Abstract
Successfully targeting the airway epithelium is essential for gene therapy of some pulmonary diseases. However, the airway epithelium is resistant to virus-mediated gene transfer with commonly used vectors. Vectors that interact with endogenously expressed receptors on the apical surface significantly increase gene transfer efficiency. However, other endogenous components involved in host immunity may hinder virus-mediated gene transfer. We tested the effect of bronchoalveolar lavage liquid (BAL) from patients with cystic fibrosis (CF), BAL from subjects without CF (non-CF BAL), Pseudomonas aeruginosa-derived proteins, and an array of inflammatory proteins on gene transfer mediated by adeno-associated virus type 5 (AAV5) and adenovirus targeted to an apically expressed glycosylphosphatidylinositol-modified coxsackie-adenovirus receptor. We found that neither CF BAL nor its components had a significant effect on gene transfer to human airway epithelium by these vectors. Non-CF BAL significantly impaired adenovirus-mediated gene transfer. Removal of immunoglobulins in non-CF BAL restored gene transfer efficiency. As virus vectors are improved and mechanisms of humoral immunity are elucidated, barriers to successful gene therapy found in the complex environment of the human lung can be circumvented.
Targeting the airway epithelium is essential for gene therapy of some pulmonary diseases, including cystic fibrosis (CF), alpha-1 antitrypsin (α1AT) deficiency, and lung cancer (11, 47, 48). However, airway epithelium is resistant to virus-mediated gene transfer with commonly used vectors, such as adenovirus types 2 and 5 and adeno-associated virus serotype 2 (AAV2) (3, 19, 33, 53). Low levels of gene transfer efficiency with these vectors may be due in part to the basolateral localization of viral receptors (16, 43, 47).
A method to increase apically applied adenovirus infection efficiency involves targeting the adenovirus to a receptor that is endogenously displayed on the apical surface of the airway epithelium (49). In this approach, adenovirus vector particles are modified with a ligand to target an endogenous, apically displayed receptor. Receptor targets of this method include the apically displayed G protein receptor family, the P2Y receptor 74 (49), and the urokinase plasminogen activator receptor (15). Also, modifications of the airway epithelium to enhance transfection have been examined. Chelating agents such as EGTA can increase gene transfer in vivo by disrupting tight junctions and allowing adenovirus vector particles access to the basolateral coxsackie-adenovirus receptor (CAR) (8). Airway epithelia that display apical recombinant CAR have been developed and demonstrate enhanced apical adenovirus infection (44). However, methods which fundamentally alter airway epithelium function may not be clinically useful.
Other virus vectors, such as AAV, are being investigated. Six primate isolates of AAV, a member of the parvovirus family, have been identified. Recombinant AAV2 was the first to be cloned into a plasmid and to be used in gene therapy studies (35, 51). Like adenovirus, AAV2 has a limited ability to infect respiratory epithelium due to the basolateral location of its receptor (16). More recently, another serotype, AAV5, has been demonstrated to infect human and murine airway epithelium more efficiently (55). In contrast to AAV2, AAV5 infection is mediated through an apically displayed receptor (45).
While virus vectors that target apically displayed receptors may improve gene transfer efficiency by overcoming one hurdle to infection, other endogenous airway components associated with host immunity in vivo need to be evaluated. Airway surface liquid has been shown to have antimicrobial and antiviral properties. Lysozyme, found at concentrations of 20 to 100 μg/ml, is one of the most abundant proteins in airway surface liquid (39). The antiprotease secretory leucoprotease inhibitor is found at concentrations of 10 to 80 μg/ml in nasal secretions (29). Immunoglobulins in airway surface liquid play an important role in limiting adenovirus infection of the airway epithelium in animal models (25, 52) and in humans (18). In our studies, we use bronchoalveolar lavage fluid (BAL) from patients with CF and BAL from subjects without CF to simulate the protein composition of the human airway surface liquid.
The airway surface liquid in CF patients may significantly affect virus vector efficiency due to high levels of neutrophil proteases and other proteins associated with inflammation (27, 36). In addition, many CF patients are also colonized with Pseudomonas aeruginosa species (50). Virulence factors secreted by P. aeruginosa, such as pyocyanin, have been shown to alter respiratory epithelium cell function (5, 12, 13, 24). These virulence factors may affect virus vector gene transfer to the CF airway epithelium. In addition, recent studies have demonstrated that CF BAL and CF sputum reduce adenovirus- and AAV2-mediated gene transfer (32, 37, 41). Although an adenovirus or an AAV targeted to an apically displayed receptor may improve gene transfer to the airway epithelium, proteins present in airway surface liquid, specifically in CF airway surface liquid, may provide additional barriers.
The aim of this study was to examine the effect of component proteins found in CF airway surface liquid through studies with CF BAL and non-CF BAL and their effects on gene transfer by adenovirus type 5 in an airway epithelium model displaying apical CAR. We also examined the effect of CF BAL on the AAV5 vector, which infects the airway epithelium via an endogenous apical receptor mechanism.
MATERIALS AND METHODS
Cells and culture.
Airway epithelium cells were obtained from the trachea and bronchi of lungs removed for organ donation. Cells were isolated by enzyme digestion as previously described (26, 56). Freshly isolated cells were seeded at a density of 5 × 105 cells/cm2 onto collagen-coated, 0.6-cm2-area Millicell polycarbonate filters (Millipore Corp., Bedford, Mass.). The cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and air. At 24 h after plating, the mucosal medium was removed, and the cells were grown at the air-liquid interface (26, 56). The culture medium consisted of a 1:1 mix of Dulbecco's modified Eagle's medium and Ham's F12 with 5% Ultroser G (Biosepra SA, Cergy-Saint-Christophe, France), 100 U of penicillin per ml, 100 μg of streptomycin per ml, 1% nonessential amino acids, and 0.12 U of insulin per ml. Airway epithelium reached confluence and had a transepithelial electrical resistance indicating the development of tight junctions with an intact barrier. Epithelia were allowed to differentiate by culturing for at least 14 days after seeding. The presence of a ciliated surface was tested by scanning electron microscopy (56).
Bronchoalveolar lavage.
Three normal volunteers who were lifetime nonsmokers (for non-CF BAL samples) with no history of acute or chronic illness and four CF patients who were clinically stable but known to be colonized with P. aeruginosa (for CF BAL samples) underwent bronchoscopy and bronchoalveolar lavage according to standard protocols (17). For the lavage procedure, five 20-ml aliquots of sterile, warmed saline were used. The lavage fluid was stored immediately on ice, filtered through two layers of gauze, and then centrifuged at 1,500 × g for 10 min. The supernatant was removed and stored at −70°C until use. This study was approved by the Committee for Investigations Involving Human Subjects at the University of Iowa.
Pseudomonas aeruginosa pyocyanin and elastase production.
Pyocyanin was isolated from a broth culture of P. aeruginosa PAO1 as previously described (9) and used at a final concentration of 50 μM. The final stock of pyocyanin had no detectable levels of P. aeruginosa lipopolysaccharide, as determined by the Limulus amoebocyte high performance assay (E-Toxate assay; Sigma, St. Louis, Mo.). Elastase derived from P. aeruginosa was a kind gift from Charles Cox, Department of Microbiology, University of Iowa. P. aeruginosa elastase was used at a final concentration of 500 μg/ml.
Recombinant adenovirus.
Airway epithelium was initially treated with EGTA to disrupt the epithelial tight junctions and to allow viral access to the basolateral side (43). Ten multiplicities of infection (MOI) of a recombinant adenovirus expressing glycosylphosphatidylinositol-modified CAR (GPI-CAR) was used (46). CAR constructs were modified with the Flag epitope tag, consisting of amino acids DYKDDDDK inserted downstream of the NH2-terminal hydrophobic leader signal sequence, as described previously (40). The GPI modification allows the receptor for adenovirus to be displayed at the apical surface of the epithelium. A recombinant adenovirus vector expressing green fluorescent protein (GFP) was a gift from Sam Wadsworth, Genzyme, Framingham, Mass.
Recombinant AAV.
Recombinant AAV5 was produced by a triple-plasmid transfection procedure described previously (1). AAV5/β-gal was prepared by triple-plasmid cotransfection of COS cells in a calcium phosphate cotransfection system (Gibco-BRL, Rockville, Md.). For every 5- to 150-mm plate, 6.1 μg of vector plasmid (p5LacZ), 6.1 μg of helper plasmid (p5RepCap), and 12.8 μg of pAd12 were precipitated with calcium phosphate. Cells were harvested and pelleted 72 h posttransfection. p5RepCap contains the cDNA for AAV5 Rep with the mouse mammary tumor virus promoter and the cDNA for CAP with the internal p40 promoter. The p5LacZ plasmid contains the inverted terminal repeats from the AAV5 serotype flanking a reporter β-galactosidase gene driven by a Rous sarcoma virus promoter.
For every 10 plates, the pellet was resuspended in 5 ml of tissue dissociation buffer (140 mM NaCl, 5 mM KCl, 0.7 mM K2HPO4, 25 mM Tris-HCl, pH 7.4) and stored at −70°C. The cell pellet was thawed at 37°C, and benzonase (Sigma Chemical Co.) was added to a final concentration of 20 U/ml. Sodium deoxycholate was then added to a final concentration of 0.5%, and the suspension was incubated for 1 h. The suspension was homogenized thoroughly (20 strokes in a Wheaton B homogenizer). Next, CsCl was added to a final density of 1.4 g/cm3, and the homogenate was centrifuged at 38,000 rpm for 65 h at 20°C. Gradient fractions with a refractive index of 1.371 to 1.373 were pooled, centrifuged again, and fractionated as described above. Recombinant AAV5 viruses were counted by Southern blot and transmission electron microscopy. The virus titers for recombinant preparations ranged between 1012 and 1013/ml. The ratio of infectious units to particles of AAV5 in COS cells ranged from 1:1,000 to 1:1,500.
Analysis of β-galactosidase expression.
For analysis of β-galactosidase expression, total β-galactosidase activity was measured with a commercially available method (Galacto-Light; Tropix, Inc., Bedford, Mass.). Briefly, 2 days postinfection, epithelium was washed with phosphate-buffered saline (PBS) and incubated with lysis buffer (25 mM Tris-phosphate [pH 7.8], 2 mM dithiothreitol, 2 mM 1, 2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, 10% glycerol, and 1% Triton X-100) for 15 min. Light emission was quantified as light units (L.U.) in a luminometer (Analytical Luminescence Laboratory, San Diego, Calif.).
Analysis of Ad/GFP expression and cell surface distribution of Flag-tagged GPI-CAR.
Apical localization of Flag-tagged GPI-CAR was evaluated by immunocytochemistry. Human airway epithelium was initially infected with adenovirus type 5 expressing GPI-CAR (Ad5/GPI-CAR) with EGTA (44). Two days later, these airway epithelia were apically infected with adenovirus expressing GFP (Ad/GFP) in the presence of either CF or non-CF BAL. Forty-eight hours later, epithelia were fixed with 4% paraformaldehyde for 15 min at 23°C. Cells were then washed twice in SuperBlock (Pierce, Rockford, Ill.), and mouse anti-Flag monoclonal antibody (1:600; Sigma, St. Louis, Mo.) was added to the epithelia for 1 h at 37°C. Cells were then washed twice with SuperBlock and subsequently incubated with donkey anti-mouse immunoglobulin G (IgG) conjugated with Texas Red fluorophore (1:600; Sigma, St. Louis, Mo.) for 1 h at 37°C. Epithelia were then washed twice with PBS for 5 min each and mounted on glass slides with Vectashield (Vector Laboratories Inc., Burlingame, Calif.). Apical staining and GFP expression were evaluated by laser confocal microscopy (MRC-1024; Bio-Rad, Hercules, Calif.) at ×60 magnification.
Effect of component proteins of BAL on adenovirus gene transfer to GPI-CAR-displaying human airway epithelium.
We tested the effect of individual components of airway surface liquid on adenovirus-mediated gene transfer. Concentrations of lysozyme (20 to 100 μg/ml) (38, 39), secretory leucoprotease inhibitor (10 to 80 μg/ml) (27, 28, 30, 42), neutrophil elastase (0.49 to 8.2 μM) (14, 28), and α1AT (150 to 300 μg/ml) (6, 10) in excess of those found in vivo were used. Human airway epithelia were infected with recombinant adenovirus expressing GPI-CAR, as described above.
Two days after transfection, when GPI-CAR expression was maximal (44), the epithelia were treated on the apical surface as follows: 50 μl of cell medium containing α1AT (1 mg/ml) (Sigma, St. Louis, Mo.), recombinant secretory leucoprotease inhibitor (1 mg/ml) (R&D Systems, Inc., Minneapolis, Minn.), lysozyme (1 mg/ml) (Calbiochem, San Diego, Calif.), or neutrophil elastase (3 μM) (Sigma, St. Louis, Mo.) or 50 μl of cell medium alone (control) for 30 min at 37°C. Then, 10 MOI of adenovirus type 5 expressing β-galactosidase (Ad5/β-gal) was added to the apical surface for 1 h at 37°C. Epithelia were examined for total β-galactosidase activity 2 days later. The data are expressed as means ± standard error of the mean (SEM), n = 12.
Since α1AT and secretory leucoprotease inhibitor can be present in CF airway surface liquid in complex with neutrophil elastase (27), we examined the effect of a solution containing either α1AT-neutrophil elastase complex or secretory leucoprotease inhibitor-neutrophil elastase complex on adenovirus gene transfer. Fifty microliters of a solution containing α1AT (1 mg/ml) and neutrophil elastase (3 μM) or secretory leucoprotease inhibitor (1 mg/ml) and neutrophil elastase (1.5 μg/ml) was added to the apical surface prior to addition of 10 MOI of Ad5/β-gal. β-Galactosidase activity was then measured 2 days later, as described above. The data are expressed as means ± SEM, n = 18. Similarly, the P. aeruginosa proteins elastase (maximum, 500 μg/ml) and pyocyanin (maximum, 50 μM) were added to the apical surface prior to adding 10 MOI of Ad5/β-gal, with subsequent measurement of β-galactosidase activity 2 days later, as described above. The data are expressed as means ± SEM, n = 6.
Effect of BAL on adenovirus gene transfer to GPI-CAR-displaying human airway epithelium.
We tested the effect of BAL on adenovirus gene transfer to human airway epithelium displaying GPI-CAR. The GPI-CAR-displaying human airway epithelia were incubated with 50 μl of non-CF BAL, CF BAL, or cell medium (control) for 30 min at 37°C. This was followed by apical application of 10 MOI of Ad5/β-gal. β-Galactosidase activity was evaluated 2 days later. The data are expressed as means ± SEM, n = 40, for three normal and four CF donors. In comparison, HeLa cells grown to 80% confluence were incubated with 100 μl of either non-CF BAL or cell medium (control) for 30 min at 37°C and then infected with 10 MOI of Ad5/β-gal for 1 h at 37°C. β-Galactosidase activity was evaluated 2 days later. The data are expressed as means ± SEM, n = 48, for three CF and three non-CF subjects.
To remove antiadenovirus antibody present in normal BAL, the BAL was treated with staphylococcal protein A-coated beads for 4 h at 4°C to allow IgG binding. After removal of IgG in complex with the beads, the BAL was added to the apical surface of GPI-CAR-displaying airway epithelium for 30 min at 37°C, as described above. Ten MOI of Ad5/β-gal was then added to the apical surface. β-Galactosidase activity was evaluated 2 days later. The data are expressed as means ± SEM, n = 6. To test the effect of BAL on AAV5 gene transfer, we added 500 particles per cell of recombinant AAV5/β-gal in the presence or absence of CF and non-CF BAL to the surface of airway epithelium. The cells were then incubated at 37°C for 14 days. After this time, β-galactosidase activity was measured, as described above. The data are expressed as means ± SEM, n = 18, for three subjects.
RESULTS
Component proteins of CF BAL have no effect on receptor-mediated adenovirus gene transfer to airway epithelium.
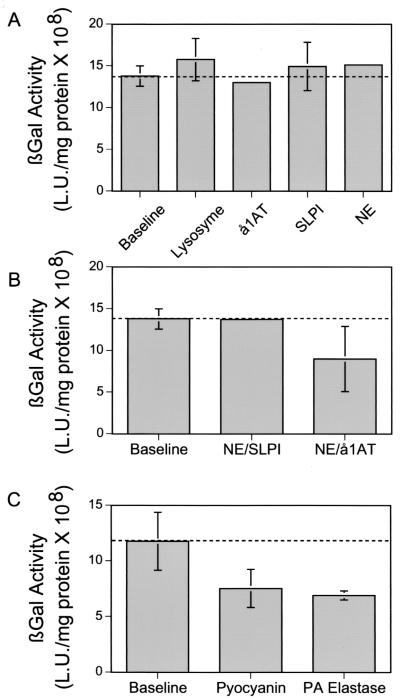
To determine whether endogenous components of CF BAL may be a barrier to gene therapy, we examined the effect of several different factors, including component proteins of innate immunity, neutrophil proteases, and bacterial virulence factors. We specifically studied the effect of lysozyme, secretory leucoprotease inhibitor, neutrophil elastase, and the antiprotease α1AT. Concentrations in excess of those found in vivo were used. Adenovirus-mediated gene transfer was not affected by any of these proteins (Fig. 1A and B).
FIG. 1.
Effect of component proteins of CF airway surface liquid on adenovirus-mediated gene transfer to human airway epithelium displaying GPI-CAR on its apical surface. Differentiated human airway epithelia were infected with 10 MOI of Ad5/GPI-CAR. Two days after infection, epithelia were incubated for 30 min with components of CF airway surface liquid prior to adding 10 MOI of Ad5/β-gal to the apical surface for 1 h. (A) Lysozyme (1 mg/ml), α1AT (1 mg/ml), secretory leucoprotease inhibitor (SLPI) (1 mg/ml), and neutrophil elastase (NE) (3 μM) were tested. The data are expressed as means ± SEM, n = 12. (B) Combinations of neutrophil elastase (3 μM) plus secretory leucoprotease inhibitor (1 mg/ml) and neutrophil elastase (3 μM) plus α1AT (1 mg/ml) were tested. The data are expressed as means ± SEM, n = 18. (C) Pyocyanin (50 μM) and elastase (500 μg/ml). Epithelia were examined for total β-galactosidase activity 2 days later. The data are expressed as mean 108 light units per milligram of protein ± SEM, n = 6.
Secretory leucoprotease inhibitor and α1AT are found in high concentrations in CF BAL and CF sputum, form protein complexes, and may have antimicrobial activity (27, 37). We tested their effect on gene transfer. As shown in Fig. 1B, neutrophil elastase plus secretory leucoprotease inhibitor did not alter adenovirus-mediated gene transfer to the airways. We found a small, statistically insignificant decrease in gene transfer with neutrophil elastase plus α1AT. Also, most CF patients are colonized with P. aeruginosa, which expresses virulence factors known to adversely affect respiratory epithelium. Therefore, we tested the effect of two common virulence factors, pyocyanin and P. aeruginosa elastase, on adenovirus gene transfer. Both of these compounds resulted in a less than 50% (P = 0.07) decline in gene transfer (Fig. 1C). Hence, components of CF BAL have little impact on adenovirus-mediated gene transfer to the airway epithelium in this model.
Non-CF BAL decreases receptor-mediated adenovirus gene transfer to airway epithelium; CF BAL has no effect on gene transfer.
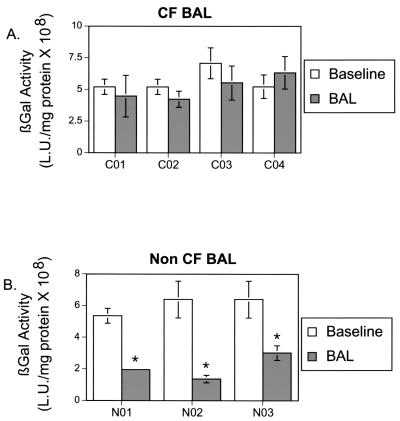
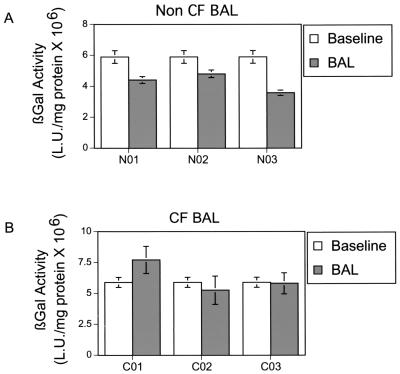
In addition to the components tested above, CF BAL may contain other substances that affect adenovirus-mediated gene transfer to the airway epithelium. Thus, we compared the effect of CF BAL, collected from four CF patients, and non-CF BAL, collected from three healthy volunteers. In the absence of BAL, adenovirus-mediated gene transfer to GPI-CAR-displaying epithelium was very efficient. The non-CF BAL, pretreated epithelium significantly inhibited gene transfer, ranging from 54% to 78%. In contrast, CF-BAL had no effect on adenovirus-mediated gene transfer (Fig. 2). These data suggest that non-CF BAL but not CF BAL contains factors that may limit adenovirus-mediated gene transfer.
FIG. 2.
Effect of BAL on Ad5/β-gal gene transfer to human airway epithelium displaying GPI-CAR on its apical surface. BAL obtained from stable CF patients (A) and from normal subjects (B) (50 μl) was applied to the apical surface of human airway epithelium displaying GPI-CAR for 30 min at 37°C. The control sample received 50 μl of medium alone. To this solution, 10 MOI of Ad5/β-gal was added for 1 h at 37°C. Epithelia were then evaluated 2 days later for β-galactosidase activity. β-Galactosidase activity is expressed as means ± SEM, n = 40, for four CF patients (C01 to C04) and n = 48 for three non-CF subjects (N01 to N03). *, P < 0.01.
Neutralizing antibodies in non-CF BAL are responsible for the decrease in gene transfer.
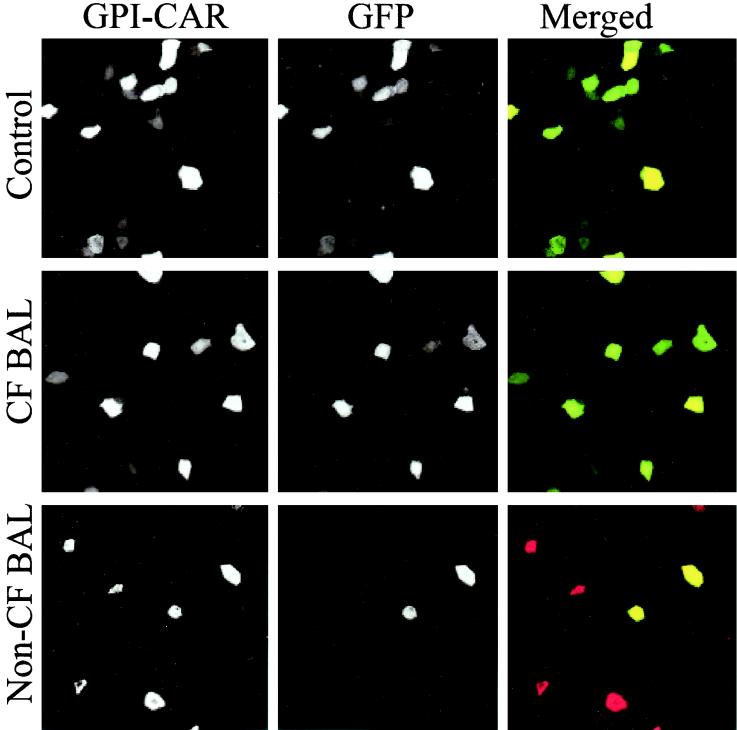
The neutralizing effect of non-CF BAL could be explained by an effect on either the GPI-CAR receptor (44) or the virus. To test this, we expressed GPI-CAR on approximately 10% of the cells of airway epithelium and then infected the epithelium with a recombinant adenovirus that expresses GFP in the presence of non-CF BAL or CF BAL. The epithelia were immunostained for GPI-CAR localization.
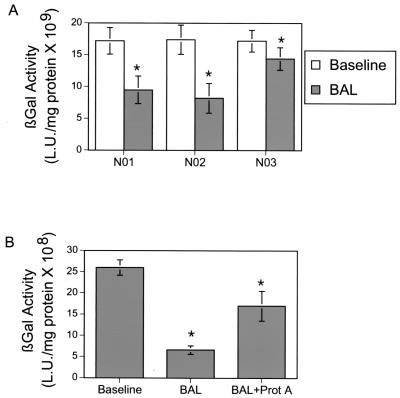
In the control epithelium, almost all epithelial cells displaying GPI-CAR also showed GFP expression, indicating that most cells displaying GPI-CAR were transfected successfully (Fig. 3A). Similarly, in the epithelium pretreated with CF BAL, almost all of the GPI-CAR-expressing cells were also successfully infected with Ad2/GFP (Fig. 3B). In contrast, the airway epithelium pretreated with non-CF BAL showed GPI-CAR expression similar to that in the control group, but GFP expression was significantly reduced (Fig. 3C). Although we cannot exclude the possibility that non-CF BAL interferes with CAR internalization, these data suggest that non-CF BAL is affecting adenovirus-mediated gene transfer by acting on the virus instead of the epithelial receptor. To ensure that the effect of non-CF BAL was not specific for the GPI-CAR receptor, we tested the effect of non-CF BAL on adenovirus infection of HeLa cells, which express wild-type CAR. The BAL reproducibly inhibited adenovirus infection (Fig. 4A).
FIG. 3.
Immunostaining of human airway epithelium displaying GPI-CAR infected with Ad2/GFP. Airway epithelium displaying GPI-CAR on the apical surface was infected with 10 MOI of Ad2/GFP in the presence of medium alone, CF BAL, and non-CF BAL. Two days later, epithelia were fixed with 4% paraformaldehyde, and a mouse anti-Flag monoclonal antibody was added to the apical surface of the epithelium for 1 h at 37°C. Subsequently, these epithelia were incubated with donkey anti-mouse IgG conjugated with Texas Red fluorophore. Apical staining for GPI-CAR and GFP expression was evaluated by laser confocal microscopy (MRC-1024; Bio-Rad) at ×60 magnification. Merged images of GPI-CAR (red) and GFP (green) are shown. Cells that express both GPI-CAR and GFP appear yellow.
FIG. 4.
(A) Effect of BAL on Ad5/β-gal gene transfer to HeLa cells. HeLa cells were grown to 80% confluence, and 100 μl of BAL obtained from normal subjects was applied to the cells. The control sample received 100 μl of HeLa medium alone. To this solution, 10 MOI of Ad5/β-gal was added for 1 h at 37°C. HeLa cells were then evaluated 2 days later for β-galactosidase activity. β-Galactosidase activity is expressed as means ± SEM, n = 48, for three subjects. *, P < 0.01. (B) Effect of treating normal BAL with staphylococcal protein A beads on adenovirus-mediated gene transfer. Normal BAL was treated with staphylococcal protein A beads for 4 h at 4°C. After removal of beads, BAL was added to the apical surface of GPI-CAR-displaying airway epithelium for 30 min at 37°C. GPI-CAR-displaying airway epithelium had equivalent amounts of either untreated or protein A-treated non-CF BAL added to the apical surface for 30 min. Ten MOI of Ad5/β-gal was then added to the apical surface for 1 h. Epithelia were then evaluated 2 days later for β-galactosidase activity. β-Galactosidase activity is expressed as means ± SEM, n = 6. *, P < 0.01.
Reduction in gene transfer caused by non-CF BAL may be due to humoral immunity towards adenovirus found generally in the human population. To test this, we used staphylococcal protein A-coated beads to precipitate immunoglobulins from the BAL fluid and to test the effect of this fluid on adenovirus infection. Immunoprecipitated BAL lost most of its activity against adenovirus infection of human airway epithelium (Fig. 4B). These data suggest that even though adenovirus-mediated gene transfer by apically targeted vectors may enhance gene transfer, immunoglobulins in non-CF BAL result in decreased gene transfer efficiency. In contrast, immunoglobulins in CF BAL may be inactivated by an overabundance of proteases. Therefore, CF BAL does not affect adenovirus gene therapy in this model.
Neither non-CF nor CF BAL decreases AAV5-mediated gene transfer to airway epithelium.
We tested the effects of human BAL on gene transfer with a virus vector, AAV5, which elicits a characteristically low human humoral response. We have recently shown that recombinant AAV5 efficiently binds and infects human airway epithelium (45). The receptor for AAV5, 2,3-sialic acid, is abundantly displayed on the apical surface of human airway epithelium. Also, human humoral response to AAV5 is generally low (22). We tested both normal and CF BAL on gene transfer by AAV5. Neither CF nor non-CF BAL had any effect on AAV5-mediated gene transfer (Fig. 5). These studies indicate that, in the absence of a humoral response, a vector targeted to a high-affinity receptor on the apical surface of the airway epithelium can mediate gene transfer despite bacterial infections and inflammation.
FIG. 5.
Effect of non-CF BAL (A) and CF BAL (B) on AAV5 gene transfer to human airway epithelium. Human airway epithelia were incubated with 50 μl of CF BAL, non-CF BAL, or vehicle alone (PBS) for 30 min at 37°C prior to the addition of 500 particles of recombinant AAV5/β-gal per cell to the apical surface. Cells were incubated with virus for 4 h at 37°C. After the incubation period, the virus suspension was removed, and the apical surface was washed twice in PBS. The cells were then incubated at 37°C for 14 days. After this time, β-galactosidase activity was measured as described above. The data are expressed as means ± SEM, n = 15, for three CF and three non-CF donors.
DISCUSSION
There are potential barriers to using virus vectors for gene transfer to the human airway epithelium in vivo. An obstacle to adenovirus vectors is the basolateral location of the CAR receptor, which limits apical entry of the vector. The presence of CAR on the apical surface circumvents this obstacle in vitro.
Another potential barrier to gene transfer is the complex environment of the human lung. We used treatment with human BAL (CF BAL and non-CF BAL) to mimic in vivo conditions in our in vitro model. As we have demonstrated in this study, non-CF BAL causes a significant reduction in adenovirus gene transfer to apically expressed GPI-CAR in the human airway epithelium model. However, CF BAL or its component proteins do not appear to have any significant effect on gene transfer to this airway epithelium. Furthermore, treatment of normal BAL with staphylococcal protein A, which removes IgG and possibly some IgA present (25), does not significantly inhibit adenovirus gene transfer. Any residual inhibitory activity is probably due to some remaining IgA.
These results support the work of previous investigations by Kaplan et al. (25) and Bastian and Bewig (4), both of whom demonstrated the ability of non-CF BAL to block adenovirus gene transfer, an effect mediated by antiadenoviral IgG and IgA antibodies. However, the identity of the antiadenovirus neutralizing antibodies present in BAL was not the focus of this work. We and others have found discrepancies between the serum antiadenovirus titers, serum neutralizing antibodies, specific adenovirus protein antibodies, and the ability to mediate gene transfer to the airways in vivo with recombinant adenoviruses (20, 54).
Interestingly, CF BAL and its component proteins had no significant effect on adenovirus gene transfer in this model. It is well recognized that most CF patients are colonized with P. aeruginosa and that P. aeruginosa produces elastase in CF patients' lungs (50). All CF patients used in this study were colonized with P. aeruginosa. P. aeruginosa elastase has been shown in in vitro studies to have protease activity against IgG and IgA as well as a variety of cytokines. Therefore, P. aeruginosa elastase could affect the activity of the antiadenoviral antibodies present in airway surface liquid. Neutrophil-derived proteases could also be responsible for affecting the activity of immunoglobulins (2, 21, 23, 31).
Neither CF nor non-CF BAL had any significant effect on AAV5 gene transfer to human airway epithelium. This may reflect a prevalence of antiadenoviral antibodies with higher activity than anti-AAV5 antibodies. Rosenecker et al. (34) demonstrated an overall prevalence of IgG against adenovirus in 95.5% of CF patients and 82% of non-CF control subjects. Chirmule et al. (7) demonstrated a prevalence of IgG against adenovirus in 95% of subjects (CF and non-CF), and yet only 55% of these subjects had antibodies with significant effect against adenovirus. The same study demonstrated antibodies to AAV-2 present in 96% of subjects, but only 32% had activity against AAV2.
In contrast to our findings, Virella-Lowell et al. (41) demonstrated that AAV2 gene transfer to a CF bronchial epithelium cell line is inhibited by CF BAL but not by non-CF BAL (41). The differences in our results may be due to the use of different vectors and different in vitro models. We use an AAV5 vector, which infects via an apically displayed receptor, in contrast to AAV2, which infects basolaterally. Also, we used a fully differentiated human airway epithelium model cultured on an insert which can present the basolateral or apical surface for our studies instead of a cell line that has apparent limitations. Finally, to date there is no evidence of cross-reactivity of antibodies against AAV2 and AAV5, indicating that antibodies against AAV2 do not block successful AAV5-mediated gene transfer (22).
In summary, we have demonstrated that non-CF BAL reduces adenovirus type 5 gene transfer to apically displayed GPI-CAR. However, successful gene transfer is predominantly restored by treating the BAL with staphylococcal protein A beads to remove IgG, suggesting a role for IgG in reducing adenovirus gene transfer. In contrast, CF BAL and component proteins of CF BAL had little effect on adenovirus gene transfer via the targeted GPI-CAR receptor. Finally, AAV5 gene transfer was not affected by either non-CF BAL or CF BAL.
As virus vectors are improved and mechanisms of humoral immunity are elucidated, barriers to successful gene therapy found in the complex environment of the human lung can be circumvented.
Acknowledgments
We thank N. G. McElvaney and S. J. O'Neill as well as C. Taggart for providing CF BAL. We thank Janice Launspach, Phil Karp, Pary Weber, Tamara Nesselhauf, Theresa Mayhew, and Rosanna Smith for excellent assistance. We thank Camille Deering for excellent editorial assistance. We especially appreciate the help of ISOPO and IIAM for the human lungs. We appreciate the support of the In Vitro Cell Models Core (supported by the NHLBI, CFF, and NIDDK via grant DK54759) and the University of Iowa Gene Transfer Vector Core (supported by the Roy J. Carver Charitable Trust, NHLBI, CFF, and NIDDK via grant DK54759).
This work was supported by NHLBI grant HL51670 (J.Z.).
REFERENCES
- 1.Alisky, J. M., S. M. Hughes, S. L. Sauter, D. Jolly, T. W. Dubensky, Jr., P. D. Staber, J. A. Chiorini, and B. L. Davidson. 2000. Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport 11:2669-2673. [DOI] [PubMed] [Google Scholar]
- 2.Bainbridge, T., and R. B. Fick, Jr. 1989. Functional importance of cystic fibrosis immunoglobulin G fragments generated by Pseudomonas aeruginosa elastase. J. Lab. Clin. Med. 114:728-733. [PubMed] [Google Scholar]
- 3.Bals, R., W. Xiao, N. Sang, D. J. Weiner, R. L. Meegalla, and J. M. Wilson. 1999. Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J. Virol. 73:6085-6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastian, A., and B. Bewig. 1999. Inhibition of adenovirus-mediated gene transfer by bronchoalveolar lavage fluid. Gene Ther. 6:637-642. [DOI] [PubMed] [Google Scholar]
- 5.Buret, A., and A. W. Cripps. 1993. The immunoevasive activities of Pseudomonas aeruginosa. Relevance for cystic fibrosis. Am. Rev. Respir. Dis. 148:793-805. [DOI] [PubMed] [Google Scholar]
- 6.Casolaro, M. A., G. Fells, M. Wewers, J. E. Pierce, F. Ogushi, R. Hubbard, S. Sellers, J. Forstrom, D. Lyons, G. Kawasaki, et al. 1987. Augmentation of lung antineutrophil elastase capacity with recombinant human alpha-1-antitrypsin. J. Appl. Physiol. 63:2015-2023. [DOI] [PubMed] [Google Scholar]
- 7.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 8.Chu, Q., J. A. St. George, M. Lukason, S. H. Cheng, R. K. Scheule, and S. J. Eastman. 2001. EGTA enhancement of adenovirus-mediated gene transfer to mouse tracheal epithelium in vivo. Hum. Gene Ther. 12:455-467. [DOI] [PubMed] [Google Scholar]
- 9.Cox, C. D. 1986. Role of pyocyanin in the acquisition of iron from transferrin. Infect. Immun. 52:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crystal, R. G., M. L. Brantly, R. C. Hubbard, D. T. Curiel, D. J. States, and M. D. Holmes. 1989. The alpha 1-antitrypsin gene and its mutations. Clinical consequences and strategies for therapy. Chest 95:196-208. [DOI] [PubMed] [Google Scholar]
- 11.Curiel, D. T., J. M. Pilewski, and S. M. Albelda. 1996. Gene therapy approaches for inherited and acquired lung diseases. Am. J. Respir. Cell Mol. Biol. 14:1-18. [DOI] [PubMed] [Google Scholar]
- 12.Denning, G. M., M. A. Railsback, G. T. Rasmussen, C. D. Cox, and B. E. Britigan. 1998. Pseudomonas pyocyanine alters calcium signaling in human airway epithelial cells. Am. J. Physiol. 274:L893-L900. [DOI] [PubMed] [Google Scholar]
- 13.Denning, G. M., L. A. Wollenweber, M. A. Railsback, C. D. Cox, L. L. Stoll, and B. E. Britigan. 1998. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect. Immun. 66:5777-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doring, G., F. Frank, C. Boudier, S. Herbert, B. Fleischer, and G. Bellon. 1995. Cleavage of lymphocyte surface antigens CD2, CD4, and CD8 by polymorphonuclear leukocyte elastase and cathepsin G in patients with cystic fibrosis. J. Immunol. 154:4842-4850. [PubMed] [Google Scholar]
- 15.Drapkin, P. T., C. R. O'Riordan, S. M. Yi, J. A. Chiorini, J. Cardella, J. Zabner, and M. J. Welsh. 2000. Targeting the urokinase plasminogen activator receptor enhances gene transfer to human airway epithelium. J. Clin. Investig. 105:589-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan, D., Y. Yue, Z. Yan, P. B. McCray, and J. F. Engelhardt. 1998. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelium. Hum. Gene Ther. 9:2761-2776. [DOI] [PubMed] [Google Scholar]
- 17.Flaherty, D. M., M. M. Monick, A. B. Carter, M. W. Peterson, and G. W. Hunninghake. 2001. GM-CSF increases AP-1 DNA binding and Ref-1 amounts in human alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 25:254-259. [DOI] [PubMed] [Google Scholar]
- 18.Gahery-Segard, H., V. Molinier-Frenkel, C. Le Boulaire, P. Saulnier, P. Opolon, R. Lengagne, E. Gautier, A. Le Cesne, L. Zitvogel, A. Venet, C. Schatz, M. Courtney, T. Le Chevalier, T. Tursz, J. G. Guillet, and F. Farace. 1997. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J. Clin. Investig. 100:2218-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grubb, B. R., R. J. Pickles, H. Ye, J. R. Yankaskas, R. N. Vick, J. F. Engelhardt, J. M. Wilson, L. G. Johnson, and R. C. J. Boucher. 1994. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelium of mice and humans. Nature 371:802-806. [DOI] [PubMed] [Google Scholar]
- 20.Harvey, B. G., P. L. Leopold, N. R. Hackett, T. M. Grasso, P. M. Williams, A. L. Tucker, R. J. Kaner, B. Ferris, I. Gonda, T. D. Sweeney, R. Ramalingam, I. Kovesdi, S. Shak, and R. G. Crystal. 1999. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J. Clin. Investig. 104:1245-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heck, L. W., P. G. Alarcon, R. M. Kulhavy, K. Morihara, M. W. Russell, and J. F. Mestecky. 1990. Degradation of IgA proteins by Pseudomonas aeruginosa elastase. J. Immunol. 144:2253-2257. [PubMed] [Google Scholar]
- 22.Hildinger, M., A. Auricchio, G. Gao, L. Wang, N. Chirmule, and J. M. Wilson. 2001. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J. Virol. 75:6199-6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holder, I. A., and R. Wheeler. 1984. Experimental studies of the pathogenesis of infections owing to Pseudomonas aeruginosa: elastase, an IgG protease. Can. J. Microbiol. 30:1118-1124. [DOI] [PubMed] [Google Scholar]
- 24.Kanthakumar, K., G. Taylor, K. W. T. Tsang, D. R. Cundell, A. Rutman, S. Smith, P. K. Jeffery, P. J. Cole, and R. Wilson. 1993. Mechanisms of action of Pseudomonas aeruginosa pyocyanin on human ciliary beat in vitro. Infect. Immun. 61:2848-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan, J. M., J. A. St. George, S. E. Pennington, L. D. Keyes, R. P. Johnson, S. C. Wadsworth, and A. E. Smith. 1996. Humoral and cellular immune responses of nonhuman primates to long-term repeated lung exposure to Ad2/CFTR-2. Gene Ther. 3:117-127. [PubMed] [Google Scholar]
- 26.Kondo, M., W. E. Finkbeiner, and J. H. Widdicombe. 1991. Simple technique for culture of highly differentiated cells from dog tracheal epithelium. Am. J. Physiol. 261:L106-L117. [DOI] [PubMed] [Google Scholar]
- 27.Konstan, M. W., K. A. Hilliard, T. M. Norvell, and M. Berger. 1994. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am. J. Respir. Crit. Care Med. 150:448-454. [DOI] [PubMed] [Google Scholar]
- 28.McElvaney, N. G., H. Nakamura, P. Birrer, C. A. Hebert, W. L. Wong, M. Alphonso, J. B. Baker, M. A. Catalano, and R. G. Crystal. 1992. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J. Clin. Investig. 90:1296-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNeely, T. B., M. Dealy, D. J. Dripps, J. M. Orenstein, S. P. Eisenberg, and S. M. Wahl. 1995. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J. Clin. Investig. 96:456-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeely, T. B., D. C. Shugars, M. Rosendahl, C. Tucker, S. P. Eisenberg, and S. M. Wahl. 1997. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood 90:1141-1149. [PubMed] [Google Scholar]
- 31.Parmely, M., A. Gale, M. Clabaugh, R. Horvat, and W. W. Zhou. 1990. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect. Immun. 58:3009-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perricone, M. A., D. D. Rees, C. R. Sacks, K. A. Smith, J. M. Kaplan, and J. A. St. George. 2000. Inhibitory effect of cystic fibrosis sputum on adenovirus-mediated gene transfer in cultured epithelial cells. Hum. Gene Ther. 11:1997-2008. [DOI] [PubMed] [Google Scholar]
- 33.Pickles, R. J., D. McCarty, H. Matsui, P. J. Hart, S. H. Randell, and R. C. Boucher. 1998. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J. Virol. 72:6014-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenecker, J., K. H. Harms, R. M. Bertele, A. Pohl-Koppe, E. von Mutius, D. Adam, and T. Nicolai. 1996. Adenovirus infection in cystic fibrosis patients: implications for the use of adenovirus vectors for gene transfer. Infection 24:5-8. [DOI] [PubMed] [Google Scholar]
- 35.Rutledge, E. A., C. L. Halbert, and D. W. Russell. 1998. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 72:309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sagel, S. D., R. Kapsner, I. Osberg, M. K. Sontag, and F. J. Accurso. 2001. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am. J. Respir. Crit. Care Med. 164:1425-1431. [DOI] [PubMed] [Google Scholar]
- 37.Stern, M., N. J. Caplen, J. E. Browning, U. Griesenbach, F. Sorgi, L. Huang, D. C. Gruenert, C. Marriot, R. G. Crystal, D. M. Geddes, and E. W. Alton. 1998. The effect of mucolytic agents on gene transfer across a CF sputum barrier in vitro. Gene Ther. 5:91-98. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, A. B., T. Bohling, F. Payvandi, and S. I. Rennard. 1990. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J. Lab. Clin. Med. 115:148-158. [PubMed] [Google Scholar]
- 39.Travis, S. M., B. A. Conway, J. Zabner, J. J. Smith, N. N. Anderson, P. K. Singh, E. P. Greenberg, and M. J. Welsh. 1999. Activity of abundant antimicrobials of the human airway. Am. J. Respir. Cell Mol. Biol. 20:872-879. [DOI] [PubMed] [Google Scholar]
- 40.van't Hof, W., and R. G. Crystal. 2001. Manipulation of the cytoplasmic and transmembrane domains alters the cell surface levels of the coxsackie-adenovirus receptor and changes the efficiency of adenovirus infection. Hum. Gene Ther. 12:25-34. [DOI] [PubMed] [Google Scholar]
- 41.Virella-Lowell, I., A. Poirier, K. A. Chesnut, M. Brantly, and T. R. Flotte. 2000. Inhibition of recombinant adeno-associated virus (rAAV) transduction by bronchial secretions from cystic fibrosis patients. Gene Ther. 7:1783-1789. [DOI] [PubMed] [Google Scholar]
- 42.Vogelmeier, C., R. C. Hubbard, G. A. Fells, H. P. Schnebli, R. C. Thompson, H. Fritz, and R. G. Crystal. 1991. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J. Clin. Investig. 87:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelium. J. Biol. Chem. 274:10219-10226. [DOI] [PubMed] [Google Scholar]
- 44.Walters, R. W., W. van't Hof, S. M. P. Yi, M. K. Schroth, J. Zabner, R. G. Crystal, and M. J. Welsh. 2001. Apical localization of the coxsackie-adenovirus receptor by glycosyl-phosphatidylinositol modification is sufficient for adenovirus-mediated gene transfer through the apical surface of human airway epithelia. J. Virol. 75:7703-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walters, R. W., S. M. Yi, S. Keshavjee, K. E. Brown, M. J. Welsh, J. A. Chiorini, and J. Zabner. 2001. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 276:20610-20616. [DOI] [PubMed] [Google Scholar]
- 46.Wang, X., and J. M. Bergelson. 1999. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J. Virol. 73:2559-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welsh, M. J. 1999. Gene transfer for cystic fibrosis. J. Clin. Investig. 104:1165-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West, J., and D. M. Rodman. 2001. Gene therapy for pulmonary diseases. Chest 119:613-617. [DOI] [PubMed] [Google Scholar]
- 49.Wickham, T. J. 2000. Targeting adenovirus. Gene Ther. 7:110-114. [DOI] [PubMed] [Google Scholar]
- 50.Wilson, R., and R. B. Dowling. 1998. Lung infections. 3. Pseudomonas aeruginosa and other related species. Thorax 53:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao, W., N. Chirmule, S. C. Berta, B. McCullough, G. Gao, and J. M. Wilson. 1999. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 73:3994-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang, Y., G. Trinchieri, and J. M. Wilson. 1995. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat. Med. 1:890-893. [DOI] [PubMed] [Google Scholar]
- 53.Zabner, J., P. Freimuth, A. Puga, A. Fabrega, and M. J. Welsh. 1997. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelium to adenovirus infection. J. Clin. Investig. 100:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zabner, J., B. W. Ramsey, D. P. Meeker, M. I. Aitken, R. P. Balfour, R. L. Gibson, J. Launspach, R. A. Moscicki, S. M. Richards, T. A. Standaert, J. Williams-Warren, S. C. Wadsworth, A. E. Smith, and M. J. Welsh. 1996. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J. Clin. Investig. 97:1504-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zabner, J., M. Seiler, R. Walters, R. M. Kotin, W. Fulgeras, B. L. Davidson, and J. A. Chiorini. 2000. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelium and facilitates gene transfer. J. Virol. 74:3852-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zabner, J., B. G. Zeiher, E. Friedman, and M. J. Welsh. 1996. Adenovirus-mediated gene transfer to ciliated airway epithelium requires prolonged incubation time. J. Virol. 70:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]