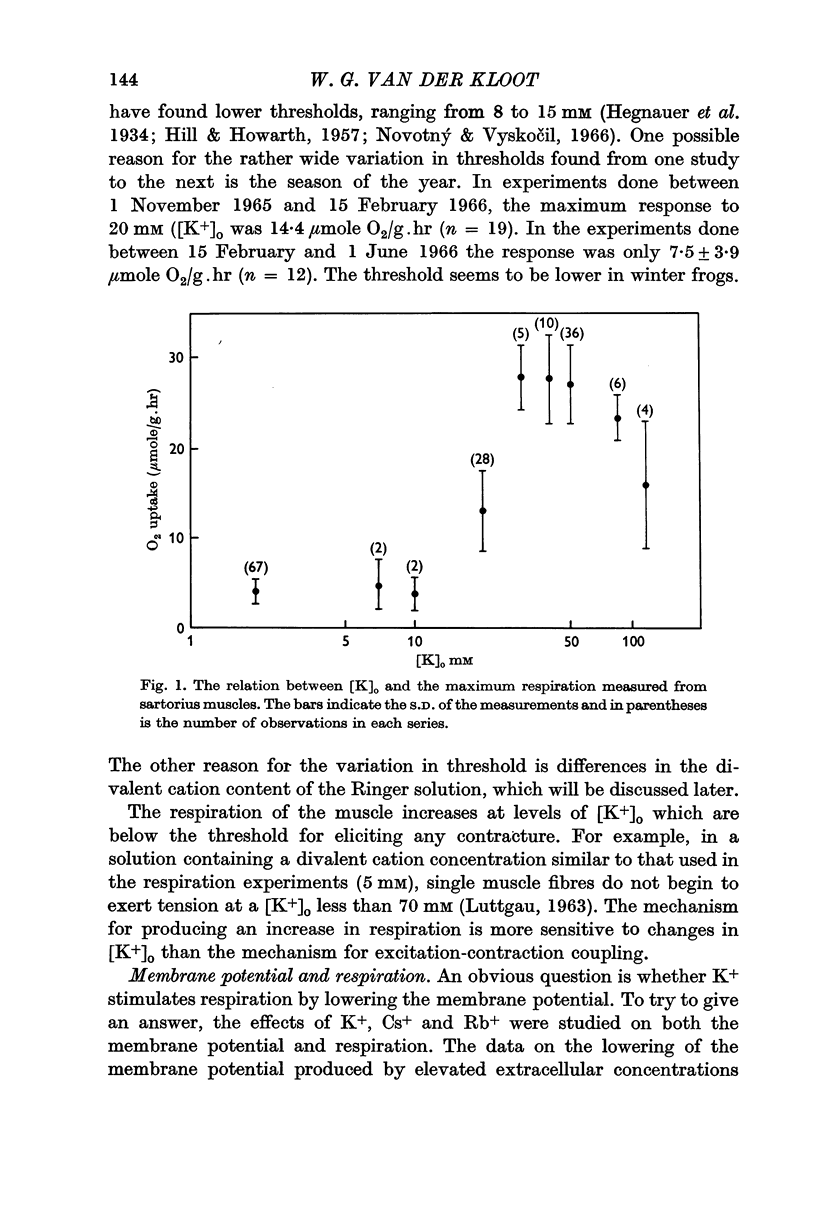
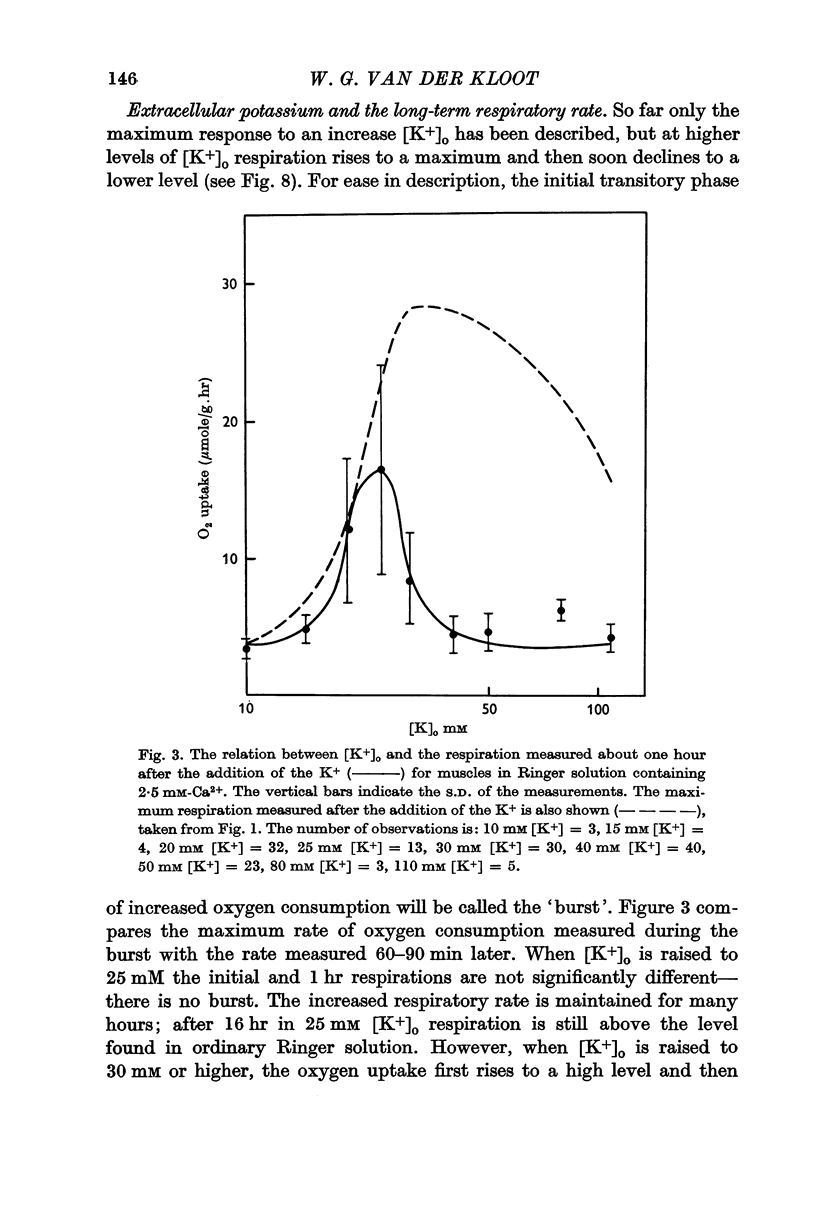
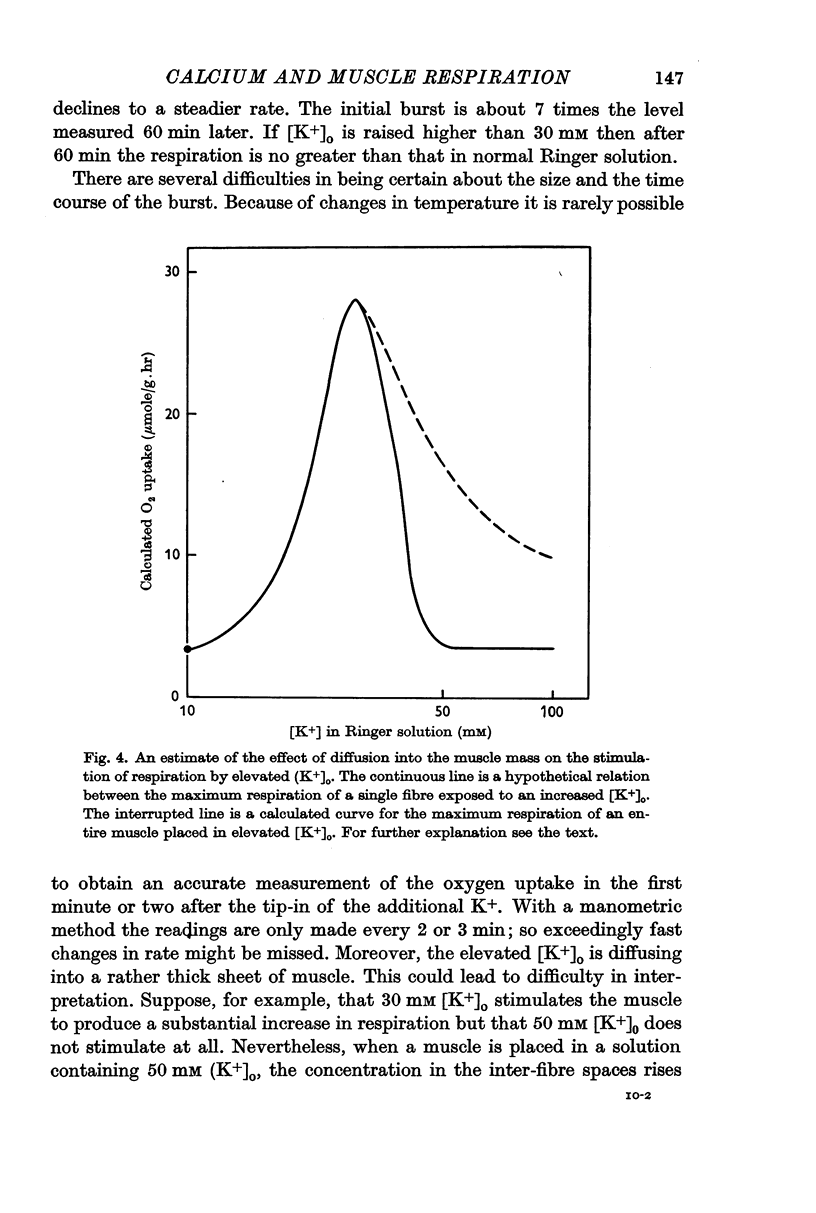
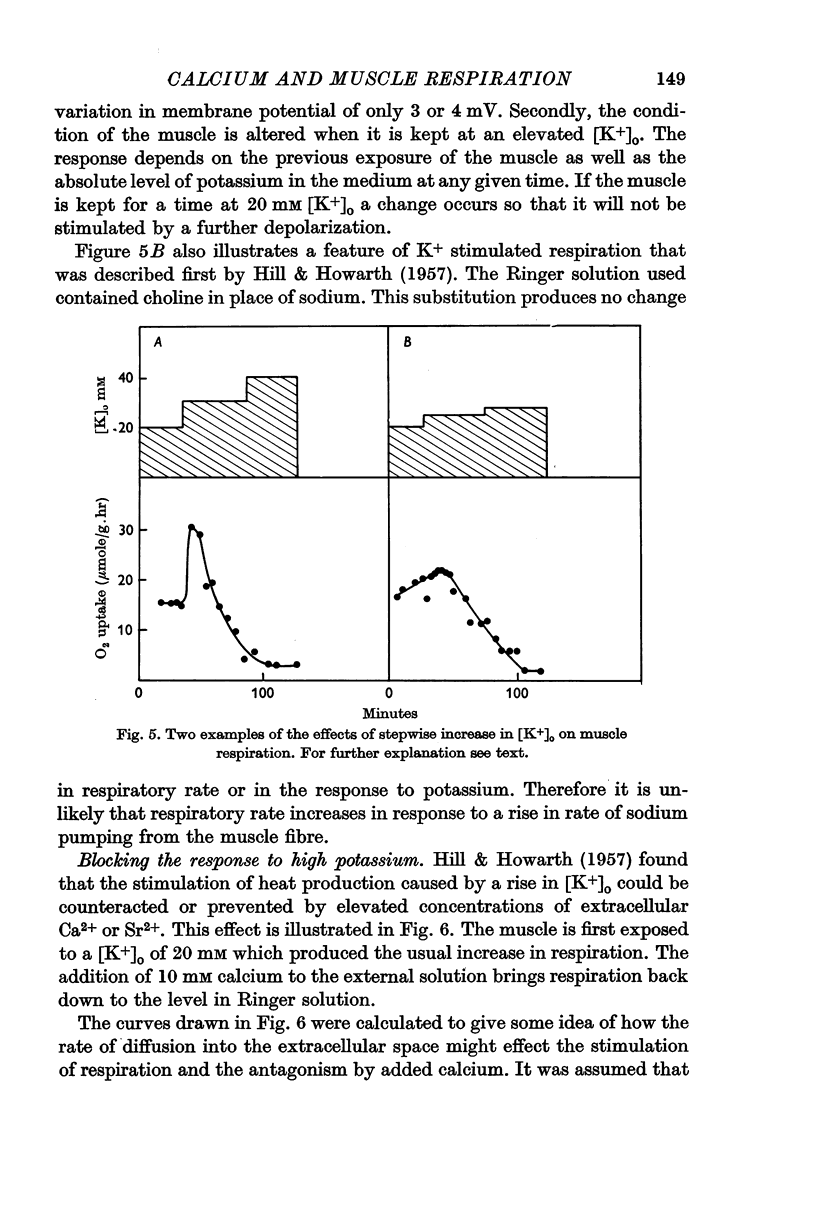
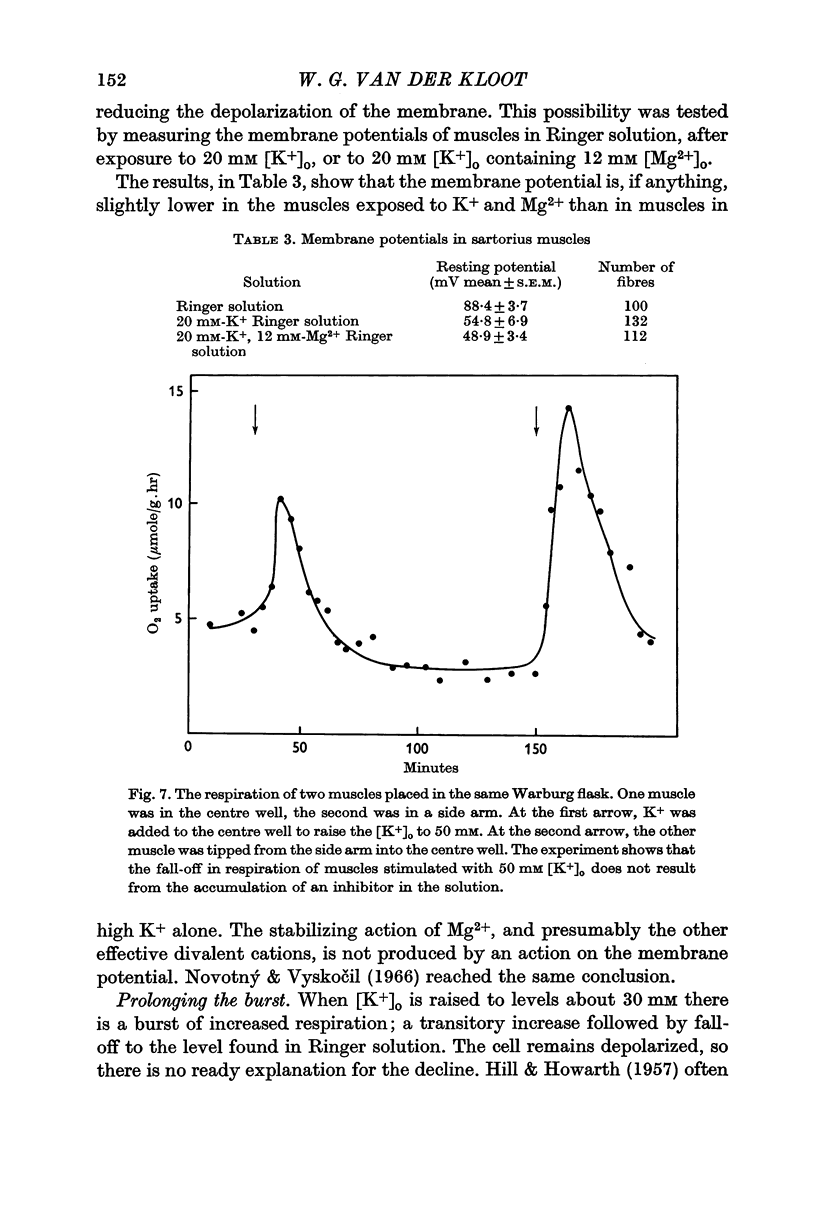
2. When [K+]o is elevated to 25 mM there is an increase in respiration which is sustained for hours. If [K+]o is 30 mM or above, there is a transitory burst of stimulated respiration, followed by a decline back to the basal level.
3. If [K+]o is raised in steps from 20 to 30 mM, there may never be a burst of increased oxygen consumption. Often a rise in [K+]o from 20 to 24 mM decreases respiration.
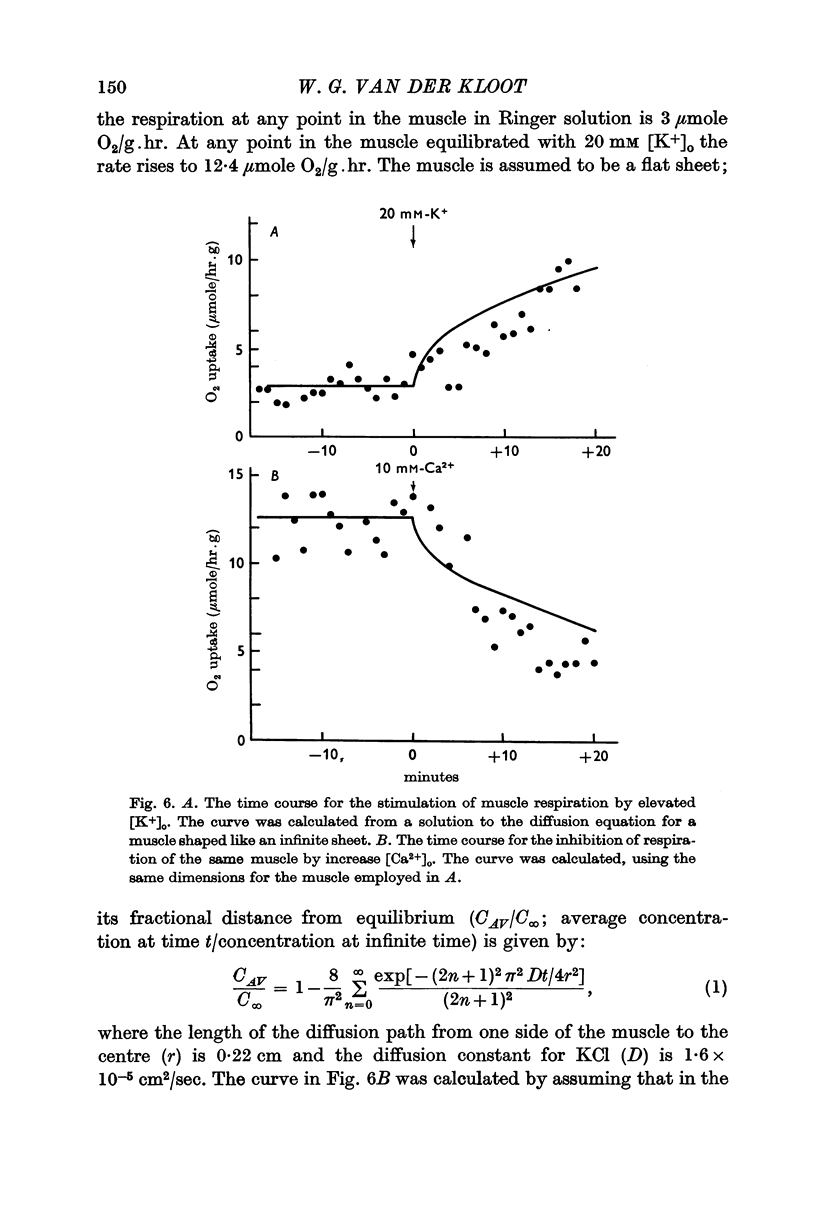
4. The response to elevated [K+]o can be blocked by divalent cations or by local anaesthetics; the blocking agents do not interfere with the depolarization of the membrane. The blocking agents act rapidly; they probably take effect soon after they contact the cell membrane.
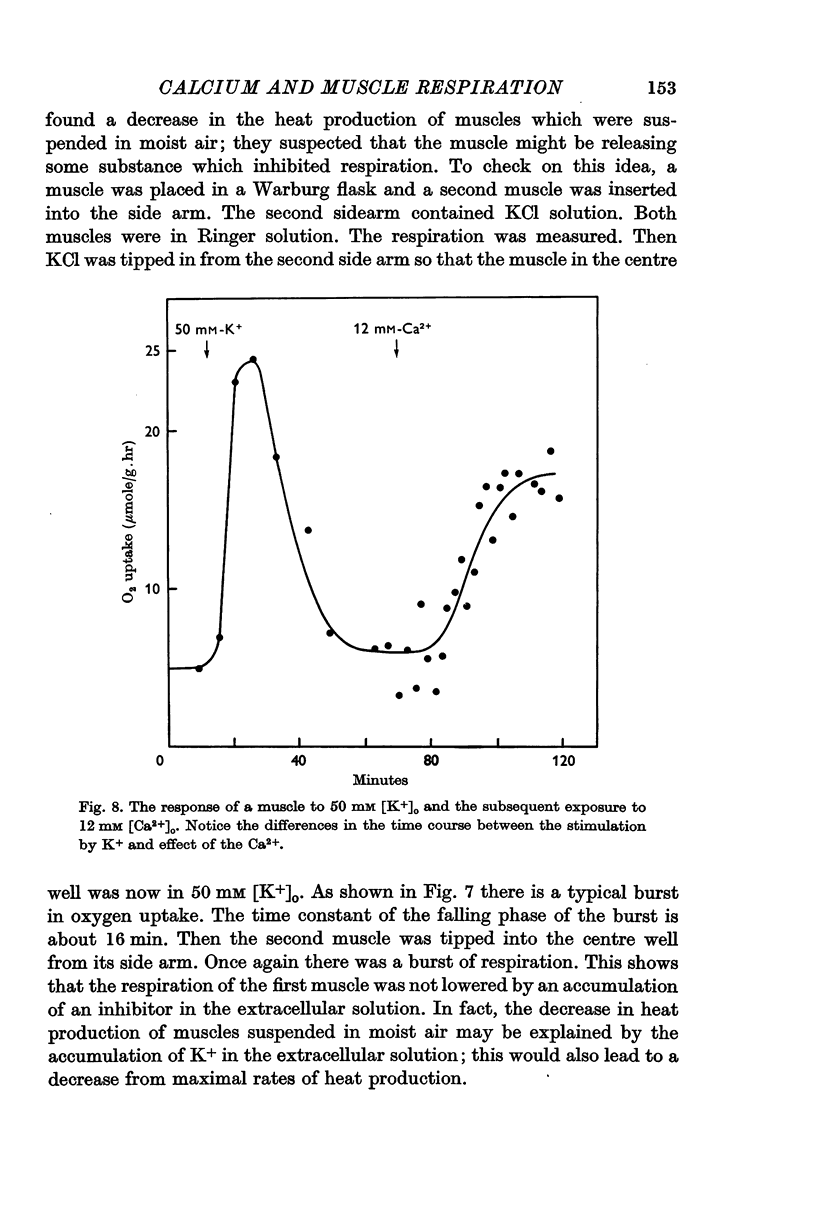
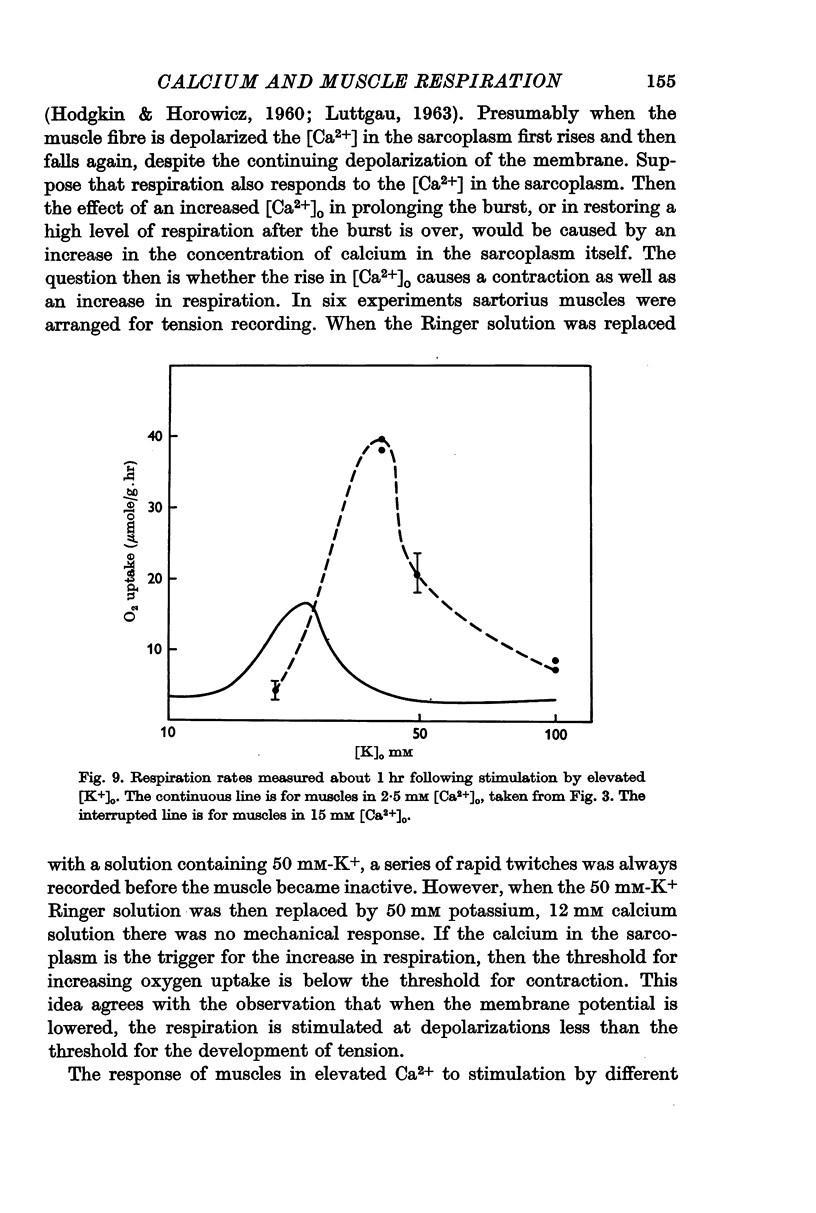
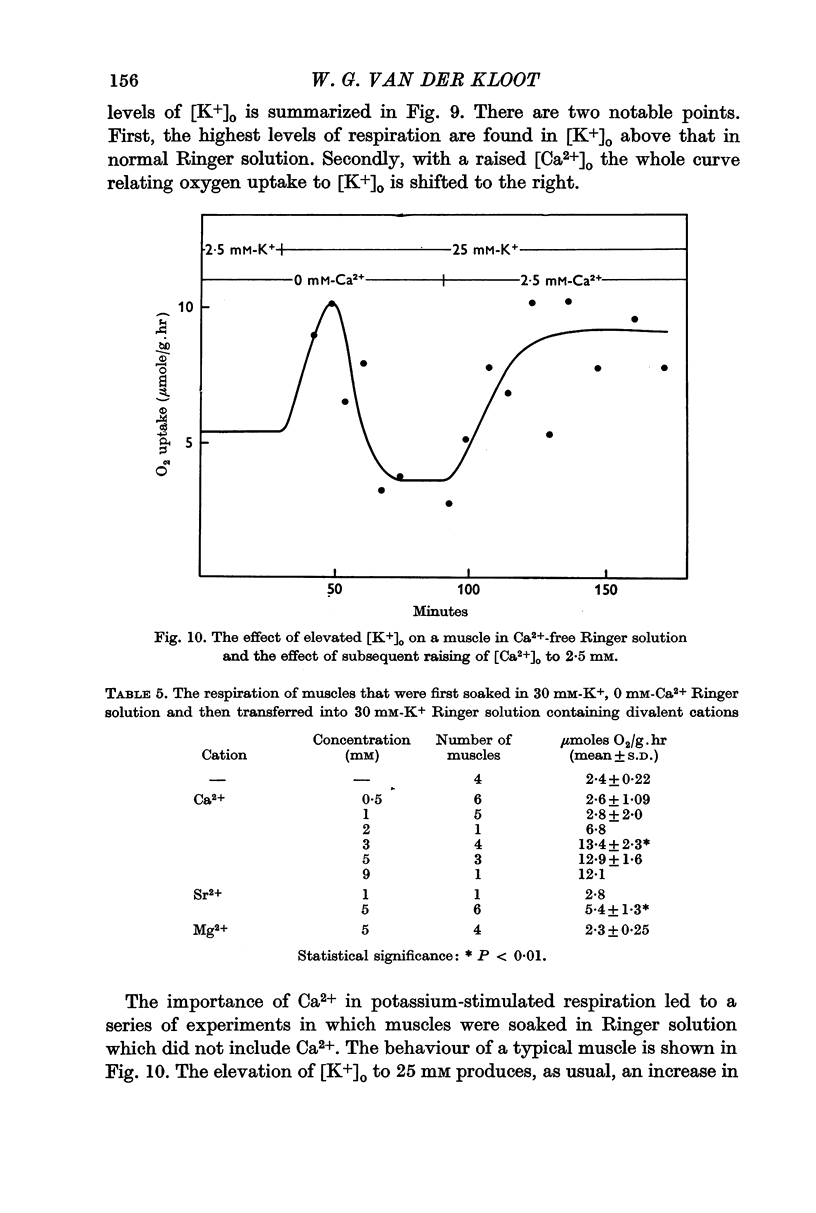
5. Either extracellular Ca2+ or Sr2+ is important for the stimulation of respiration. The burst produced by 50 mM [K+]o is transformed into a sustained rise in respiration when [Ca2+]o or [Sr2+]o are also raised. If a muscle is stimulated with 25 mM [K+]o in the absence of extracellular calcium, respiration is elevated as usual, but now the rise is transitory unless Ca2+ or Sr2+ are added back to the extracellular solution.
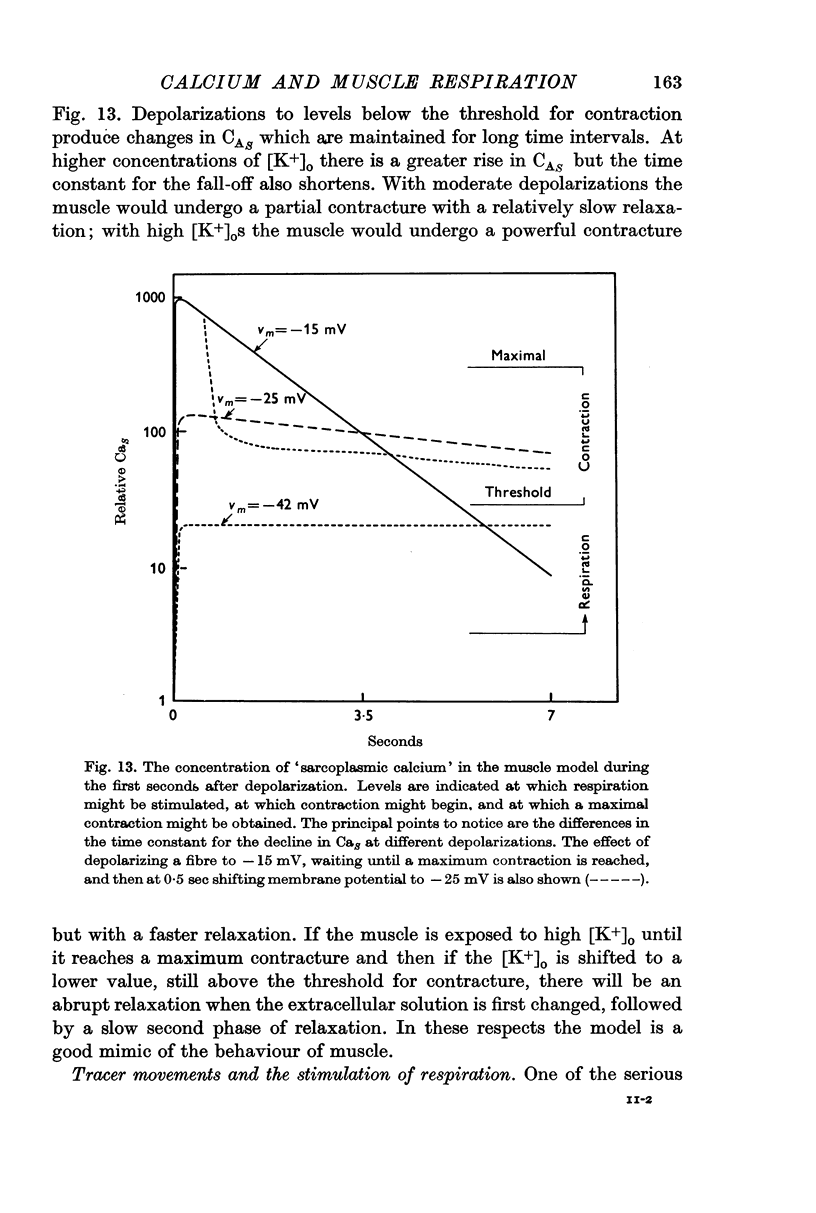
6. Depolarization seems to stimulate respiration by causing the release of Ca2+ into the sarcoplasm. Since respiration is increased by a depolarization below the threshold for producing a contracture, respiration is more sensitive than contraction as an indicator of sarcoplasmic calcium concentration.
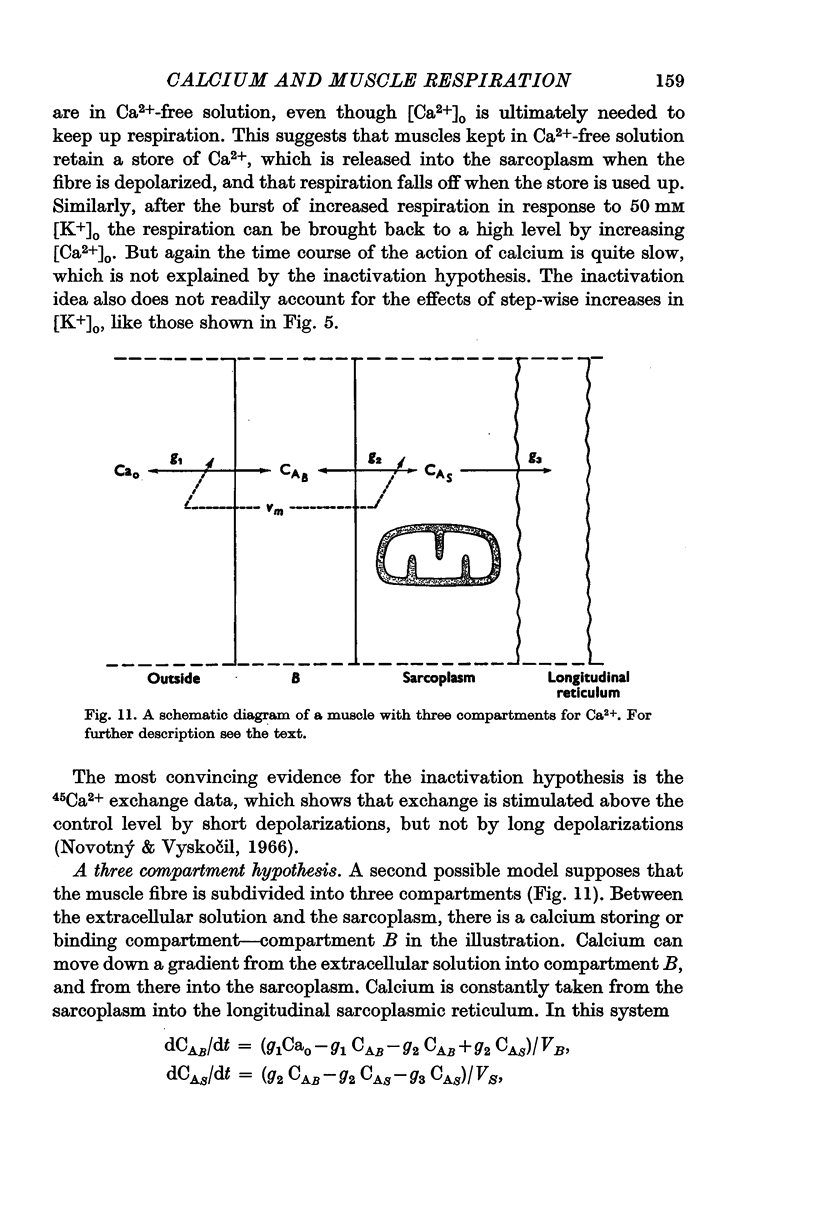
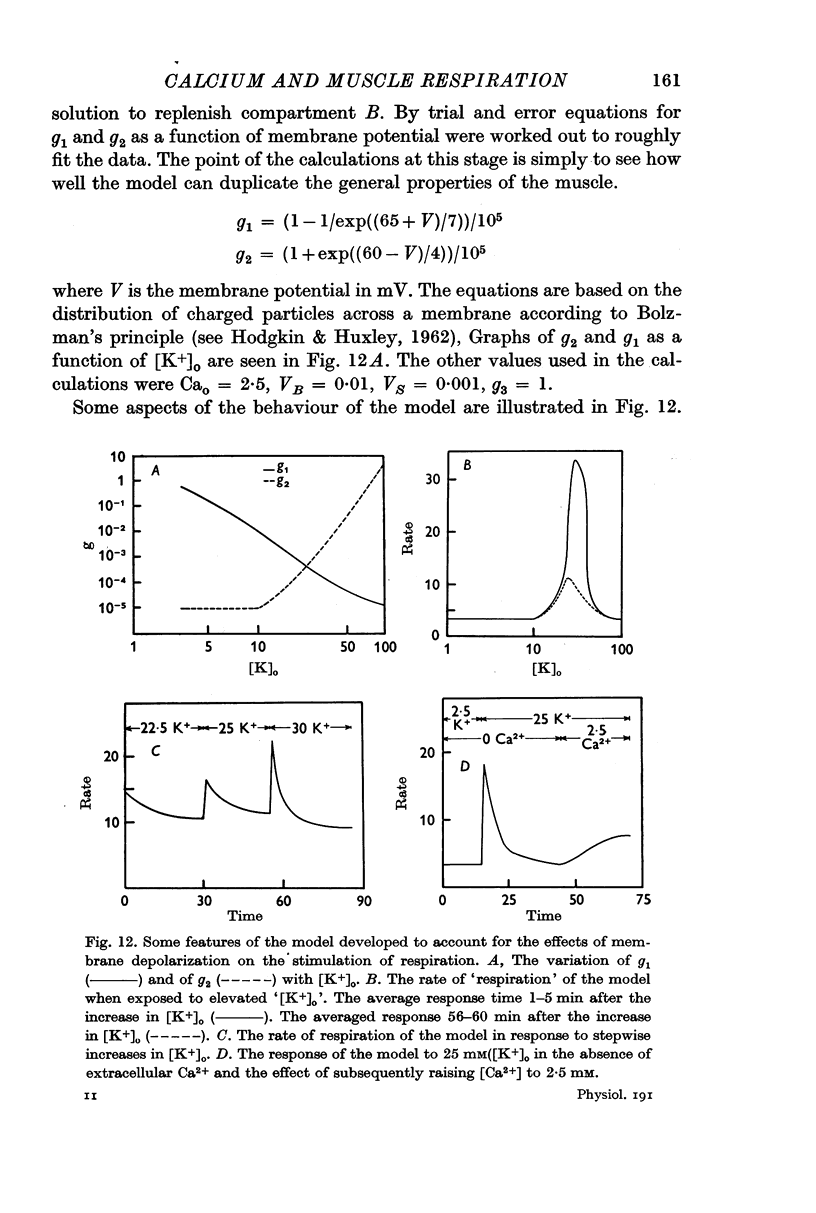
7. A model for the relation between sarcoplasmic calcium and membrane potential is proposed. The model accounts for the time course of the stimulation of respiration and also for much of the available data on potassium contracture. In the model, Ca2+ is released into the sarcoplasm from a store in the cell. With depolarization, the release of Ca2+ from the store is increased, but the rate at which extracellular Ca2+ can replenish the store is decreased. The ability of the longitudinal reticulum to pump Ca2+ from the sarcoplasm does not vary with membrane potential.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DANFORTH W. H., HELMREICH E. REGULATION OF GLYCOLYSIS IN MUSCLE. I. THE CONVERSION OF PHOSPHORYLASE BETA TO PHOSPHORYLASE ALPHA IN FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3133–3138. [PubMed] [Google Scholar]
- Edwards C., Lorković H., Weber A. The effect of the replacement of calcium by strontium on excitation-contraction coupling in frog skeletal muscle. J Physiol. 1966 Oct;186(2):295–306. doi: 10.1113/jphysiol.1966.sp008035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulks J. G., Perry F. A. The relation between external potassium concentration and the relaxation rate of potassium-induced contractures in frog skeletal muscle. J Physiol. 1966 Oct;186(2):243–260. doi: 10.1113/jphysiol.1966.sp008032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V., HOWARTH J. V. The effect of potassium on the resting metabolism of the frog's sartorius. Proc R Soc Lond B Biol Sci. 1957 Aug 24;147(926):21–43. doi: 10.1098/rspb.1957.0034. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAYE L., MOMMAERTS W. F. The role of calcium ions in the acceleration of resting muscle glycolysis by extracellular potassium. J Gen Physiol. 1960 Nov;44:405–413. doi: 10.1085/jgp.44.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE ACTION OF CALCIUM IONS ON POTASSIUM CONTRACTURES OF SINGLE MUSCLE FIBRES. J Physiol. 1963 Oct;168:679–697. doi: 10.1113/jphysiol.1963.sp007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. Mitochondrial ion transport: mechanism and physiological significance. Fed Proc. 1966 May-Jun;25(3):903–911. [PubMed] [Google Scholar]
- SIEKEVITZ P., POTTER V. R. Intramitochondrial regulation of oxidative rate. J Biol Chem. 1953 Mar;201(1):1–13. [PubMed] [Google Scholar]
- SLATER E. C., CLELAND K. W. The effect of calcium on the respiratory and phosphorylative activities of heart-muscle sarcosomes. Biochem J. 1953 Nov;55(4):566–590. doi: 10.1042/bj0550566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. G., Solandt D. Y. The relation of contracture to the increment in the resting heat production of muscle under the influence of potassium. J Physiol. 1938 Sep 16;93(4):305–311. doi: 10.1113/jphysiol.1938.sp003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solandt D. Y. The effect of potassium on the excitability and resting metabolism of frog's muscle. J Physiol. 1936 Feb 8;86(2):162–170. doi: 10.1113/jphysiol.1936.sp003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. G., Glovsky J. The uptake of Ca2+ and Sr2+ by fractions from lobster muscle. Comp Biochem Physiol. 1965 Aug;15(4):547–565. doi: 10.1016/0010-406x(65)90154-4. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. G. The exchange of radioactive cations by somatic and cardiac muscles of the crayfish. Comp Biochem Physiol. 1966 Mar;17(3):1019–1043. doi: 10.1016/0010-406x(66)90140-x. [DOI] [PubMed] [Google Scholar]


