Abstract
Human T-cell lymphotropic virus type 1 (HTLV-1) establishes persistent infection and is associated with lymphoproliferative or neurodegenerative diseases. As a complex retrovirus, HTLV-1 contains typical structural and enzymatic genes, as well as regulatory and accessory genes encoded in the pX region. The early events necessary for HTLV-1 to establish infection in lymphocytes, its primary target cells, remain unresolved. Recent studies have demonstrated the importance of regulatory and accessory gene products in determining this virus-host interaction. Among these, pX open reading frame I, which encodes two proteins, p12I and p27I, is required for establishing persistent infection in vivo and for infection in quiescent primary lymphocytes. In addition, p12I localizes in the endoplasmic reticulum (ER) and cis-Golgi apparatus and associates with a calcium binding protein, calreticulin. We recently reported that p12I expression induces the calcium-responsive T-cell transcription factor, nuclear factor of activated T cells (NFAT), in the presence of phorbol ester activation. Based on these studies, we hypothesize that p12I may modulate calcium release from the ER. Here, we report that p12I expression increases basal cytoplasmic calcium and concurrently diminishes calcium available for release from the ER stores. Overexpression of calreticulin, a calcium buffer protein, blocked p12I-mediated NFAT activation independently of its ability to bind p12I. Chemical inhibition studies using inhibitors of inositol 1,4,5-triphosphate receptor and calcium release-activated calcium channels suggest that inositol 1,4,5-triphosphate receptor in the ER membrane and calcium release-activated calcium channels in the plasma membrane contribute to p12I-mediated NFAT activation. Collectively, our results are the first to demonstrate the role of p12I in elevating cytoplasmic calcium, an antecedent to T-cell activation, and further support the important role of this accessory protein in the early events of HTLV-1 infection.
Human T-lymphotropic virus type 1 (HTLV-1), the etiologic agent of adult T-cell leukemia/lymphoma, immortalizes and eventually transforms primary T lymphocytes after long-term culture (3, 20, 24, 46, 50). Studies designed to investigate the mechanism of these immortalization and transformation events have been primarily focused on Tax, a pleiotropic viral and cellular transcriptional activator encoded by pX open reading frame (ORF) IV (26, 39, 42, 55). However, the details of virus-cell interactions that allow establishment of the viral infection in lymphocytes are unclear.
Recent studies have provided important new data that indicate a role for the highly conserved pX ORF I-encoded protein p12I in the early stages of HTLV-1 infection. ORF I mRNA can be detected in HTLV-1-infected cells derived from adult T-cell leukemia/lymphoma patients, as well as asymptomatic carriers (5, 7-9, 22, 32). Antibodies that recognize p12I and cytotoxic T lymphocytes reactive to peptides representing regions of p12I have been detected in patients and asymptomatic carriers (13, 45). Thus, p12I expression appears to be sustained throughout the natural viral infection. More importantly, it has been shown that selective ablation of ORF I mRNA dramatically decreases the viral infectivity of ACH, an infectious molecular clone of HTLV-1, in a rabbit model of infection (11). Additionally, ORF I expression is required for HTLV-1 infection in quiescent primary lymphocytes (1). These findings indicate the involvement of p12I in the early stage of viral infection in lymphocytes.
The putative structural domains of p12I suggest the possibility that this protein regulates or interacts with cell signaling pathways. The viral protein contains two putative transmembrane regions and four potential SH3 domain binding motifs (PXXP). p12I associates with the H+ vacuolar ATPase (21), as well as the interleukin-2 (IL-2) receptor β- and γ-chains (40). Although it does not appear to dysregulate IL-2 receptor signaling pathways in HTLV-1-immortalized cell lines (10), the transduction of primary lymphocytes with a retrovirus vector expressing p12I causes a modest increase in Stat5 phosphorylation and may reduce IL-2 requirements for T-cell proliferation (43). It was recently demonstrated that the expression of p12I selectively activates nuclear factor of activated T cells (NFAT) (2). As a key transcription factor in T cells, NFAT is dephosphorylated by the phosphatase calcineurin, which is activated by the increase of cytoplasmic calcium. T-cell receptor ligation leads to a series of events that eventually trigger calcium release from endoplasmic reticulum (ER) stores. This transient increase in cytoplasmic calcium itself is insufficient to promote transcriptional activation in T cells (41, 49). However, the depleted ER store further triggers the opening of store-operated calcium channels on the plasma membrane and results in calcium influx, which eventually activates calcineurin and, subsequently, NFAT (23, 33). Dephosphorylated NFAT translocates from the cytoplasm to the nucleus to induce the expression of downstream genes, e.g., that for IL-2 (12, 28, 52). Interestingly, p12I localizes to the ER and associates with an ER luminal protein, calreticulin (14), which is a calcium binding protein that regulates calcium homeostasis in a variety of cell types (27, 38, 54). Taken together, these findings support the hypothesis that p12I regulates calcium signaling to activate T lymphocytes during the early stages of HTLV-1 infection.
Here, we report that p12I expression elevates basal intracellular calcium in transfected Jurkat T cells. Parallel studies of p12I-transfected Jurkat T cells treated with thapsigargin (TG), an inhibitor of Ca2+-ATPase, demonstrated reduced calcium release from the ER stores compared to the control cells. In addition, calreticulin expression blocked p12I-mediated NFAT activation, possibly through its known property as a calcium buffer protein. An inositol 1,4,5-triphosphate (IP3) receptor inhibitor, 2-APB, partially blocked p12I-mediated NFAT activation, implying that p12I enhances calcium release through the IP3 receptor. Furthermore, chemical inhibition of calcium release-activated calcium (CRAC) channels in the plasma membrane demonstrated that extracellular-calcium influx contributes to p12I-mediated NFAT activation. Our data describe a mechanism of NFAT activation mediated by p12I and support the tenet that p12I is a key accessory protein that regulates calcium signals and subsequent lymphocyte activation to facilitate early viral infection. These findings are consistent with previous studies that demonstrated a requirement for HTLV-1 p12I in viral infection both in vivo and in vitro (1, 11).
MATERIALS AND METHODS
Cell lines.
Jurkat T cells (clone E6-1; American Type Culture Collection catalog number TIB-152) were maintained in RPMI 1640 medium (Gibco, Rockville, Md.) supplemented with 15% fetal bovine serum, 100 μg of streptomycin-penicillin/ml, 2 mM l-glutamine, and 10 mM HEPES (Gibco) (complete RPMI [cRPMI]). HeLa-Tat is a human cervical carcinoma cell line, HeLa, which stably expresses human immunodeficiency virus type 1 (HIV-1) Tat protein and was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institutes of Health, from B. Felber and G. Pavlakis (HLtat; catalog number 1293). The 293T cell line is the 293 cell line (catalog number 1573; American Type Culture Collection) which stably expresses the simian virus 40 T antigen (obtained from G. Franchini, National Institutes of Health). Both 293T and HeLa-Tat were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 100 μg of streptomycin-penicillin/ml, and 2 mM l-glutamine as previously described (14).
Plasmids.
The pME-18S and pME-p12 plasmids (40) were provided by G. Franchini (National Cancer Institute, National Institutes of Health). The pME-p12 plasmid expresses the fusion protein of HTLV-1 p12I tagged with the influenza virus hemagglutinin (HA1) tag. The AP-1 luciferase reporter plasmid was purchased from Stratagene (La Jolla, Calif.). The NFAT luciferase construct pNFAT-Luc contains a trimerized human distal IL-2 NFAT site inserted into the minimal IL-2 promoter and was a gift from G. Crabtree (Stanford University). Calreticulin expression plasmids, pcDNA-CRT and VR1012-CRT, were kindly provided by M. Michalak (University of Alberta, Edmonton, Alberta, Canada) and P. Pizzo (University of Padua, Padua, Italy), respectively. The VR1012-CRT plasmid (18) contains the calreticulin sequence tagged with HA1. The p12EGFP plasmid was generated by inserting the p12I sequence into the BglII and BamHI sites in the pEGFP-N1 vector (Clontech, Palo Alto, Calif.). The p12I sequence in p12EGFP was confirmed to be in frame by Sanger sequencing. p12I mutants generated in the pME-18S vector were previously described (14). The β-galactosidase (β-Gal)-encoding plasmid pCMV · SPORT-β-gal was used as a transfection efficiency control and was purchased from Gibco. The pcDNA 3.1(+) plasmid was purchased from Invitrogen (Carlsbad, Calif.).
Jurkat T-cell transfection and luciferase assay.
Unless otherwise indicated, transient transfections were done by electroporation of 107 cells in cRPMI (350 V; 975 μF; Bio-Rad Gene Pulser II) with 0 to 30 μg of pME-p12 in the absence or presence of 0 to 10 μg of pcDNA-CRT expression plasmid as indicated in the figure legends, 10 μg of reporter plasmid (NFAT-Luc or AP-1 Luc), and 1 μg of pCMV · SPORT-β-gal plasmid to analyze the NFAT or AP-1 transcriptional activity. The corresponding control vectors, pME-18S and pcDNA 3.1(+), were used as carrier DNA to equilibrate the total amount of DNA for each transfection. The transfected cells were seeded in six-well plates at a density of 5 × 105/ml and were stimulated with 20 ng of phorbol myristate acetate (PMA; Sigma, St. Louis, Mo.)/ml or with 2 μM ionomycin (Sigma) at 6 h posttransfection. Following 18 h of stimulation, the cells were lysed with Cell Culture Lysis Reagent (Promega, Madison, Wis.), and the cell lysates were analyzed for luciferase activity according to the manufacturer's protocol. Values were normalized for transfection efficiency based on β-Gal activity, which was determined using Lumi-Gal (Lumigen, Southfield, Mich.). Data points were expressed as the mean of triplicate samples from at least two independent experiments. Statistical analysis was performed using Student's t test.
To investigate whether binding between p12I and calreticulin is required for NFAT activation, Jurkat T cells were cotransfected with 30 μg of pME-p12 or the mutant p12I 15-99 or p12I 15-69 in the presence or absence of 2.5 μg of pcDNA-CRT plus reporter plasmids as described above, followed by the measurement of luciferase activity.
Calcium inhibition studies.
To test the contribution of the IP3 receptor in the NFAT activation induced by p12I, the IP3 receptor inhibitor 2-aminoethoxydiphenyl borate (2-APB; Sigma), used at a concentration of 100 μM (37), was added to the transfected cells 6 h posttransfection for 30 min at 37°C followed by PMA stimulation for 18 h, and the cells were further lysed for luciferase assay. To investigate the role of the CRAC channels in the p12I-mediated NFAT activation, the CRAC channel inhibitor SKF 96365 (Calbiochem, San Diego, Calif.) was used at a concentration of 20 μM (29) to treat the transfected cells 20 h posttransfection for 30 min followed by PMA stimulation for 4 h, and the cells were lysed for luciferase assay.
Immunoblot assay.
The expression of p12I and p12I truncation mutants was analyzed by immunoblot assay. Briefly, approximately 1.5 × 106 transfected Jurkat cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris [pH 8.0], 10 mM EDTA, 10 mM NaF, 10 mM Na4P2O7 · H2O, 1% NP-40, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate [SDS], 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and Complete Protease Inhibitor [Roche, Indianapolis, Ind.]), and the cell lysates were cleared by centrifugation. Fifty micrograms of cell lysates were separated by SDS-15% polyacrylamide gel electrophoresis followed by transfer to nitrocellulose membranes. The membranes were blocked with 5% milk for 2 h, incubated with monoclonal anti-HA antibody (clone 16B-12; Covance, Richmond, Calif.) overnight at 4°C, and developed using horseradish peroxidase-labeled secondary antibody and enhanced chemiluminescence (Cell Signaling Technologies, Beverly, Mass.).
Immunoprecipitation assay.
To test the calreticulin binding region in p12I, approximately 2 million 293T cells were transfected with 15 μg of pME-p12, pME-18S, or p12I truncation mutants using Lipofectamine Plus (Invitrogen) and were lysed in RIPA buffer 48 h posttransfection. Cell lysates (1 mg) were precleared with 30 μl of protein A/G-agarose beads (Santa Cruz Biotechnology, Santa Cruz, Calif.) and 3 μl of normal rabbit serum for 6 h, followed by incubation with 1:150-diluted rabbit polyclonal calreticulin antibody (Affinity Bioreagents, Golden, Colo.) overnight. The immune complex mixture was then incubated with 40 μl of protein A/G-agarose beads for 2 h. The beads were washed twice in RIPA buffer and boiled in SDS sample buffer, and the supernatants were collected to analyze the associated p12I and p12I truncation mutants by using an immunoblot assay with anti-HA monoclonal antibody. The expression of full-length p12I and the truncation mutants was tested by immunoblot assay as described above. The endogenous expression of calreticulin was tested by immunoblot assay with rabbit polyclonal calreticulin antibody.
Indirect immunofluorescence assay.
To examine the intracellular expression of transfected calreticulin and p12I, HeLa-Tat cells were seeded into LAB-TAK chamber slides (Nalgene Nuc International, Rochester, N.Y.) and transfected with 4 μg of VR1012-CRT using Lipofectamine Plus. The cells were fixed for 15 min in 4% paraformaldehyde at 24 h posttransfection, followed by incubation with mouse anti-HA monoclonal antibody in antibody dilution buffer (10 mM NaPO4, 0.5 M NaCl, 0.5% Triton X-100, 2% bovine serum albumin, 5% normal goat serum) for 1 h at room temperature. After being washed three times, the cells were incubated with indocarbo cyanine-3-labeled anti-mouse antibody in antibody dilution buffer for 1 h before final washing and mounting in glycerol. The cell nuclei were stained with bisbenzimide H 33258 (Calbiochem). Fluorescence microscopy was performed using an Axioplan2 fluorescent microscope (Carl Zeiss Optical, Chester, Va.), and images were collected using a SPOT camera (model 1.4.0; Diagnostic Instruments Inc., Sterling Heights, Mich.) with Adobe Photoshop 5.0 software.
Intracellular-calcium measurement.
To measure the basal intracellular-calcium concentration, intracellular-store calcium release, and capacitative calcium entry, indo-1 acetoxymethyl ester (indo-1/AM) and fura-2/AM were purchased from Molecular Probes (Eugene, Oreg.) and used in flow cytometry and calcium measurement experiments, respectively.
Flow cytometry analysis.
For the measurement of the baseline intracellular-calcium concentration, 107 Jurkat cells were electroporated in cRPMI with 30 μg of p12EGFP or pEGFP-N1 plasmid. At 6 h posttransfection, cells from four transfection experiments were pooled together and live cells were separated by Ficoll purification. At 24 h posttransfection, the cells were resuspended at a concentration of 5 × 106 per ml in Hanks' balanced salt solution (HBSS) buffer (Gibco) in the presence or absence of 1 mM Ca2+, followed by staining with 6 μM indo-1/AM at 37°C for 30 min. The cells were analyzed on an ELITE ESP flow cytometer (Beckman Coulter, Miami, Fla.) with a UV laser for indo-1 excitation. Approximately 3.0 × 104 cells were analyzed, and the calcium concentration in green fluorescent protein (GFP)-positive cells was determined from the ratio of light emitted from calcium-bound dye (wavelength, 405 nm) to that from unbound indo-1 (wavelength, 495 nm). The mean ratios were calculated over time by using standard ELITE analysis software. Statistical analysis was performed by Student's t test.
Measurement of calcium release from the ER store.
For the measurement of intracellular-store calcium release and calcium entry, Jurkat cells were transfected with 30 μg of p12EGFP or pEGFP-N1 and the live cells were purified as described above. At 24 h posttransfection, cells pooled from six transfection experiments were resuspended in cRPMI, and the cells with positive GFP fluorescence were sorted on an ELITE ESP flow cytometer with a 488-nm-wavelength laser and collected in cRPMI. At 48 h posttransfection, the sorted cells were loaded with 2 μM fura-2/AM in HBSS buffer containing 1 mM Ca2+ at room temperature for 40 min, washed once, and left in the same buffer for 90 min for fura-2/AM hydrolysis. The cells were then seeded at a density of 2 × 105 per well in HBSS buffer containing 1 mM Ca2+ into LAB-TAK chamber slides coated with CELL-TAK (BD Biosciences, Bedford, Mass.) according to the manufacturer's protocol. The cells were alternately excited at 340 and 380 nm by a motor-driven beam chopper (Photon Technology International, Monmouth Junction, N.J.) connected to a Diaphot-TMD inverted microscope (Nikon Corporation, Tokyo, Japan). Fluorescence emission at 510 nm was captured by a microscope photometer (Photon Technology International). The cytoplasmic calcium concentration was represented by the ratio of emitted fluorescence when the cells were excited at 340 nm to that when the cells were excited at 380 nm. The data were analyzed by using commercial software (FeliX; Photon Technology International). For each experiment, the emitted 340- to 380-nm fluorescence ratios from approximately 10 to 20 cells were collected and analyzed. After measurement of the baseline calcium concentration in HBSS buffer containing 1 mM Ca2+, the bathing solution was replaced with HBSS buffer without calcium by adding 2 mM EGTA. To measure the intracellular-store calcium release, an ER Ca2+-ATPase inhibitor, TG, was used at a concentration of 500 nM to trigger ER calcium depletion following determination of the baseline calcium concentration in the bathing solution. Finally, extracellular-calcium entry was measured by restoring the calcium concentration to 2 and 5 mM in the bathing solution.
To test the effect of the inhibitor of IP3 receptor on calcium release from ER stores triggered by T-cell receptor ligation, Jurkat T cells were treated with 100 μM 2-APB followed by stimulation with 8 μg of monoclonal anti-human CD3 antibody (Clone HIT3a; BD Pharmingen, San Diego, Calif.)/ml or left untreated. The resultant changes in calcium concentration were recorded as described above. To test the role of the inhibitor of CRAC channels on the phytohemagglutinin (PHA)-induced calcium influx, 2 μg of PHA/ml was added to the Jurkat T cells, followed by the addition of SKF 96365. The calcium concentration was measured as described above.
RESULTS
p12I expression increases basal intracellular calcium in Jurkat T cells.
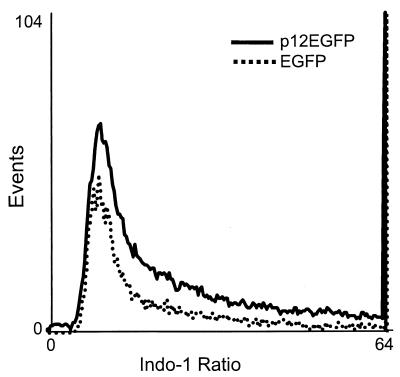
It was reported that p12I expression in Jurkat T cells activates NFAT, a major T-cell transcription factor, and that this activation is dependent on cytoplasmic Ca2+ (2). To investigate potential mechanisms that mediate NFAT activation, we tested the effect of p12I expression on basal [Ca2+]i (the intracellular-calcium concentration). Jurkat T cells transiently transfected with the p12EGFP or pEGFP-N1 plasmid were loaded with indo-1/AM, and the ratio of indo-1 emissions at 405 and 495 nm on GFP-positive cells was measured by a flow cytometer as an indication of the [Ca2+]i. Jurkat T cells expressing p12EGFP displayed an elevated indo-1 ratio compared to EGFP-expressing cells in medium containing 1 mM Ca2+ (P < 0.05) (Fig. 1). The mean ratio in p12EGFP-expressing cells (10.27 ± 0.23; mean ± standard error of the mean [SEM]; n = 3) was higher than that in the vector control pEGFP-N1-transfected cells (9.13 ± 0.58; mean ± SEM; n = 3). These data indicated a modest, but consistent, increase in intracellular calcium in p12I-expressing cells. This finding is consistent with other reports (33, 34) which demonstrated that NFAT as a transcription factor is responsive to small and oscillating increases in cytoplasmic calcium. However, the mean ratios in p12EGFP- and pEGFP-N1-expressing cells in Ca2+-free media were not significantly different (data not shown). Therefore, p12I expression increased the [Ca2+]i and was dependent on extracellular Ca2+, suggesting the involvement of extracellular Ca2+ influx.
FIG. 1.
p12I increases the basal intracellular calcium in transfected Jurkat T cells. Jurkat T cells transiently transfected with p12EGFP or pEGFP-N1 plasmid were stained with indo-1/AM at 24 h posttransfection and the ratio of indo-1 emissions at 405 and 495 nm on GFP-positive cells was measured by an ELITE ESP flow cytometer. The mean ratio (10.27 ± 0.23; mean ± SEM; n = 3) in p12EGFP-transfected cells was slightly higher than the ratio in vector control pEGFP-N1-transfected cells (9.13 ± 0.58; mean ± SEM; n = 3). These data are representative of three independent experiments.
p12I reduces the amount of calcium released from ER stores.
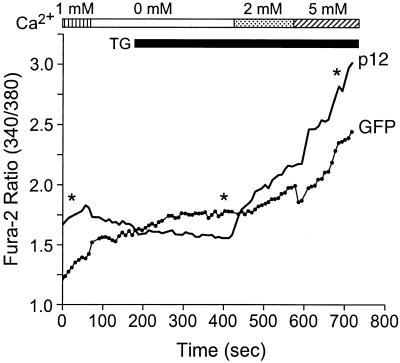
As an approach to measure the amount of Ca2+ capable of being released from the ER Ca2+ store in cells expressing p12I, TG was used to block ER Ca2+ uptake through the Ca2+- ATPase pump. In the presence of the drug, Ca2+ leaking from the ER accumulates in the cytosol and the [Ca2+]i can be used as an indirect measure of the ER Ca2+ store (19, 48). The addition of TG to the vector control cells transfected with pEGFP-N1 in Ca2+-free medium led to an increase in the [Ca2+]i, reflecting the release of Ca2+ from the ER. In transiently p12EGFP-transfected cells, the amplitude of the TG-induced Ca2+ release was markedly reduced (P < 0.05) (Fig. 2), suggesting that the ER store Ca2+ content was reduced due to the Ca2+ release induced by p12I expression. The addition of Ca2+ back into the medium following the TG-induced response elicited a higher (P < 0.05) Ca2+ entry in p12EGFP-transfected cells than in vector control-transfected cells (Fig. 2), implying that the increased [Ca2+]i is due to capacitative Ca2+ entry from the calcium channel at the plasma membrane responding to the lower ER Ca2+ content.
FIG. 2.
p12I diminishes calcium release from ER stores and subsequently enhances extracellular-calcium entry. Jurkat T cells transiently transfected with p12EGFP or pEGFP-N1 were loaded with fura-2/AM 48 h posttransfection, and the basal [Ca2+]i, calcium release following TG addition, and calcium entry were measured as described in Materials and Methods. The data points along the curves are means of four experiments. Statistical significances were analyzed by Student's t test. ∗, P < 0.05. Solid line, p12EGFP-transfected Jurkat T cells; dotted line, vector-transfected Jurkat T cells. Note that Jurkat T cells expressing p12EGFP have relatively higher basal [Ca2+]i and calcium entry but lower calcium release following TG addition than the control cells expressing EGFP.
Importantly, these data, collected using a completely different system to measure the cytoplasmic calcium, confirmed that p12I expression elevates basal intracellular calcium in Jurkat T cells.
Calreticulin blocks NFAT activation induced by p12I.
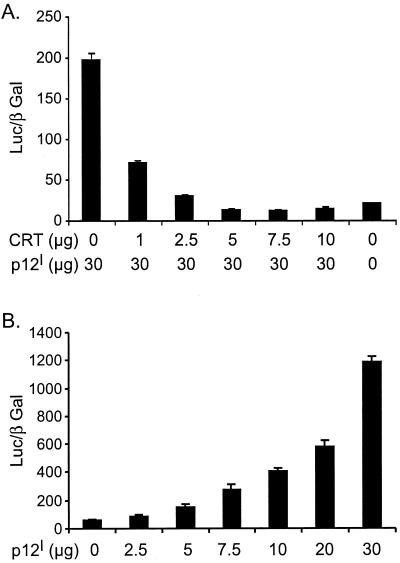
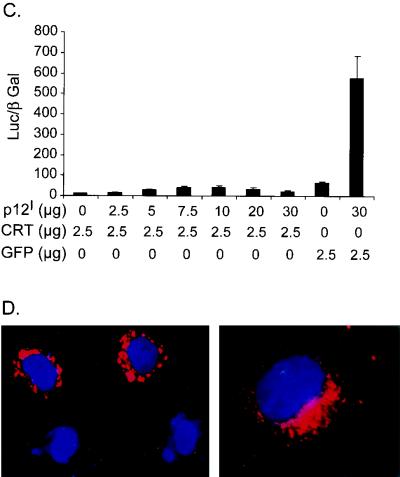
Calreticulin, a primary calcium-buffering protein, binds p12I in the ER (14). Overexpression of calreticulin increases the whole-cell calcium content and reduces capacitative calcium entry (38, 54). To identify the effect of calreticulin on p12I-mediated NFAT activation, Jurkat T cells were transfected with 30 μg of pME-p12 in combination with increasing doses of pcDNA-CRT. Calreticulin expression inhibited the NFAT activation induced by p12I in a dose-dependent manner (Fig. 3A). While p12I expression alone dose-dependently activated NFAT transcriptional activity (Fig. 3B), 2.5 μg of pcDNA-CRT transfection abolished the dose-dependent activation of NFAT by p12I (Fig. 3C). A control protein, EGFP, did not affect the NFAT activation mediated by p12I. The expression of p12I remained unchanged as detected in immunoblot assays (data not shown). The calreticulin block of p12I-mediated NFAT activation could be explained if calreticulin affected the p12I function by direct binding or if calreticulin reduced calcium release from the ER due to its calcium-buffering capability. We also confirmed that the expressed calreticulin displayed a reticular pattern in the perinuclear region of transfected HeLa-Tat cells, which was similar to p12I localization corresponding to the ER (Fig. 3D).
FIG. 3.
Calreticulin (CRT) expression blocks p12I-mediated NFAT activation. (A) Calreticulin blocks NFAT activation induced by p12I in a dose-dependent manner. Jurkat T cells were transfected with 30 μg of pME-p12 plus increasing amounts of pcDNA-CRT plasmid, as well as NFAT reporter plasmid, and the luciferase activities were analyzed 18 h post-PMA treatment using the method described in Materials and Methods. (B) p12I dose-dependently activates NFAT in transfected Jurkat T cells. (C) Transfection with 2.5 μg of calreticulin plasmid abolishes the dose-dependent activation of NFAT induced by p12I. The GFP vector control plasmid did not affect p12I-mediated NFAT activation. The values are the means (plus SEM) of triplicate samples of at least duplicate experiments. (D) Calreticulin expression in HeLa-Tat cells is in the perinuclear region (red), consistent with its known ER localization property (27).
Binding between p12I and calreticulin is not required for calreticulin inhibition of p12I-mediated NFAT activation.
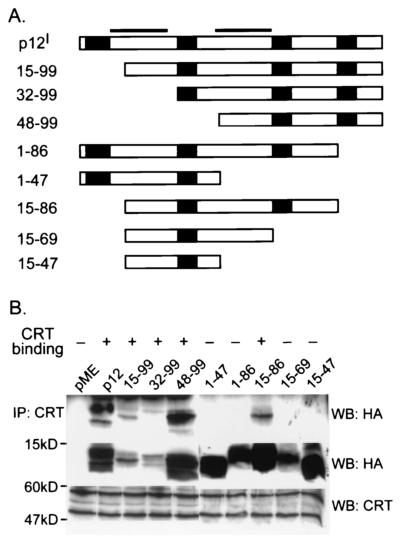
To further clarify the blocking effect of calreticulin on p12I-mediated NFAT activation, we performed coimmunoprecipitation assays to map the calreticulin binding region in p12I (Fig. 4). Three N-terminal-truncation mutants, p12I 15-99, p12I 32-99, and p12I 48-99, were precipitated by polyclonal anti-calreticulin antibody, indicating that the calreticulin binding site is in the C-terminal half of p12I. The C-terminal-truncation mutant p12I 15-86 also coprecipitated with calreticulin. However, p12I 15-69, p12I 15-47, and p12I 1-47 did not associate with calreticulin. Collectively, these data indicate that the region between amino acids (aa) 69 and 86 of p12I is a binding region between the viral protein and calreticulin. Mutant p12I 1-86 did not bind to calreticulin in the immunoprecipitation experiment. This could be due to the presence of an inhibitory region (aa 1 to 15) in the N terminus of p12I which could prevent the binding of this protein with calreticulin.
FIG. 4.
Calreticulin (CRT) binds the region from aa 69 to 86 of p12I. (A) Schematic representation of full-length p12I and a series of p12I truncation mutants. Soild bars, transmembrane regions. Solid boxes, SH3 binding domains (PXXP motif). (B) 293T cells transfected with pME-p12 and p12I truncation mutants were lysed, and the cell lysates were precipitated with polyclonal calreticulin antibody followed by immunoblot assay to map the region in p12I associated with calreticulin (top). An aliquot of cell lysates (50 μg) was used to test the expression of p12I and the truncation mutants by immunoblot assay (middle). The same blot was stripped, and the expression of calreticulin was tested by immunoblot assay (bottom). The larger band (∼60 kDa) in the bottom blot represents native calreticulin. A smaller isoform of calreticulin (∼50 kDa) was also detected.
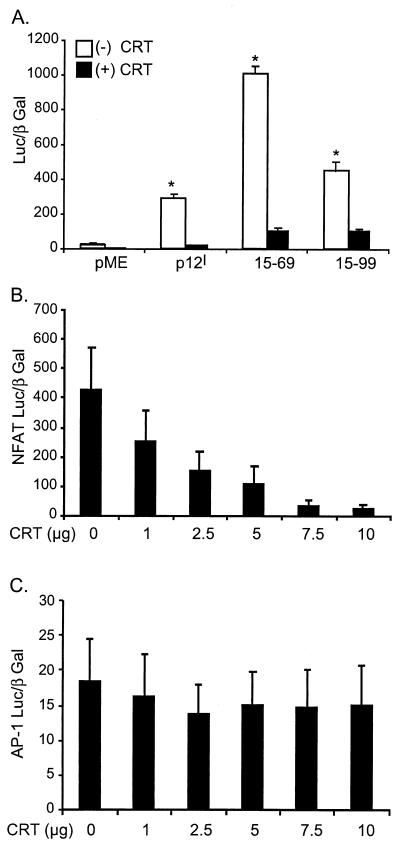
To test if the association between calreticulin and p12I is correlated with the known buffering capacity of calreticulin, Jurkat T cells were cotransfected with or without 2.5 μg of pcDNA-CRT in combination with the mutant p12I 15-99, which bound to calreticulin, or the mutant p12I 15-69, which did not bind to calreticulin. NFAT transcriptional activities were analyzed to assess the influence of calreticulin on p12I function. Both mutants reproducibly elicited hyperactivated NFAT luciferase activities, indicating that binding does not correlate with NFAT activation mediated by p12I mutants. Interestingly, both mutants displayed significantly reduced NFAT luciferase activities (Fig. 5A) in the presence of calreticulin, implying that binding does not correlate with the inhibitory effect of calreticulin. In addition, calreticulin alone inhibited the NFAT luciferase activity induced by 2 μM ionomycin in a dose-dependent manner (Fig. 5B), while the transcriptional activity of a non-calcium-responsive transcription factor, AP-1, was not affected (Fig. 5C). Thus, the inhibitory effect of calreticulin on p12I-mediated NFAT activation is likely indirect through its known Ca2+-buffering capability, which would overcome ER calcium release induced by p12I.
FIG. 5.
Calreticulin inhibition of p12I-mediated NFAT activation is independent of its association with p12I. (A) Calreticulin blocks the NFAT activation mediated by both a calreticulin binding mutant, 15-99, and a non-calreticulin binding mutant, 15-69. Thirty micrograms of pME-p12, p12I 15-99, or p12I 15-69 was used to transfect Jurkat T cells in the absence [(−) CRT] or presence [(+) CRT]of 2.5 μg of pcDNA-CRT, and the NFAT luciferase activities were analyzed 18 h post-PMA stimulation to test the effect of calreticulin on the NFAT activation mediated by both mutants. Statistical significances were analyzed by Student's t test. ∗, P < 0.05. (B) Calreticulin dose-dependently inhibits the NFAT activation induced by ionomycin treatment. Jurkat T cells were transfected with increasing amounts of pcDNA-CRT plus 10 μg of NFAT-Luc, and the NFAT luciferase activities were analyzed 18 h post-ionomycin treatment. (C) Calreticulin does not affect AP-1-responsive reporter gene. Jurkat T cells were transfected with increasing amounts of pcDNA-CRT plus 10 μg of AP-1 Luc, and the luciferase activities were analyzed 18 h post-ionomycin treatment. The values (arbitrary light units) are the means (plus SEM) of triplicate samples of at least duplicate experiments.
IP3 receptor contributes to p12I-mediated NFAT activation.
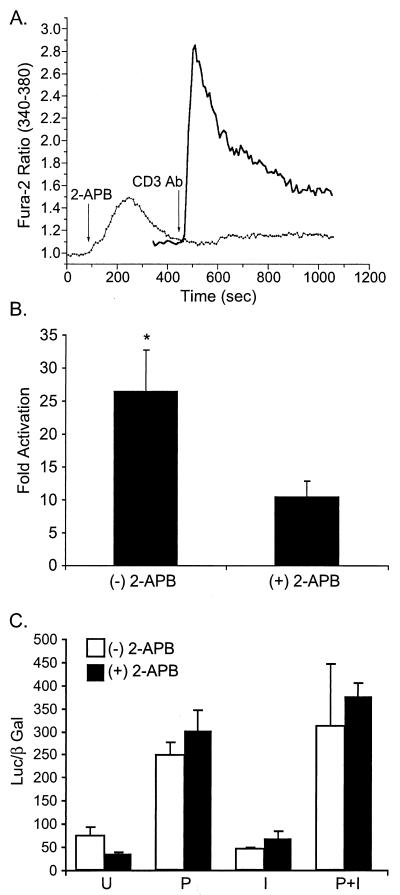
Following engagement of the T-cell receptor, a series of activation events lead to IP3 elevation. As a major regulator in calcium-related signaling in T cells, IP3 binds to its receptor, an intracellular-calcium channel on the ER, to induce the calcium release that further triggers prolonged calcium influx through the store-operated Ca2+ channels in the plasma membrane (23, 33). The mechanism for store depletion-triggered capacitative calcium entry remains unclear. To test if IP3 receptor plays a major role in the NFAT activation induced by p12I, pME-p12-transfected Jurkat T cells were treated with an IP3 receptor inhibitor, 2-APB, before the stimulation of PMA addition, and the NFAT luciferase activities were analyzed. The inhibitor 2-APB (35) blocked intracellular-calcium release elicited by CD3 antibody (Fig. 6A). 2-APB also blocked p12I-mediated NFAT activation (Fig. 6B). However, the activity of a non-calcium-responsive transcription factor, AP-1, remained unchanged in the presence of 2-APB (Fig. 6C), suggesting that the IP3 receptor is involved in the NFAT activation induced by p12I.
FIG. 6.
Inhibition of IP3 receptor reduces p12I-mediated NFAT activation. (A) IP3 receptor inhibitor, 2-APB, blocks intracellular-calcium increase following the CD3 antibody stimulation of Jurkat T cells. Solid line, Jurkat T cells stimulated with the CD3 antibody; dotted line, Jurkat T cells pretreated with 100 μM 2-APB 5 min before CD3 antibody stimulation. (B) 2-APB treatment 30 min before the addition of PMA inhibits the NFAT activation induced by p12I. (C) 2-APB treatment [(+) 2-APB] did not significantly affect AP-1 Luc activity. P, PMA; I, ionomycin; U, unstimulated. The values represent the data means (plus SEM) collected from two samples of three independent experiments. Statistical significances were analyzed by Student's t test. ∗, P < 0.05.
CRAC channel inhibitor partially blocks p12I-mediated NFAT activation.
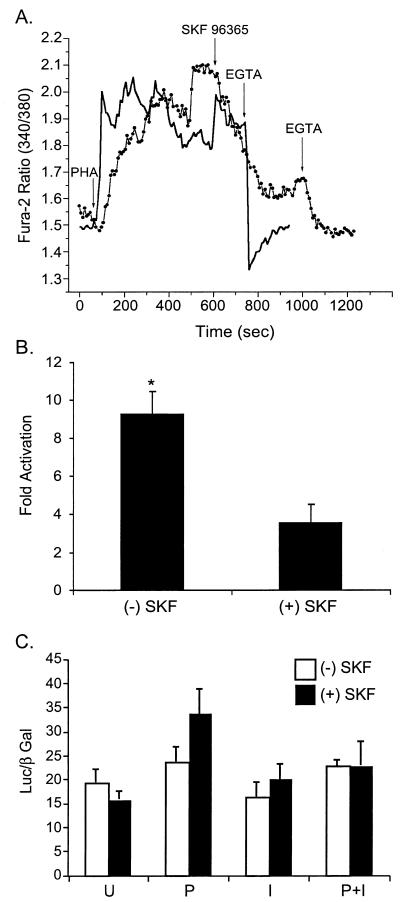
Activation of T-cell receptor by antigens or mitogens, such as PHA, leads to a biphasic increase in the [Ca2+]i resulting from an initial IP3-dependent release of calcium from the ER store followed by an influx of calcium across the plasma membrane. Recent studies suggest that calcium influx across the plasma membrane is carried out by store-operated calcium channels (56), whose biophysical properties are very similar to those of the calcium release-activated calcium current of mast cells (25). The fact that the basal [Ca2+]i increase in p12I-transfected cells was observed only in 1 mM Ca2+ solution suggests the importance of the Ca2+ influx in p12I-mediated NFAT activation. Thus, a widely used inhibitor of CRAC channels, SKF 96365, was used to treat p12I-transfected Jurkat T cells before PMA stimulation to test the role of CRAC channels in the NFAT activation induced by p12I. A concentration of 20 μM SKF 96365 that blocked the Ca2+ influx induced by PHA (Fig. 7A) reduced the p12I-mediated NFAT activation by half (Fig. 7B). However, the transcriptional activity of AP-1 remained unchanged in the presence of SKF 96365 (Fig. 7C), indicating that the Ca2+ influx from the plasma membrane contributes to the NFAT activation induced by p12I.
FIG. 7.
Inhibition of calcium release-activated calcium channel partially blocks p12I-mediated NFAT activation. (A) CRAC channel inhibitor, SKF 96365, reduces the length of the plateau phase induced by PHA stimulation. Solid line, Jurkat T cells stimulated with 2 μg of PHA/ml followed by EGTA addition. Dotted line, Jurkat T cells stimulated with PHA followed by the addition of SKF 96365 and, subsequently, EGTA. (B) Inhibition of CRAC channels partially blocks the NFAT activation mediated by p12I. SKF, SKF 96365; (+), present; (−), absent. The values represent the means (plus SEM) of triplicate samples of two independent experiments. Statistical significances were analyzed by Student's t test. ∗, P < 0.05. (C) SKF 96365 treatment did not affect AP-1 Luc activities. P, PMA, I, ionomycin, U, unstimulated.
DISCUSSION
In this study, we report that the expression of p12I increases the [Ca2+]i and that p12I expression reduces calcium content in the ER stores, which is consistent with the previous finding that p12I can replace a low concentration of TG to cause NFAT activation (2). These results suggest that an increase in the ER Ca2+ permeability mediates the increase of the [Ca2+]i in p12I-expressing cells and a decrease in the ER calcium content. Further study is required to test this possibility. Our data demonstrated that the IP3 receptor inhibitor, 2-APB, blocked the effect of NFAT activation induced by p12I, indicating the contribution of IP3 receptor in p12I-mediated NFAT activation. This observation could be explained if p12I facilitates Ca2+ release via the ER Ca2+ channel (IP3 receptor). However, we cannot exclude the possibility that p12I interacts with IP3 receptor or itself forms a channel. A recent study suggested that in addition to IP3 receptor, 2-APB may also inhibit the function of CRAC channels in the plasma membrane (6). Our study also detected a significantly higher calcium entry following TG addition in p12I-expressed cells and that the CRAC channel inhibitor, SKF 96365, reduced the NFAT activation induced by p12I by half, indicating the importance of Ca2+ influx on p12I-mediated NFAT activation. Due to the location of this protein, the higher calcium entry is possibly secondary to the reduced calcium content in the ER store. Similar mechanisms are also used by a cellular protein, Bcl-2, to regulate intracellular-calcium homeostasis. While localized in both the ER and mitochondria, Bcl-2 enhances the Ca2+ permeability of the ER membranes and subsequently reduces the ER store content. The reduced Ca2+ content in ER stores further triggers the increased extracellular Ca2+ entry in Bcl-2-expressing cells (19). The modulation of Ca2+ homeostasis is also believed to be a key determinant for the antiapoptotic function of Bcl-2 (44). Therefore, it is reasonable to speculate that p12I, a HTLV-1 accessory protein, could mimic the function of Bcl-2 to regulate calcium homeostasis in host cells to facilitate viral infection.
As an ER-resident and calcium binding protein, calreticulin serves as a calcium buffer. The expression of calreticulin increases the whole-cell calcium content and reduces calcium entry (38). Additionally, calreticulin expression decreases ER calcium release (54). When calreticulin and p12I were coexpressed in our study, calreticulin blocked p12I activation in a dose-dependent manner. Using the calreticulin binding and nonbinding p12I mutants, we found that the binding between p12I and calreticulin did not correlate with NFAT activation mediated by p12I. The inhibitory effect of calreticulin on p12I-mediated NFAT activation is likely indirect, by preventing effective calcium release from the ER or by reducing calcium entry through the plasma membrane. This tenet is supported by the finding that calreticulin inhibited the NFAT activation induced by ionomycin in a dose-dependent manner. Taken together, these results show that the lower ER Ca2+ content, most likely resulting from increasing ER membrane permeability induced by p12I, can be blocked by the expression of the calcium buffer protein calreticulin. However, we could not exclude the possibility of additional cellular proteins contributing to NFAT activation mediated by p12I.
The elevation of intracellular free Ca2+ is an essential signal for T-cell activation by antigens and the other stimuli that cross-link the T-cell receptor. Short-term Ca2+ increase helps to stabilize contacts between T cells and antigen-presenting cells through cytoskeleton reorganization. Long-term Ca2+ signals (over periods of hours) increase the efficiency and specificity of gene activation, such as NFAT translocation. Dolmetsch et al. and Lewis showed that the low plateau phase generated by ionomycin plus PMA treatment activates NFAT, a transcription factor responsive to low-amplitude [Ca2+]i oscillations (15, 33). The modest increase of the [Ca2+]i and the subsequent NFAT activation mediated by p12I are independent of T-cell receptor activation. This finding raises the intriguing possibility that p12I expression in HTLV-1-infected T lymphocytes increases the activation of these cells in response to weak stimuli, which would normally not activate the T-cell receptor signal pathway. Activation of these cells likely triggers cell division, which would enhance HTLV-1 proviral DNA integration and the establishment of persistent infection. In addition to promoting T-cell activation, Ca2+ signaling and NFAT activation could also contribute to retrovirus replication. Interestingly, Kinoshita and colleagues (31) recently reported that the expression of NFAT in primary peripheral T lymphocytes induced a highly permissive state to overcome the blockade at reverse transcription and permitted replication of a similar retrovirus, HIV, in primary CD4+ T cells.
In addition to HTLV-1, other viruses encode proteins regulating Ca2+-related signals by analogous or different mechanisms in T lymphocytes or other cell types. Nef, a regulatory protein in HIV, is functionally and structurally similar to p12I and synergistically activates NFAT with the Ras/MAPK pathway (36). Though the underlying mechanism to regulate the Ca2+ signals may be different, the effects of these two proteins on T-cell activation and viral infection are strikingly similar. Hepatitis C virus core protein, an ER-localized viral protein, activates the transcription of the IL-2 promoter in Jurkat cells by activating NFAT (4). In addition, two other ER-localized viral proteins, rotavirus nonstructural protein NSP4 (16, 48) and coxsackievirus protein 2B (51), increase intracellular-calcium release in fibroblasts by enhancing the ER membrane permeability and plasma membrane permeability, respectively. Therefore, the modulation of Ca2+ signal to activate viral target cells is a common and potent mechanism for viruses to facilitate infection.
In future studies, it will be important to test if p12I is incorporated in viral particles or if the protein is selectively expressed during early stages of viral infection. Wu and Marsh (53) recently reported that HIV infection leads to selective transcription of the nef and tat genes before integration. This preintegration transcription in quiescent cells leads to increased T-cell activation and viral replication. Like p12I, Nef contains an SH3 binding motif that facilitates the functional interaction of this protein with multiple cellular signal proteins (47) and is essential for efficient viral infectivity in vivo (17, 30).
In summary, p12I increases intracellular Ca2+, likely by sustained release of calcium from ER stores, and enhances extracellular-calcium entry to induce NFAT activation in T cells. These data are consistent with the role of pX ORF I gene products in viral infection in a rabbit model and in quiescent human T lymphocytes. Thus, p12I, a highly conserved viral protein, appears to be critical during the early stage of HTLV-1 infection in T cells.
Acknowledgments
This work was supported by National Institute of Health grants RR-14324, AI-01474, and CA-92009 awarded to M. D. Lairmore and CA-70529 from the National Cancer Institute, awarded through the Ohio State University Comprehensive Cancer Center. B. Albrecht was sponsored by a Boehringer Ingelheim Predoctoral Fellowship.
We thank A. Oberyszyn and other members of the Analytical Cytometry Laboratory of the OSU Comprehensive Cancer Center. We also thank T. Vojt for the preparation of figures and G. Franchini, G. Crabtree, M. Mickalak, and P. Pizzo for sharing valuable reagents.
REFERENCES
- 1.Albrecht, B., N. D. Collins, M. T. Burniston, J. W. Nisbet, L. Ratner, P. L. Green, and M. D. Lairmore. 2000. Human T-lymphotropic virus type 1 open reading frame I p12I is required for efficient viral infectivity in primary lymphocytes. J. Virol. 74:9828-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht, B., C. D. D'Souza, W. Ding, S. Tridandapani, K. M. Coggeshall, and M. D. Lairmore. 2002. Activation of nuclear factor of activated T cells (NFAT) by human T-lymphotropic virus type 1 (HTLV-1) accessory protein p12. J. Virol. 76:3493-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangham, C. R. 2000. HTLV-1 infections. J. Clin. Pathol. 53:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergqvist, A., and C. M. Rice. 2001. Transcriptional activation of the interleukin-2 promoter by hepatitis C virus core protein. J. Virol. 75:772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berneman, Z. N., R. B. Gartenhaus, M. S. Reitz, W. A. Blattner, A. Manns, B. Hanchard, O. Ikehara, R. C. Gallo, and M. E. Klotman. 1992. Expression of alternatively spliced human T-lymphotropic virus type 1 pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc. Natl. Acad. Sci. USA 89:3005-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broad, L. M., F. J. Braun, J. P. Lievremont, G. S. Bird, T. Kurosaki, and J. W. Putney, Jr. 2001. Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J. Biol. Chem. 276:15945-15952. [DOI] [PubMed] [Google Scholar]
- 7.Cereseto, A., Z. Berneman, I. Koralnik, J. Vaughn, G. Franchini, and M. E. Klotman. 1997. Differential expression of alternatively spliced pX mRNAs in HTLV-I-infected cell lines. Leukemia 11:866-870. [DOI] [PubMed] [Google Scholar]
- 8.Ciminale, V., D. D'Agostino, L. Zotti, G. Franchini, B. K. Felber, and L. Chieco-Bianchi. 1996. Expression and characterization of proteins produced by mRNAs spliced into the X region of the human T-cell leukemia/lymphotropic virus type II. Virology 209:445-456. [DOI] [PubMed] [Google Scholar]
- 9.Ciminale, V., G. N. Pavlakis, D. Derse, C. P. Cunningham, and B. K. Felber. 1992. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J. Virol. 66:1737-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, N. D., C. D'Souza, B. Albrecht, M. D. Robek, L. Ratner, W. Ding, P. L. Green, and M. D. Lairmore. 1999. Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T-cell lymphotropic virus type 1 is independent of open reading frame I expression. J. Virol. 73:9642-9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins, N. D., G. C. Newbound, B. Albrecht, J. L. Beard, L. Ratner, and M. D. Lairmore. 1998. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood 91:4701-4707. [PubMed] [Google Scholar]
- 12.Crabtree, G. R. 2000. Calcium, calcineurin and the control of transcription. J. Biol. Chem. 276:2313-2316. [DOI] [PubMed] [Google Scholar]
- 13.Dekaban, G. A., A. A. Peters, J. C. Mulloy, J. M. Johnson, R. Trovato, E. Rivadeneira, and G. Franchini. 2000. The HTLV-I ORF I protein is recognized by serum antibodies from naturally infected humans and experimentally infected rabbits. Virology 274:86-93. [DOI] [PubMed] [Google Scholar]
- 14.Ding, W., B. Albrecht, R. Luo, W. Zhang, J. R. Stanley, G. C. Newbound, and M. D. Lairmore. 2001. Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12I: association with calreticulin and calnexin. J. Virol. 75:7672-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolmetsch, R. E., R. S. Lewis, C. C. Goodnow, and J. I. Healy. 1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386:855-858. [DOI] [PubMed] [Google Scholar]
- 16.Dong, Y., C. Q. Zeng, J. M. Ball, M. K. Estes, and A. P. Morris. 1997. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc. Natl. Acad. Sci. USA 94:3960-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du, Z. J., S. M. Lang, V. G. Sasseville, A. A. Lackner, P. O. Ilyinskii, M. D. Daniel, J. U. Jung, and R. C. Desrosiers. 1995. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665-674. [DOI] [PubMed] [Google Scholar]
- 18.Fasolato, C., P. Pizzo, and T. Pozzan. 1998. Delayed activation of the store-operated calcium current induced by calreticulin overexpression in RBL-1 cells. Mol. Biol. Cell 9:1513-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foyouzi-Youssefi, R., S. Arnaudeau, C. Borner, W. L. Kelley, J. Tschopp, D. P. Lew, N. Demaurex, and K. H. Krause. 2000. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 97:5723-5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franchini, G. 1995. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood 86:3619-3639. [PubMed] [Google Scholar]
- 21.Franchini, G., J. C. Mulloy, I. J. Koralnik, A. Lo Monico, J. J. Sparkowski, T. Andresson, D. J. Goldstein, and R. Schlegel. 1993. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J. Virol. 67:7701-7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furukawa, K., K. Furukawa, and H. Shiku. 1991. Alternatively spliced mRNA of the pX region of human T lymphotropic virus type I proviral genome. FEBS Lett. 295:141-145. [DOI] [PubMed] [Google Scholar]
- 23.Guse, A. H. 1998. Ca2+ signaling in T-lymphocytes. Crit. Rev. Immunol. 18:419-448. [DOI] [PubMed] [Google Scholar]
- 24.Hollsberg, P. 1999. Mechanisms of T-cell activation by human T-cell lymphotropic virus type I. Microbiol. Mol. Biol. Rev. 63:308-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoth, M., and R. Penner. 1992. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355:353-356. [DOI] [PubMed] [Google Scholar]
- 26.Jeang, K. T. 2001. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-κB. Cytokine Growth Factor Rev. 12:207-217. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, S., M. Michalak, M. Opas, and P. Eggleton. 2001. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 11:122-129. [DOI] [PubMed] [Google Scholar]
- 28.Kane, L. P., J. Lin, and A. Weiss. 2000. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12:242-249. [DOI] [PubMed] [Google Scholar]
- 29.Kanno, T., and U. Siebenlist. 1996. Activation of nuclear factor-κB via T cell receptor requires a Raf kinase and Ca2+ influx. Functional synergy between Raf and calcineurin. J. Immunol. 157:5277-5283. [PubMed] [Google Scholar]
- 30.Kestler, H. W., D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high viral loads and for the development of AIDS. Cell 65:651.. [DOI] [PubMed] [Google Scholar]
- 31.Kinoshita, S., B. K. Chen, H. Kaneshima, and G. P. Nolan. 1998. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell 95:595-604. [DOI] [PubMed] [Google Scholar]
- 32.Koralnik, I. J., A. Gessain, M. E. Klotman, A. Lo Monico, Z. N. Berneman, and G. Franchini. 1992. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type 1. Proc. Natl. Acad. Sci. USA 89:8813-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis, R. S. 2001. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19:497-521. [DOI] [PubMed] [Google Scholar]
- 34.Li, W., J. Llopis, M. Whitney, G. Zlokarnik, and R. Y. Tsien. 1998. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392:936-941. [DOI] [PubMed] [Google Scholar]
- 35.Ma, H. T., R. L. Patterson, D. B. van Rossum, L. Birnbaumer, K. Mikoshiba, and D. L. Gill. 2000. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science 287:1647-1651. [DOI] [PubMed] [Google Scholar]
- 36.Manninen, A., R. G. Herma, and K. Saksela. 2000. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J. Biol. Chem. 275:16513-16517. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama, T., T. Kanaji, S. Nakade, T. Kanno, and K. Mikoshiba. 1997. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. (Tokyo) 122:498-505. [DOI] [PubMed] [Google Scholar]
- 38.Mery, L., N. Mesaeli, M. Michalak, M. Opas, D. P. Lew, and K. H. Krause. 1996. Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J. Biol. Chem. 271:9332-9339. [DOI] [PubMed] [Google Scholar]
- 39.Mesnard, J. M., and C. Devaux. 1999. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 Tax protein. Virology 257:277-284. [DOI] [PubMed] [Google Scholar]
- 40.Mulloy, J. C., R. W. Crowley, J. Fullen, W. J. Leonard, and G. Franchini. 1996. The human T-cell leukemia/lymphotropic virus type 1 p12I protein binds the interleukin-2 receptor beta and gamma(c) chains and affects their expression on the cell surface. J. Virol. 70:3599-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negulescu, P. A., N. Shastri, and M. D. Cahalan. 1994. Intracellular calcium dependence of gene expression in single T lymphocytes. Proc. Natl. Acad. Sci. USA 91:2873-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuveut, C., K. G. Low, F. Maldarelli, I. Schmitt, F. Majone, R. Grassmann, and K. T. Jeang. 1998. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol. Cell. Biol. 18:3620-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicot, C., J. C. Mulloy, M. G. Ferrari, J. M. Johnson, K. Fu, R. Fukumoto, R. Trovato, J. Fullen, W. J. Leonard, and G. Franchini. 2001. HTLV-1 p12I protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mononuclear cells. Blood 98:823-829. [DOI] [PubMed] [Google Scholar]
- 44.Pinton, P., D. Ferrari, E. Rapizzi, F. D. Di Virgilio, T. Pozzan, and R. Rizzuto. 2001. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 20:2690-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pique, C., A. Ureta-Vidal, A. Gessain, B. Chancerel, O. Gout, R. Tamouza, F. Agis, and M. C. Dokhelar. 2000. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J. Exp. Med. 191:567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renkema, H. G., and K. Saksela. 2000. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front. Biosci. 5:D268-D283. [DOI] [PubMed] [Google Scholar]
- 48.Tian, P., M. K. Estes, Y. Hu, J. M. Ball, C. Q. Zeng, and W. P. Schilling. 1995. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J. Virol. 69:5763-5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Timmerman, L. A., N. A. Clipstone, S. N. Ho, J. P. Northrop, and G. R. Crabtree. 1996. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature 383:837-840. [DOI] [PubMed] [Google Scholar]
- 50.Uchiyama, T. 1997. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu. Rev. Immunol. 15:15-37. [DOI] [PubMed] [Google Scholar]
- 51.van Kuppeveld, F. J., J. G. Hoenderop, R. L. Smeets, P. H. Willems, H. B. Dijkman, J. M. Galama, and W. J. Melchers. 1997. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 16:3519-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Oers, N. S. 1999. T cell receptor-mediated signs and signals governing T cell development. Semin. Immunol. 11:227-237. [DOI] [PubMed] [Google Scholar]
- 53.Wu, Y., and J. W. Marsh. 2001. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 293:1503-1506. [DOI] [PubMed] [Google Scholar]
- 54.Xu, W., F. J. Longo, M. R. Wintermantel, X. Jiang, R. A. Clark, and S. DeLisle. 2000. Calreticulin modulates capacitative Ca2+ influx by controlling the extent of inositol 1,4,5-trisphosphate-induced Ca2+ store depletion. J. Biol. Chem. 275:36676-36682. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida, M. 2001. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu. Rev. Immunol. 19:475-496. [DOI] [PubMed] [Google Scholar]
- 56.Zweifach, A., and R. S. Lewis. 1993. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA 90:6295-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]