Abstract
Early events during human immunodeficiency virus infections are considered to reflect the capacity of the host to control infection. We have studied early virus and host parameters during the early phase of simian immunodeficiency virus SIVmnd-1 nonpathogenic infection in its natural host, Mandrillus sphinx. Four mandrills were experimentally infected with a primary SIVmnd-1 strain derived from a naturally infected mandrill. Two noninfected control animals were monitored in parallel. Blood and lymph nodes were collected at three time points before infection, twice a week during the first month, and at days 60, 180, and 360 postinfection (p.i.). Anti-SIVmnd-1 antibodies were detected starting from days 28 to 32 p.i. Neither elevated temperature nor increased lymph node size were observed. The viral load in plasma peaked between days 7 to 10 p.i. (2 × 106 to 2 × 108 RNA equivalents/ml). Viremia then decreased 10- to 1,000-fold, reaching the viral set point between days 30 to 60 p.i. The levels during the chronic phase of infection were similar to that in the naturally infected donor mandrill (2 × 105 RNA equivalents/ml). The CD4+ cell numbers and percentages in blood and lymph nodes decreased slightly (<10%) during primary infection, and CD8+ cell numbers increased transiently. All values returned to preinfection infection levels by day 30 p.i. CD8+ cell numbers or percentages, in peripheral blood and lymph nodes, did not increase during the 1 year of follow-up. In conclusion, SIVmnd-1 has the capacity for rapid and extensive replication in mandrills. Despite high levels of viremia, CD4+ and CD8+ cell numbers remained stable in the post-acute phase of infection, raising questions regarding the susceptibility of mandrill T cells to activation and/or cell death in response to SIVmnd-1 infection in vivo.
African nonhuman primates are natural hosts for simian immunodeficiency viruses (SIVs). To date, SIV infections have been detected in 20 distinct species (24). SIVs were shown to cluster in six major, approximately equidistant lineages (17, 53). Human immunodeficiency virus type 1 (HIV-1) and HIV-2 belong to two of these clusters and emerged most likely following zoonotic transmissions of, respectively, SIVcpz from chimpanzees and SIVsm from sooty mangabeys (14, 16, 21). The remaining clusters are formed by SIVs isolated from African green monkeys (AGMs), Syke's monkeys, l'hoest monkeys, and colobus monkeys (4, 17, 20, 27, 39, 60). The phylogeny of many SIVs resembles that of their host species, suggesting a coevolution (1, 21, 39). In contrast to these, some viruses (SIVrcm, SIVagm.sab, SIVmnd-2, and SIVdrl) cluster in different lineages according to the genomic region analyzed (15, 22, 31, 55). These viruses most likely result from recombination events in monkeys dually infected by SIVs of two distinct lineages (15, 22, 31, 55). Such dual infections suppose the existence of cross-species transmissions of SIV in nature. It has indeed been shown that wild patas monkeys and baboons can acquire SIVagm (7, 32, 61). It remains unclear if these two latter species are infected with species-specific SIVs (54). This was also questioned for mandrills (Mandrillus sphinx). The first SIVmnd strain (GB1), now defined as SIVmnd-1, was isolated 13 years ago from a wild-born mandrill (59). This virus was the only representative of the SIVmnd lineage until two Cercopithecinae viruses, from l'hoest (Cercopithecus l'hoesti l'hoesti) and sun-tailed monkeys (Cercopithecus l'hoesti solatus) were shown to cluster in the same lineage as SIVmnd GB1 (4, 29). As the genome of SIVmnd GB1 had the organization of Cercopithecus SIVs and not that of Papionini SIVs, and because the habitat of mandrills and sun-tailed monkeys overlaps in Gabon, it was suggested that SIVmnd GB1 represents a virus cross-transmitted from sun-tailed monkeys to mandrills, and the lineage was renamed the l'hoest lineage (4, 5). It was subsequently demonstrated that SIVmnd GB1-related viruses are commonly present in wild adult mandrills, indicating that the virus is capable, at least today, of efficiently spreading between animals of this species (55). Mandrills infected with SIVmnd-1 are separated by the Ogooué River from mandrills infected with SIVmnd type 2 (55). This latter virus has a genomic organization distinct from those of SIVmnd GB1 and SIVlhoest and identical to that of SIVs of other Papio monkeys, such as SIVsm and SIVdrl (15, 55).
It is unknown why SIV infections are generally nonpathogenic in African nonhuman primates. SIVmnd-2 infections in mandrills have not been associated with signs of AIDS. SIVmnd-1 infection, which apparently results from SIV transmission from C. l'hoesti to mandrills, is also considered to be asymptomatic, although a possible exception has been reported (44). SIVlhoest, which is genetically close to SIVmnd-1 and is also associated with asymptomatic infection in its natural host, appears to induce AIDS in macaques (29). In both HIV-1-infected humans and SIVmac-infected macaques, it was shown that early virus-host interactions are predictive of the outcome of infection. Predictive markers are in particular gag-specific T-helper responses and viral load levels in the post-acute phase of infection (36, 50). In macaques, the steady-state level of plasma viremia 5 to 6 weeks after exposure to the virus is an excellent predictor of the subsequent disease course (28, 35, 42, 63). RNA levels in plasma measured early in HIV-1 infection are also highly predictive of subsequent rates of disease progression (36, 37, 43). However, although this observation is broadly acknowledged and used as the main indication for treating HIV-1-infected patients early in the course of the disease (10), little is still known about the driving mechanism(s) directing this phenomenon. Studies of the early events during nonpathogenic infections in natural host species can help to elucidate such mechanisms. So far, studies during the early phase of infection in African nonhuman primates are limited (58). Studies in SIVagm.sab92018-infected AGMs have revealed for the first time an extensive replication during the acute and post-acute phases (18). Many naturally infected AGMs analyzed during the chronic phase also show continuously high levels of viral RNA in plasma (9, 23). Moreover, virus load in the blood and lymph nodes (LN) of mangabeys during chronic infection is at levels equivalent to that in macaques and humans progressing to AIDS, despite the lack of clinical signs of AIDS (49). The absence of AIDS in these monkeys therefore seems to be paradoxical in the presence of such a high viral load. However, it is not clear whether the high viral replication observed is a general feature of nonpathogenic infections in natural host species (3, 23). Moreover, the precise dynamics of CD4+ and CD8+ cells during primary infection have not been reported so far.
Our primary objective for the present study was to investigate the acute and post-acute phases of SIVmnd-1 infection in mandrills. SIVmnd-1 is thought to represent the result of an ancient cross-species transmission in the wild (4, 6), and we investigated whether it would represent an intermediate model between the pathogenic and nonpathogenic models of lentiviral infections. We analyzed viral dynamics and corroborated the virological study with the analyses of CD4+- and CD8+-T-cell changes over time in the blood and LN.
MATERIALS AND METHODS
Animals.
All mandrills used in this study originated from a semi-free range colony at the International Center of Medical Research in Franceville, Gabon. One of the founder mandrills was a wild-captured juvenile female (F17) introduced into the colony in 1985. F17 was seropositive for SIVmnd-1 at the time of capture and seronegative for simian T-cell leukemia virus. The prototype SIVmnd-1 strain (GB1) was isolated from this mandrill in 1988 (59, 60). The other 7 monkeys included in our study were males (16E, 12C2, 2C2, and 12A4) and females (10G, 5H, and 2I) born in the semi-free range colony. SIV and simian T-cell leukemia virus seronegativity prior to inoculation were demonstrated by Western blotting (New Lav Blot II; Diagnostics Pasteur, Marne-la-Coquette, France) (HTLV Blot 2.3; Genelabs Diagnostic). All of these animals were young adults aged between 8 and 9 years old at the time when the protocol began, with an average mean weight of 14.3 kg for males and 7.7 kg for females. Housing and handling were in accordance with national guidelines and institutional policies.
Virus and infections.
To avoid selection of viral variants in vitro, all inocula used in this study consisted of plasma obtained from SIVmnd-1-infected mandrills. SIVmnd-1 virus was obtained from its original host (F17) while alive and healthy. Ten milliliters of blood was collected, and plasma was separated by centrifugation, aliquoted, and frozen at −80°C. The plasma was then titrated on SupT1 cells. Briefly, serial 10-fold dilutions of the plasma were added to 0.5 × 106 SupT1 cells (four wells per dilution). Virus production was measured by detection of reverse transcription (RT) activity (Cavidi Tech, Uppsala, Sweden) in the supernatant of each well at days 14 and 28 of culture (19). The infectious titer of the plasma was then expressed as the 50% tissue culture infectious dose (TCID50) determined by the Karber calculation method. The plasma of chronically infected mandrill F17 had a titer of 4 TCID50/ml. In order to obtain a plasma sample with a higher titer, mandrill 16E was inoculated with 5 ml of plasma (20 TCID50) from F17. Twenty-five milliliters of blood was collected from 16E at day 11 postinfection (p.i.). One-milliliter aliquots of plasma samples were prepared and frozen at −70°C. The infectious titer of the plasma from 16E on SupT1 cells, determined by the limiting dilution assay described above, was 3,000 TCID50/ml.
Animals were anesthetized with 10 mg of ketamine-HCl/ml. Four mandrills (12A4, 10G, 12C2, and 2C2) were inoculated intravenously with 3,000 TCID50 of plasma from 16E. Two other mandrills (5H and 2I) were used as negative controls. They received the same amount of plasma (1 ml) from mandrill 16E sampled prior to SIVmnd-1 inoculation.
Specimen collection.
Whole blood (7 to 10 ml) was collected from every monkey in EDTA-K2 tubes before infection (at days −30, −15, and 0), twice weekly throughout the first 3 weeks p.i. (days 4, 7, 10, 14, 17, and 21), and finally at days 28, 32, 60, 180, and 360 p.i. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque gradient. PBMC were frozen in dimethyl sulfoxide, and plasma aliquots were stored at −70°C. Excisional axillar and inguinal LN biopsies were collected before the infection at days −30, −15, and 0; during primary infection at days 7, 10, 14, 17, 21, and 28; and then during chronic infection at days 60, 180, and 360 p.i. After excision, the LNs were dilacerated. Cells were separated from the remaining tissue by passage through a 100-μm-pore size nylon mesh, resuspended in RPMI, centrifuged for 10 min at 800 × g, and frozen in dimethyl sulfoxide at −80°C until used.
Antibody detection.
Antibody responses to SIVmnd-1 GB1 were monitored by an HIV-1-HIV-2 enzyme-linked immunosorbent assay (ELISA) (Genelavia Mixt; Sanofi-Diagnostics Pasteur) and confirmed by a primate immunodeficiency virus-specific enzyme immunoassay (EIA) (PIV-EIA) designed to detect antibodies directed towards the immunodominant region of gp36 and the highly variable V3 loop (54). Reactivity was also confirmed by Western blotting (New Lav Blot; Sanofi-Diagnostics Pasteur).
Virus isolation.
One milliliter of plasma was added to 2 × 106 SupT1 cells and incubated for 1 h at 37°C. SupT1 cells were then cultured for 28 days, and culture supernatants were monitored for RT activity (Cavidi).
Quantification of viral RNA in plasma.
The RNA quantification was performed by a limiting dilution RT-PCR assay specific for SIVmnd-1. The primers were MP5 (5′-CCA GAT AAG TGG AAG ATA GAA AAG-3′) and MP6 (5′-CAC CAA TCT TCC CAT ATC TCC C-3′), which allowed the amplification of a 476-bp region within the SIVmnd-1 GB1 pol gene as previously described (41).
RNA was extracted from 540 μl of plasma by using the QIAamp Viral RNA Mini kit (Qiagen, Courtaboeuf, France). Seven 10-fold serial dilutions of the RNA extract were submitted to RT-PCR (one-step RT-PCR kit; Qiagen). RT was performed at 50°C for 30 min in the presence of MP5 and MP6 and followed by 47 cycles of 95°C for 15 s, 55°C for 45 s, and 72°C for 1 min, with a final extension of 7 min at 72°C. The absolute target copy numbers were determined by the amplification in parallel of known amounts of an SIVmnd-1 RNA external standard. The standard was obtained by amplifying the 476-bp region of SIVmnd-1 GB1 pol from F17 genomic DNA. The PCR product was then cloned into the pCR2.1 vector with the topoTA cloning kit (Invitrogen, Groningen, The Netherlands). The 5′ to 3′ sense orientation of the insert was verified by sequence analyses. A clone containing the insert in 5′ to 3′ orientation was chosen and subjected to PCR with the primers pCRT7S (5′-GCG TAA TAC GAC TCA CTA TAG GGA GAG GA GCTC GAG CGG CCG CCA GTG TG-3′) and pCRT7AS (5′-ATT ACG CCA AGC TTG GTA CCG AG-3′), which hybridize to regions just upstream and downstream of the pCR2.1 plasmid polylinker sequence. Cycling conditions for this PCR consisted of an initial DNA denaturation step followed by 40 cycles that included a denaturation step at 92°C for 30 min, primer annealing at 62°C for 45 min, and DNA synthesis at 72°C for 45 min, with a final extension of 7 min at 72°C. The resulting PCR product contained the cloned insert together with a T7 promoter sequence at its 5′ end. After clean up of the PCR product (PCR purification kit; Qiagen), this DNA was used as a template for an in vitro transcription (MEGAscript kit; Ambion, Austin, Tex.). The resulting RNA was DNase digested, and nonincorporated nucleotides were eliminated with a Chroma Spin-30 DEPC H2O column (Clontech, Palo Alto, Calif.). The size and purity of the synthesized RNA were verified on a denaturing polyacrylamide gel electrophoresis gel. The concentration of the in vitro transcript was determined by a highly sensitive and specific Gene Quant II spectrophotometric operation system as already described (18). Aliquots were stored at −80°C until use. Immediately before usage, one aliquot of RNA was thawed and diluted in RNase-free H2O and five dilutions of the RNA were used as external standards in each experiment.
As a control to determine whether the sensitivity of the RT-PCR could be affected by the presence of putative, naturally occurring inhibitory molecules in plasma, we mixed individual plasma samples, obtained before infection of the animals, with the SIVmnd standard RNA, extracted the RNA as usual, and subjected the extracts to limiting dilution RT-PCR. No changes in the sensitivity of the RT-PCRs were observed when the standard RNA was previously mixed with plasma in comparison to standard RNA alone (data not shown). The sensitivity of the assay was 2 × 102 RNA copies/ml of plasma.
Flow cytometry analysis of CD4+ and CD8+ cells in blood and LNs.
PBMC (2 × 105) isolated from whole blood and the same number of cells separated from the LNs were stained with 5 μl of the appropriate monoclonal antibody (anti-CD4 Leu-3a or anti-CD8 Leu-2a; Becton Dickinson, Mountain View, Calif.) and relevant negative controls (immunoglobulin G1 [IgG1] fluorescein isothiocyanate [PN0639; Immunotech], IgG2a phycoerythrin, and IgG1 phycoerythrin [Becton Dickinson]). Cells were incubated with the antibody for 20 min at 4°C. This was followed by a lysis step of 5 min with a solution containing 0.9 mM NH4CO3H and 131 mM NH4Cl in order to eliminate the erythrocytes. Cells were then washed two times in phosphate-buffered saline and fixed with 1% paraformaldehyde. Samples were analyzed on a FACScalibur flow cytometer with Cell Quest software (Becton Dickinson).
Statistical analysis.
CD4+ and CD8+ values from the first sampling point before infection for each monkey were designated 100%, and values for subsequent measurements were expressed proportionally. Two groups were analyzed: the first group corresponded to all four infected monkeys and the second group corresponded to the two noninfected control monkeys. For each group, we used the Wilcoxon signed rank test to evaluate the statistical significance of changes in CD4+ and CD8+ values following inoculation when compared to the three preinoculation values (Statview; Abacus Concept, Berkeley, Calif.).
RESULTS
Absence of primary infection syndrome in mandrills experimentally infected with SIVmnd-1.
The animals were monitored until day 360 p.i. After inoculation with SIVmnd-1 GB1, none of the animals developed fever. No loss of weight was observed during the follow-up in infected animals. Neither increase in size of LNs nor opportunistic infection was detected. One mandrill (12C2) died at day 200 p.i. The autopsy did not reveal signs of visceral infection associated with AIDS. The unique possible fatal event was a gastric dilatation, which is frequently reported in literature to be the cause of the death in captive monkeys (62). Transient variations in the CD4+ and CD8+ cell levels were observed in mandrill 12C2, but there was no progressive CD4+ decrease or increase in viral load (see below).
SIVmnd-1-infected mandrills rapidly develop antibodies directed against SIVmnd.
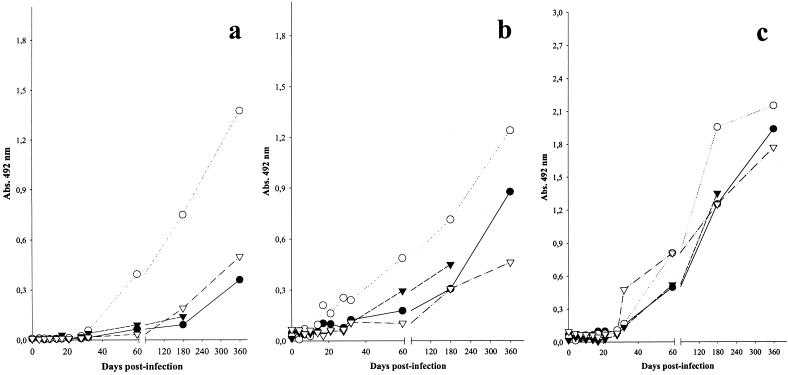
In a commercial ELISA screening assay (HIV-1-HIV-2; Genelavia; Sanofi Diagnostic Pasteur, Marne-la-Coquette, France) all animals inoculated with SIVmnd-1 showed detectable anti-gp36 antibodies starting from days 28 and 32 p.i. and maintained a sustained antibody response (Fig. 1a). Similar patterns of reactivity (appearance of anti-p26 at day 28 p.i. and appearance of anti-gp105 by day 32 p.i.) were also observed by using the HIV-2 Western blot antibody detection assay (data not shown). The observed reactivity against p26 was similar to the anti-gp105 reactivity in this assay. In order to test reactivity specifically directed against SIVmnd antigens, we used the PIV-EIA. The reactivities directed towards the SIVmnd peptides were indeed significantly stronger than those directed towards peptides of other SIVs and HIVs (data not shown). Antibodies directed against gp36 of SIVmnd-1 were detected from days 28 to 32 p.i. on (Fig. 1b) while anti-SIVmnd-1 V3 antibodies were detected starting from day 32 p.i. (Fig. 1c). This difference is not surprising, as it was already shown that V3 reactivity generally appears later than gp41/36 reactivity (54). The PIV-ELISA gave both stronger and earlier reactivity than the commercial HIV-1-HIV-2 ELISA (Genelavia), which maps the HIV gp41/36 immunodominant regions. The commercial ELISA uses a conjugated antibody, enabling the detection of IgMs also, in contrast to the PIV-EIA, which detects only IgG. However, the Genelavia ELISA contains only HIV-1-HIV-2 antigens, whereas the PIV-EIA includes SIVmnd-1-specific peptides, which probably explains its greater sensitivity. In conclusion, the serological data showed that in mandrills infected with SIVmnd-1 GB1, anti-SIV antibodies were detected as early as in macaques infected with SIVmac (56).
FIG. 1.
Antibody responses during SIVmnd-1 infection in M. sphinx. (a) Commercial screening assay (Genelavia Mixt; Bio-Rad). (b) Anti-SIVmnd-1 gp36. (c) V3 antibodies detected by PIV-EIA. Animals: •, 12A4; ○, 10G; ▾, 12C2; ▵, 2C2. Abs., absorbance.
High viral load in plasma during SIVmnd-1 infection.
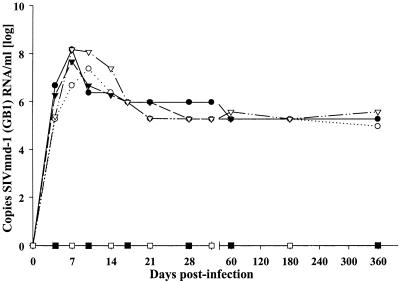
In order to study the extent of early virus growth during SIVmnd-1 infection in mandrills, and to compare SIVmnd-1 replication levels to those of pathogenic SIVmac in macaques and nonpathogenic SIVagm in AGMs, we investigated the dynamics of plasma viremia with an SIVmnd-1-specific quantitative RT-PCR assay. A peak of viral RNA in plasma was observed very early at day 7 or 10 p.i. that ranged from 4.5 × 106 to 1.5 × 108 copies/ml (Fig. 2). Viral RNA in plasma then declined rapidly; a decline of 10- to 1,000-fold was observed for all animals between days 10 and 21 p.i., followed by a further 10-fold decline in one mandrill (12A4) before day 60 p.i. (Fig. 2). For three animals, the time point at which the viral load stabilized (viral set point) was already reached between days 21 and 28 p.i. No significant variations in viral RNA loads in plasma were noted during the chronic phase. Thus, viral loads in plasma remained around 2 × 105 RNA copies/ml in all 4 animals between days 60 and 360 p.i., with only slight variations (Fig. 2). In order to confirm that the RNA profiles correlate with levels of infectious virus, we performed virus isolation close to the peak (day 11) and after the viral set point (day 60) in all four animals. Virus could always be isolated more readily in plasma collected at day 11 p.i. than at day 60 p.i. (data not shown). The naturally infected mandrill which served as a donor animal (F17) had an RNA viral load in plasma in a similar range (5 × 105 copies/ml) at the time of sampling (data not shown), suggesting that the experimentally induced infections reproduced the features of the natural infection.
FIG. 2.
Viral load in plasma during experimental SIVmnd-1 infection in M. sphinx. The viral RNA copy numbers were determined in animals 12A4 (•), 10G (○), 12C2 (▾), and 2C2 (▵).
Absence of persistent quantitative changes of CD4+ and CD8+ cells despite high levels of SIVmnd-1 viremia.
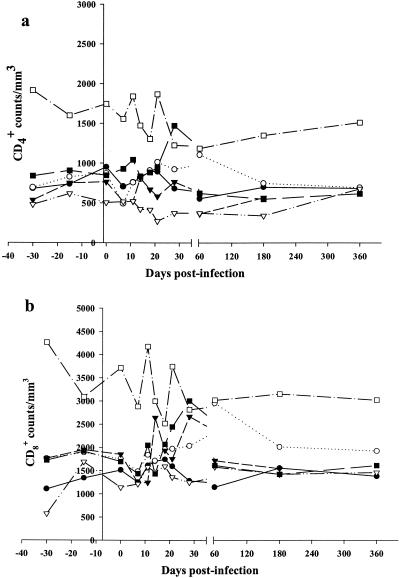
Our data reveal extensive SIVmnd-1 replication during the primary infection, which after a rapid decrease, stabilizes at still relatively high levels. This contrasts with the presumed nonpathogenic outcome of the infection. In order to study biological markers for progression, we evaluated CD4+ and CD8+ cell numbers over time. In addition, we addressed the question of whether the viral replication profiles are associated with cell target numbers and/or whether the rapid decrease of viral load correlates with the increase in CD8+ cells or not. We therefore evaluated the quantitative changes of CD4+ and CD8+ cells in the blood at the same time points as those analyzed for viral load. We performed analysis of three time points for each animal before inoculation in order to determine normal values of CD4+ and CD8+ cells for each individual and to assess intraindividual variations. In addition, we submitted two mock-infected animals to the same follow-up as controls. Before infection, the CD4+ and CD8+ values in the blood of the four animals that were subsequently exposed to SIVmnd-1 ranged from 506 to 952 cells/μl for CD4+ cells and 1,041 to 1,949 cells/μl for CD8+ cells. The mean values before infection for these animals were of 702 ± 23 CD4+ cells/μl and of 1,568 ± 89 CD8+ cells/μl (Fig. 3). One of the two mandrills included in the control group (5H) had similar mean values (774 ± 240 CD4+ cells/μl and 1,868 ± 473 CD8+ cells/μl). The other control mandrill (2I), although of the same age as the others, displayed higher baseline CD4+ and CD8+ values. These higher counts were constantly recorded during the follow-up, the mean CD4+ level in animal 2I was 1,602 ± 310 cells/μl and the mean CD8+ count was 3,210 ± 596 cells/μl. In general, the CD4+ and CD8+ numbers in mandrills were thus similar to those described in macaques (38, 51).
FIG. 3.
Peripheral CD4+ and CD8+ cell numbers during experimental SIVmnd-1 infection in M. sphinx. (a) CD4+ cell counts in infected and control animals; (b) CD8+ cell numbers in infected and control animals. Infected animals: •, 12A4; ○, 10G; ▾, 12C2; ▵, 2C2. Control animals: ▪, 5H; □, 2I.
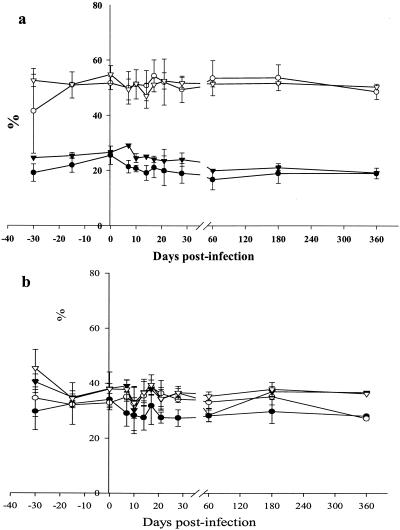
We then followed relative changes in CD4+ and CD8+ cells after infection. During primary infection, CD4+ counts presented a statistically significant decrease at day 7 p.i. (P = 0.01) (Fig. 3a). This moderate, transient decrease of CD4+ cells around the peak of viral replication was followed by a statistically significant increase in CD8+ counts at days 14 and 17 p.i. (P = 0.02) (Fig. 3b). The slight decrease of CD4+ cells at day 21 p.i. was not statistically significant. When evaluating CD4+ and CD8+ cell percentages in blood, no statistically significant changes were observed (Fig. 4a). During the post-acute and chronic phases of infection, the CD4+ counts were constant and presenting at day 360 p.i. similar levels as before the inoculation (Fig. 3a). CD8+ counts were also stable during the first year p.i.
FIG. 4.
CD4+ and CD8+ percentages in SIVmnd-1-infected mandrills and control animals. The mean values for the infected and control animals are shown. The mean values were calculated for the four SIVmnd-1-infected animals (CD4+ [•] and CD8+ [○] cells) and the two noninfected animals (CD4+ [▾] and CD8+ [▵] cells). (a) PBMC; (b) LNs.
In the LNs, a significant reduction in the CD4 percentage was recorded at days 21 and 28 p.i. (P = 0.01), as was a significant increase in the CD8+ percentage at days 17 and 21 p.i. (P = 0.01 and 0.02) (Fig. 4b). This resulted in a reduction in the CD4+/CD8+ ratio of up to 0.3 observed between days 21 and 28 p.i. However, during the post-acute phase until day 360 p.i., the CD4+ percentages showed similar levels as those observed prior to inoculation. Interestingly, the CD8+ percentages in LNs were not increased with respect to the baseline values.
DISCUSSION
The mechanism of nonpathogenicity of SIV infections in their natural hosts is unknown. We studied the early SIVmnd-1 infection in mandrills, as early events have been shown to be predictive for the outcome of the infection and might reveal insights into the balance between the virus and the host. We first evaluated the level of SIVmnd-1 replication during early infection by measuring distinct viral parameters. The RNA copy numbers in plasma revealed that the viral load was very high, similar to the values generally described for pathogenic infections (11, 57). A peak of SIVmnd-1 replication was consistently detected between days 7 and 10 p.i. and followed by a rapid 2 to 3 log decrease in viral load. The viral dynamic profiles and levels were remarkably similar among the distinct individuals. The viral set point had already been reached for three out of four animals at day 28 p.i., suggesting that the balance between host and virus is established rapidly.
After the viral set point and during the chronic phase of infection (studied here until day 360 p.i.), viremia levels remained relatively high. This high replication of SIVmnd-1 during the primary and chronic infection indicates that SIVmnd-1 is highly adapted to mandrills, although it originated from an SIV that infects Cercopithecus monkeys (4, 5, 29). This observation is in agreement with epidemiological studies showing that SIVmnd-1 efficiently spreads among wild mandrills (55). This might suggest either that SIVmnd-1 has been present in mandrills for a long time and/or that mandrills have host-intrinsic factors distinct from macaques which would explain the distinct outcome of SIV infection in the two species. These could be common with other African nonhuman primates such as sooty mangabeys and AGMs. AGMs and mangabeys naturally infected with SIVagm and SIVsm, respectively, show similar or even higher loads in plasma (9, 13, 18, 23, 49). For HIV-1 and SIVmac infections, such levels of 105 to 107 were associated with a high risk of progression (36, 56). This study confirms that African monkeys are in a nonpathogenic equilibrium with their corresponding viruses and that the high levels of virus in the blood observed during the HIV infection are not the only factors accounting for the progressive immunodeficiency.
Pathogenic lentiviral infections are characterized by a rapid turnover of both virus and activated CD4+ T cells concomitant with an expansion of the CD8+-T-lymphocyte subset (26, 30, 38, 45, 48, 51, 64). We evaluated here the CD4+ and CD8+ cell numbers in mandrills before and after infection with SIVmnd-1. We compared the cell numbers in noninfected mandrills to those described in macaques (38, 51). The CD4+ and CD8+ cell numbers were in a similar range in mandrills and in macaques. We then evaluated the changes in CD4+ and CD8+ levels in mandrills in response to SIVmnd-1 infection. Our study presents the first report on the dynamics of CD4+ and CD8+ cells during primary infection of a nonpathogenic infection in African monkeys. Although we did not observe a sustained drop in CD4+ cell numbers during the acute stage of infection, a slight reduction in CD4+ cells, concomitant with the peak of viral replication, was detected. This reduction might be due to virus-induced cell death and/or cell sequestration in the LNs as suggested for HIV infection (33, 34, 40, 46, 52, 65). However, in the LNs, the CD4+ percentages did not increase in the infected animals, although this does not exclude an eventual increase in total numbers of CD4+ cells if the numbers of CD8+ T and/or B lymphocytes increased in parallel.
The decrease in CD4+ cells was only transient and moderate in contrast to what is generally observed in SIVmac-infected macaques (38, 51). Thus, the CD4+ cell numbers in the blood of SIVmnd-1-infected mandrills rapidly returned to the baseline values, and at 1 year p.i., they were still close to the values recorded before infection. No significant differences were observed in blood from infected animals and blood from the control group. Additional analyses should be done to determine if a decline in CD4+ peripheral cells will occur later in chronically infected mandrills. In both AGMs and mangabeys infected with SIVagm and SIVsm, respectively, a slight decline in peripheral CD4+-T-cell counts compared to those of uninfected animals was described (2, 13). However, these moderate decreases have not been reported to compromise the clinical status of the animals (9).
An increase in CD8+ counts was observed at days 14 and 17 p.i. This increase might be due to either a homeostatic reaction following the drop in CD4+ counts and/or a transitory immune activation due to the SIVmnd-1 infection. However, there was no increase in CD8+ cells in the blood and LNs in the post-acute phase of infection in contrast to those of SIVmac-infected macaques (48) despite equivalent levels of viral replication. The lower expansion of CD8+ cells in the mandrills could be indicative of a lower chronic immune activation. Indeed, we observed no signs of fever or of hyperplasia in peripheral LNs that could be markers for a strong immune activation. In addition, the viral replication after the set point, although up to 3 log lower than during primary infection, was not strongly suppressed but rather goes on at considerably high levels. The absence of a strong immune activation might be beneficial for the host by preventing tissue damage and accelerated T-cell turnover. In mangabeys, a species closely related to mandrills (25), studied after long-term infection with SIVsm, a normal level of T-cell turnover was indeed reported (12). Our study suggests that early infection is already characterized by a lower activation state. Supplementary investigations are needed to understand the mechanism underlying the particular virus-host equilibrium in these models. A hypothesis for explaining these features would be that the susceptibility of mandrill cells to virus-driven activation is different from that observed for both humans and macaques. A host-specific difference with the virus has indeed been described in mangabeys as they apparently show a relative resistance to CD4+ cell anergy in response to activation stimuli in vitro (8).
Altogether, our data show that SIVmnd-1 is well adapted to mandrills. This study extends the concept that high viral load alone is not sufficient for disease progression. We showed that in SIVmnd-1-infected mandrills, CD4+ and CD8+ levels in both blood and LNs are stable during the first year. These data suggest a lower general state of activation of the immune system in vivo early on. The investigation of the factors associated with the balance between the virus and the host should provide valuable explanations for understanding the progressive loss of CD4+ cell function in HIV-infected humans and/or the discrepancies between virological and immunological responses observed in about 20% of patients undergoing highly active antiretroviral therapy (47). Understanding the factors which are responsible for maintaining the steady state of infection in African primates should provide help in elaborating valuable immunostrategies against AIDS.
Acknowledgments
We thank Preston Marx for critical reading of the manuscript and Paul Ngari for excellent technical help.
This work was supported by grants from the French Agency for AIDS Research (ANRS) and by funds from the Centre International de Recherches Medicales Franceville (CIRMF), Franceville, Gabon. The CIRMF is supported by the Government of Gabon, Total-Fina-Elf Gabon, and the Ministère de la Coopération Française. C.K. was the recipient of a fellowship from the Foundation for Medical Research (Sidaction).
REFERENCES
- 1.Allan, J. S., M. Short, M. E. Taylor, S. Su, V. M. Hirsch, P. R. Johnson, G. M. Shaw, and B. H. Hahn. 1991. Species-specific diversity among simian immunodeficiency viruses from African green monkeys. J. Virol. 65:2816-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beer, B., J. Denner, C. R. Brown, S. Norley, J. zur Megede, C. Coulibaly, R. R. Plesker, S. Holzammer, M. Bayer, V. M. Hirsch, and R. Kurth. 1998. Simian immunodeficiency virus of African green monkeys is apathogenic in the newborn natural host. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:210-220. [DOI] [PubMed] [Google Scholar]
- 3.Beer, B. E., J. Scherer, J. zur Megede, S. Norley, M. Baier, and R. Kurth. 1996. Lack of dichotomy between virus load of peripheral blood and lymph nodes during long-term simian immunodeficiency virus infection of African green monkeys. Virology 219:367-375. [DOI] [PubMed] [Google Scholar]
- 4.Beer, B. E., E. Bailes, R. Goeken, G. Dapolito, C. Coulibaly, S. G. Norley, R. Kurth, J. P. Gautier, A. Gautier-Hion, D. Vallet, P. M. Sharp, and V. M. Hirsch. 1999. Simian immunodeficiency virus (SIV) from sun-tailed monkeys (Cercopithecus solatus): evidence for host-dependent evolution of SIV within the C. lhoesti superspecies. J. Virol. 73:7734-7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer, B. E., E. Bailes, G. Dapolito, B. J. Campbell, R. M. Goeken, M. K. Axthelm, P. D. Markham, J. Bernard, D. Zagury, G. Franchini, P. M. Sharp, and V. M. Hirsch. 2000. Patterns of genomic sequence diversity among their simian immunodeficiency viruses suggest that L'Hoest monkeys (Cercopithecus lhoesti) are a natural lentivirus reservoir. J. Virol. 74:3892-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer, B. E., E. Bailes, P. M. Sharp, and V. M. Hirsch. 2000. Diversity and evolution of primate lentiviruses, p. 460-474. In C. Kuiken, B. Foley, B. Hahn, P. A. Marx, F. McCutchan, J. Mellors, J. Mullins, S. Wolinsky, B. Korber (ed.), Human retroviruses and AIDS. 1999. A compilation and analyses of nucleic acid and amino acid sequences. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 7.Bibollet-Ruche, F., A. Galat-Luong, G. Cuny, P. Sarni-Manchado, G. Galat, J. P. Durand, X. Pourrut, and F. Veas. 1996. Simian immunodeficiency virus infection in a patas monkey (Erythrocebus patas): evidence for cross-species transmission from African green monkeys (Cercopithecus aethiops sabaeus) in the wild. J. Gen. Virol. 77:773-781. [DOI] [PubMed] [Google Scholar]
- 8.Bostik, P., A. E. Mayne, F. Villinger, K. P. Greenberg, J. D. Powell, and A. A. Ansari. 2001. Relative resistance in the development of T cell anergy in CD4+ T cells from simian immunodeficiency virus disease-resistant sooty mangabeys. J. Immunol. 166:506-516. [DOI] [PubMed] [Google Scholar]
- 9.Broussard, S. R., S. I. Staprans, R. White, E. M. Whitehead, M. B. Feinberg, and J. S. Allan. 2001. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J. Virol. 75:2262-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter, C. C., D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, S. M. Hammer, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 2000. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society—USA Panel. JAMA 283:381-390. [DOI] [PubMed] [Google Scholar]
- 11.Chakrabarti, L. A., V. Baptiste, E. Khatissian, M. C. Cumont, A. M. Aubertin, L. Montagnier, and B. Hurtel. 1995. Limited viral spread and rapid immune response in lymph nodes of macaques inoculated with attenuated simian immunodeficiency virus. Virology 213:535-548. [DOI] [PubMed] [Google Scholar]
- 12.Chakrabarti, L. A., S. R. Lewin, L. Zhang, G. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 74:1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Age-dependent changes in T cell homeostasis and SIV load in sooty mangabeys. J. Med. Primatol. 29:158-165. [DOI] [PubMed] [Google Scholar]
- 14.Chen, Z., A. Luckay, D. L. Sodora, P. T. Telfer, P. Reed, A. Gettie, J. M. Kanu, R. F. Sadek, J. A. Yee, D. D. Ho, L. Zhang, and P. A. Marx. 1997. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabeys. J. Virol. 71:3953-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clewley, J. P., J. C. M. Lewis, D. W. G. Brown, and E. L. Gadsby. 1998. A novel simian immunodeficiency virus (SIVdrl) pol sequence from the drill monkey, Mandrillus leucophaeus. J. Virol. 72:10305-10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbet, S., M. C. Müller-Trutwin, P. Versmisse, S. Delarue, A. Ayouba, J. Lewis, S. Brunak, P. Martin, F. Brun-Vezinet, F. Simon, F. Barre-Sinoussi, and P. Mauclere. 2000. env sequences of simian immunodeficiency virus from chimpanzees in Cameroon are strongly related to those of human immunodeficiency virus group N from the same geographic area. J. Virol. 74:529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courgnaud, V., X. Pourrut, F. Bibollet-Ruche, E. Mpoudi-Ngole, A. Bourgeois, E. Delaporte, and M. Peeters. 2001. Characterization of a novel simian immunodeficiency virus from guereza colobus monkeys (Colobus guereza) in Cameroon: a new lineage in the nonhuman primate lentivirus family. J. Virol. 75:857-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diop, O. M., A. Gueye, M. Dias-Tavares, C. Kornfeld, A. Faye, P. Ave, M. Huerre, S. Corbet, F. Barré-Sinoussi, and M. C. Müller-Trutwin. 2000. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J. Virol. 74:7538-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekstrand, D. H. L., D. Böttiger, H. Anderson, J. S. Gronowitz, and C. F. R. Källander. 1997. Reverse transcriptase and corresponding activity-blocking antibody in monitoring SIVsm infection in macaques. AIDS Res. Hum. Retrovir. 13:601-610. [DOI] [PubMed] [Google Scholar]
- 20.Emau, P., H. M. McClure, M. Isahakia, J. G. Else, and P. N. Fultz. 1991. Isolation from African Sykes' monkeys (Cercopithecus mitis) of a lentivirus related to human and simian immunodeficiency viruses. J. Virol 65:2135-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, F., E. Bailes, D. L. Robertson, Y. Chen, C. M. Rodenburg, S. F. Michael, L. B. Cummins, L. O. Arthur, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397:436-441. [DOI] [PubMed] [Google Scholar]
- 22.Georges-Courbot, M. C., C. Y. Lu, M. Makuwa, P. Telfer, R. Onanga, G. Dubreuil, Z. Chen, S. M. Smith, A. Georges, F. Gao, B. H. Hahn, and P. A. Marx. 1998. Natural infection of a household pet red-capped mangabey (Cercocebus torquatus torquatus) with a new simian immunodeficiency virus. J. Virol. 72:600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein, S., I. Ourmanov, C. R. Brown, B. E. Beer, W. R. Elkins, R. Plishka, A. Buckler-White, and V. M. Hirsch. 2000. Wide range of viral load in healthy African green monkeys naturally infected with simian immunodeficiency virus. J. Virol. 74:11744-11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn, B. H., G. M. Shaw, K. M. De Cock, and P. M. Sharp. 2000. AIDS as a zoonosis: scientific and public health implications. Science 287:607-614. [DOI] [PubMed] [Google Scholar]
- 25.Harris, E. E., and T. R. Disotell. 1998. Nuclear gene trees and the phylogenetic relationship of the mangabeys (Primates: Papionini). Mol. Biol. Evol. 15:892-900. [DOI] [PubMed] [Google Scholar]
- 26.Hellerstein, M., M. B. Hanley, D. Cesar, S. Siler, C. Papageorgopoulos, E. Wieder, D. Schmidt, R. Hoh, R. Neese, D. Macallan, S. Deeks, and J. M. McClune. 1999. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat. Med. 5:83-89. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch, V. M., G. A. Dapolito, S. Goldstein, H. McClure, P. Emau, P. N. Fultz, M. Isahakia, R. Lenroot, G. Myers, and P. R. Johnson. 1993. A distinct African lentivirus from Sykes' monkeys. J. Virol. 67:1517-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch, V. M., B. J. Campbell, E. Bailes, R. Goeken, C. Brown, W. R. Elkins, M. Axthelm, M. Murphey-Corb, and P. M. Sharp. 1999. Characterization of a novel simian immunodeficiency virus (SIV) from L'Hoest monkeys (Cercopithecus l'hoesti): implications for the origin of SIVmnd and other primate lentiviruses. J. Virol. 73:1036-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho, D. D., A. Neumann, A. Perelson, W. Chen, J. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 31.Jin, M. J., H. Hui, D. L. Robertson, M. C. Müller-Trutwin, F. Barre-Sinoussi, V. M. Hirsch, J. S. Allan, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Mosaic genome structure of simian immunodeficiency virus from West African green monkeys. EMBO J. 13:2935-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin, M. J., J. Rogers, J. E. Phillips-Conroy, J. S. Allan, R. C. Desrosiers, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Infection of a yellow baboon with simian immunodeficiency virus from African green monkeys: evidence for cross-species transmission in the wild. J. Virol. 68:8454-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koup, R. A., and D. D. Ho. 1994. Shutting down HIV. Nature 370:416.. [DOI] [PubMed] [Google Scholar]
- 34.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lifson, J. D., M. A. Nowak, S. Goldstein, J. L. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. L. Lloyd, J. Williams, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 71:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis of HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 37.Mellors, J. W., A. Munoz, J. V. Giorgi, J. B. Margolick, C. J. Tassoni, P. Gupta, L. A. Kingsley, J. A. Todd, A. J. Saah, R. Detels, J. P. Phair, and C. R. Rinaldo, Jr. 1997. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann. Intern. Med. 126:946-954. [DOI] [PubMed] [Google Scholar]
- 38.Mohri, H., S. Bonhoeffer, S. Monard, A. S. Perelson, and D. D. Ho. 1998. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science 279:1223-1227. [DOI] [PubMed] [Google Scholar]
- 39.Müller, M. C., N. K. Saksena, E. Nerrienet, C. Chappey, V. M. A. Herve, J. P. Durand, P. Legal-Campodonico, M.-C. Lang, J.-P. Digoutte, A. J. Georges, M.-C. Georges-Courbot, P. Sonigo, and F. Barre-Sinoussi. 1993. Simian immunodeficiency viruses from Central and Western Africa: evidence for a new species-specific lentivirus in tantalus monkeys. J. Virol. 67:1227-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 41.Nerrienet, E., X. Amouretti, M. C. Müller-Trutwin, V. Poaty-Mavoungou, I. Bedjebaga, H. T. Nguyen, G. Dubreuil, S. Corbet, E. J. Wickings, F. Barre-Sinoussi, A. J. Georges, and M. C. Georges-Courbot. 1998. Phylogenetic analysis of SIV and STLV type I in mandrills (Mandrillus sphinx): indications that intracolony transmissions are predominantly the result of male-to-male aggressive contacts. AIDS Res. Hum. Retrovir. 14:785-796. [DOI] [PubMed] [Google Scholar]
- 42.Nowak, M. A., A. L. Lloyd, G. M. Vasquez, T. A. Wiltrout, L. M. Wahl, N. Bischofberger, J. Williams, A. Kinter, A. S. Fauci, V. M. Hirsch, and J. D. Lifson. 1997. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J. Virol. 71:7518-7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Brien, T., W. A. Blattner, D. Waters, E. Eyster, M. W. Hilgartner, A. R. Cohen, N. Luban, A. Hatzakis, L. M. Aledort, P. S. Rosenberg, W. J. Miley, B. L. Kroner, and J. J. Goedert. 1996. Serum HIV-1 RNA levels and time to development of AIDS in the multicenter hemophilia cohort study. JAMA 276:105-110. [PubMed] [Google Scholar]
- 44.Pandrea, I., R. Onanga, P. Rouquet, O. Bourry, P. Ngari, E. J. Wickings, P. Roques, and C. Apetrei. 2001. Chronic SIV infection ultimately causes immunodeficiency in African non-human primates. AIDS 15:2461-2462. [DOI] [PubMed] [Google Scholar]
- 45.Perelson, A., A. Neumann, M. Markowitz, J. Leonard, and D. D. Ho. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582-1586. [DOI] [PubMed] [Google Scholar]
- 46.Phillips, A. N. 1996. Reduction of HIV concentration during acute infection: independence from a specific immune response. Science 271:497-499. [DOI] [PubMed] [Google Scholar]
- 47.Piketty, C., P. Castiel, L. Belec, D. Batisse, A. Si Mohamed, J. Gilquin, G. Gonzalez-Canali, D. Jayle, M. Karmochkine, L. Weiss, J. P. Aboulker, M. D. Kazatchkine, T. W. Chun, L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1998. Discrepant responses to triple combination antiretroviral therapy in advanced HIV disease. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. AIDS 12:745-750. [DOI] [PubMed] [Google Scholar]
- 48.Reimann, K. A., K. Tenner-Racz, P. Racz, D. C. Montefiori, Y. Yasutomi, W. Lin, B. J. Ransil, and N. L. Letvin. 1994. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Virol. 68:2362-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rey-Cuille, M. A., J. L. Berthier, M. C. Bomsel-Demontoy, Y. Chaduc, L. Montagnier, A. G. Hovanessian, and L. A. Chakrabarti. 1998. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J. Virol. 72:3872-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberg, E. S., and B. D. Walker. 1998. HIV type 1-specific helper T cells: a critical host defense. AIDS Res. Hum. Retrovir. 14:S143-S147. [PubMed] [Google Scholar]
- 51.Rosenzweig, M., M. A. Demaria, D. M. Harper, S. Friedrich, R. K. Jain, and R. P. Johnson. 1998. Increased rates of CD4+ and CD8+ T lymphocyte turnover in simian immunodeficiency virus-infected macaques. Proc. Natl. Acad. Sci. USA 95:6388-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Safrit, J. T., and R. A. Koup. 1995. The immunology of primary HIV infection: which immune responses control HIV replication? Curr. Opin. Immunol. 7:456-461. [DOI] [PubMed] [Google Scholar]
- 53.Sharp, P. M., L. Bailes, D. L. Robertson, F. Gao, and B. H. Hahn. 1999. Origins and evolution of AIDS viruses. Biol. Bull. 196:338-342. [DOI] [PubMed] [Google Scholar]
- 54.Simon, F., S. Souquière, F. Damond, M. Makuwa, E. Leroy, P. Rouquet, J.-L. Berthier, J. Rigoulet, A. Lecu, P. T. Telfer, I. Pandrea, J. C. Plantier, F. Barré-Sinoussi, P. Roques, M. C. Müller-Trutwin, and C. Apetrei. 2001. A synthetic peptide strategy for the detection of and discrimination among highly divergent primate lentiviruses. AIDS Res. Hum. Retrovir. 17:937-952. [DOI] [PubMed] [Google Scholar]
- 55.Souquière, S., F. Bibollet-Ruche, D. L. Robertson, M. Makuwa, C. Apetrei, R. Onanga, C. Kornfeld, J. C. Plantier, F. Gao, K. Abernethy, L. J. T. White, W. Karesh, P. T. Telfer, E. J. Wickings, P. Mauclère, P. A. Marx, F. Barré-Sinoussi, B. H. Hahn, M. C. Müller-Trutwin, and F. Simon. 2001. Wild Mandrillus sphinx are carriers of two types of lentiviruses. J. Virol. 75:7086-7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staprans, S. I., P. J. Dailey, A. Rosenthal, C. Horton, R. M. Grant, N. Lerche, and M. B. Feinberg. 1999. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J. Virol. 73:4829-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ten Haaft, P., B. Verstrepen, K. Uberla, B. Rosenwirth, and J. Heeney. 1998. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J. Virol. 72:10281-10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ten Haaft, P., K. Murthy, M. Salas, H. McClure, R. Dubbes, W. Koornstra, H. Niphuis, D. Davis, G. van der Groen, and J. Heeney. 2001. Differences in early virus loads with different phenotypic variants of HIV-1 and SIVcpz in chimpanzees. AIDS 15:2085-2092. [DOI] [PubMed] [Google Scholar]
- 59.Tsujimoto, H., R. W. Cooper, T. Kodama, M. Fukasawa, T. Miura, Y. Ohta, K. Ishikawa, M. Nakai, E. Frost, G. E. Roelants, J. Roffi, and M. Hayami. 1988. Isolation and characterization of simian immunodeficiency virus from mandrills in Africa and its relationship to other human and simian immunodeficiency viruses. J. Virol. 62:4044-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsujimoto, H., A. Hasegawa, N. Maki, M. Fukasawa, T. Miura, S. Spiedel, R. W. Cooper, E. N. Moriyama, T. Gojobori, and M. Hayami. 1989. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature 341:539-541. [DOI] [PubMed] [Google Scholar]
- 61.Van Rensburg, E. J., S. Engelbrecht, J. Mwenda, J. D. Laten, B. A. Robson, T. Stander, and G. K. Chege. 1998. Simian immunodeficiency viruses (SIVs) from eastern and southern Africa: detection of a SIVagm variant from a chacma baboon. J. Gen. Virol. 79:1809-1814. [DOI] [PubMed] [Google Scholar]
- 62.Wallach, J. D. 1979. The mechanics of nutrition for exotic pets. Vet. Clin. N. Am. Small Anim. Pract. 9:405-414. [DOI] [PubMed] [Google Scholar]
- 63.Watson, A., J. Ranchalis, B. Travis, J. McClure, W. Sutton, P. R. Johnson, S.-L. Hu, and N. L. Haigwood. 1997. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J. Virol. 71:284-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, M. S. Saag, and G. M. Shaw. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 65.Zaitseva, M., A. Blauvelt, S. Lee, C. K. Lapham, V. Klaus-Kovtun, H. Mostowski, J. Manischewitz, and H. Golding. 1997. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat. Med. 3:1369-1375. [DOI] [PubMed] [Google Scholar]