Abstract
We observed two patterns of chemokine expression in the lungs of mice infected with murine gammaherpesvirus 68: peaks of chemokine expression correlated with or occurred after the peak of viral gene expression. Chemokine expression remained elevated through 29 days postinfection.
Murine gammaherpesvirus 68 (MHV-68) infection of mice provides a model to study the pathogeneses of the human gammaherpesviruses, Epstein-Barr virus and Kaposi's sarcoma herpesvirus. MHV-68 is a naturally occurring rodent pathogen which, following intranasal inoculation in mice, causes an acute infection and replicates in the epithelial cells of lungs (2, 3, 18, 29, 30). Virus is cleared from the lungs by 10 days postinfection (dpi). Viral latency is established in the mediastinal lymph nodes as early as 3 dpi and in the spleen by 10 dpi (5, 19, 23, 28). Interestingly, the inflammatory response to MHV-68 infection in the lungs is not seen until after the virus is cleared (5, 25, 27).
Chemokines are chemotactic cytokines that orchestrate the movement of leukocytes into and out of sites of inflammation along a concentration gradient (12, 20, 31). Infection with respiratory viruses has been associated with lung inflammation and the induction of chemokines critical to the inflammatory response (7, 8, 10, 14, 32). Furthermore, induction of chemokine synthesis occurred early after infection in these studies and was postulated to be part of the innate immune response to viral infection. Underscoring the importance of chemokines in viral pathogenesis is the fact that several viruses, including members of both the herpesvirus and poxvirus families, encode proteins that are chemokine homologues, chemokine receptor homologues, or chemokine binding proteins (1, 15-17, 22, 24, 33, 37).
The identification of the patterns of the chemokine response to viral infection is essential to an understanding of the host immune response to MHV-68 as well as to elucidate the role of the virally encoded immunomodulatory proteins. In this study, we determined the kinetics of the chemokine response in the lungs of mice during acute infection with MHV-68. In addition, we examined whether the MHV-68 M3 protein modulated chemokine responses in the lungs.
Kinetics of the chemokine response in lungs of mice infected with MHV-68.
To determine the temporal patterns of chemokine gene expression in the lung following infection, 4- to 6-week-old BALB/c mice (Harlan Sprague, Indianapolis, Ind.) were infected with 4 × 104 PFU of MHV-68. RNA was extracted from lungs harvested every day on dpi 1 through 8 and on dpi 10, 13, 15, and 29. Between 3 and 11 mice were used per time point, with the exception of 2, 3, and 4 dpi, when 2 mice were used. Isolation of RNA was as described previously (6, 19). RNA was analyzed by an RNase protection assay (RPA) to measure gene transcripts for the CC chemokines RANTES, MIP-1α, MIP-1β, MCP-1, and eotaxin (Fig. 1A); the CXC chemokines MIP-2 and IP-10 (Fig. 1B); and the C chemokine Ltn (Fig. 1C) with the mCK-5 template set (PharMingen, San Diego, Calif.). To quantify levels of expression of individual transcripts relative to each other, we determined the PhosphorImager (PI) units for each protected probe fragment and expressed the PI units as percentages of the PI units of the housekeeping gene L32. Baseline levels of chemokine expression were measured from lungs of uninfected animals. Further controls included mock infections of two to three mice per time point with supernatant from mock-infected owl-monkey kidney cells at the same dilution in phosphate-buffered saline. Baseline levels of chemokine gene expression were negligible except with Ltn, which was constitutively expressed in small amounts in the two uninfected mice tested. No or minimal increases in chemokine gene expression were measured at all time points in mock-infected animals. To verify that chemokine mRNA expression as measured by RPA correlated with levels of chemokine proteins, levels of representative CC (Fig. 2A) and CXC (Fig. 2B) chemokines in lung homogenates from MHV-68- or mock-infected mice were measured by a sandwich enzyme-linked immunosorbent assay (ELISA) as previously described (11, 36).
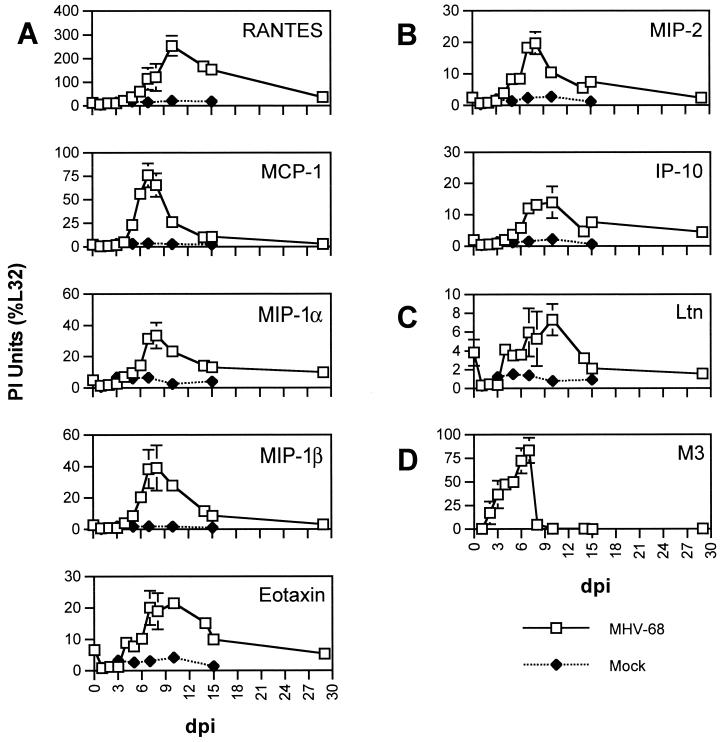
FIG. 1.
Chemokine and viral gene expression in lungs of MHV-68-infected mice. RNA extracted from the lungs of MHV-68-infected mice (□) and mock-infected mice (⧫) was analyzed by RPA with the mCK-5 riboprobe template (A to C) or the γ-3 riboprobe template. PI units were obtained for protected probe fragments corresponding to CC (A), CXC (B), and C (C) chemokines, and the data are presented as percentages of the internal housekeeping signal L32. Expression of the viral gene M3 is presented for direct comparison of host chemokine gene expression to viral gene expression (D). Means ± standard errors of the means (SEM) are shown for each time point.
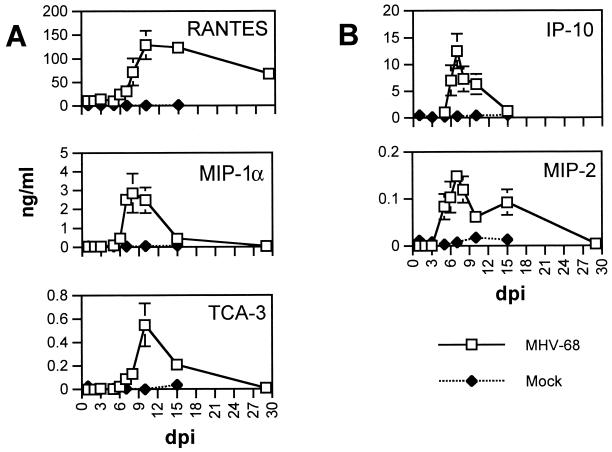
FIG. 2.
Chemokine protein production in lungs of MHV-68-infected mice. ELISA was performed on lung homogenate from MHV-68-infected and mock-infected mice for representative CC (A) and CXC (B) chemokines. Means ± SEM are shown for each time point.
For all chemokines measured, only a low level of induction of chemokine gene expression was observed between 1 and 4 dpi following infection with MHV-68 (Fig. 1). Levels of Ltn expression were slightly lower than baseline levels during this time period. Likewise, only low or negligible levels of chemokine protein were detected early in infection (before 4 dpi) (Fig. 2). Gene expression of Ltn and eotaxin began to increase by 4 dpi, while gene expression of other chemokines did not substantially increase until 5 dpi. Levels of all measured transcripts except RANTES, IP-10, and Ltn were at their maximal levels between 7 and 8 dpi. The peaks of RANTES and Ltn expression did not occur until 10 dpi. Levels of chemokine transcripts gradually declined and MIP-1β and MCP-1 transcripts were at baseline levels by 29 dpi. In contrast, levels of RANTES, MIP-1α, eotaxin, IP-10, MIP-2, and Ltn transcripts remained above baseline levels at 29 dpi, suggesting the presence of a prolonged inflammatory stimulus. Levels of RANTES protein remained elevated above 50 ng/ml through 29 dpi. The kinetics of chemokine protein production therefore mirrored levels of chemokine mRNA expression.
As a marker of viral replication, we simultaneously measured selected MHV-68 gene transcripts using the γ-3 riboprobe template (19) to directly compare the patterns of chemokine expression to that of viral gene expression. The MHV-68 gene M3 is shown in Fig. 1D as a representative marker for viral gene expression; the kinetics of expression in the lungs of all MHV-68 genes tested were similar (19; additional data not shown). Expression of M3 was detectable as early as 2 dpi, rapidly increased through 7 dpi, and precipitously fell to undetectable levels at 10 dpi (Fig. 1D). Levels of M3 were elevated prior to the induction of chemokine expression, while the peak expression of M3 corresponded to the peak expression of most chemokines measured. The clearance of M3 expression was markedly more rapid than the decline in chemokine expression.
To determine if infectious virions could contribute to the inflammatory stimulus, plaque assays were performed on whole-lung homogenates at 5 and 10 dpi. Although infectious virus was easily detected at 5 dpi, no plaques were observed at 10 dpi (data not shown), thus confirming other reports that virus is cleared from the lungs by 10 dpi (5, 27). PCR was performed to determine whether viral DNA persisted after viral clearance as measured by plaque assay. DNA was extracted from the lungs of two mice at 6 dpi and of three mice at 12 dpi with a DNEasy kit (QIAGEN Inc., Valencia, Calif.). PCR was performed to amplify the M9 region by using methods and primers as previously described (19). PCR-amplified DNA was easily detected in the lungs of both mice at 6 dpi. Interestingly, a faint PCR product was detected in the lungs of two out of three mice at 12 dpi (data not shown). No PCR product was detected in the negative control.
Two overall patterns of chemokine expression emerged from these data. In the first pattern there was a rapid rise in chemokine production, the kinetics of which had the same pattern as but was slower than that of viral gene expression. Chemokines exhibiting this pattern were MIP-1α, MIP-1β, MCP-1, MIP-2, and IP-10. In the second pattern, chemokine induction was slower to emerge, the peak of chemokine expression was delayed relative to the peak of viral gene expression, and a prolonged production of chemokines lasted long after viral gene expression was undetectable in the lungs. Chemokines exhibiting this pattern were RANTES, TCA-3, Ltn, and eotaxin.
The types of chemokines induced in the lungs of MHV-68-infected mice confirm and extend the findings of Sarawar and colleagues (21) and are similar to those seen following infection with other respiratory viruses (7, 8, 14, 32), suggesting a common pattern of innate immune response to viral infections of the lungs. Thus, the early expression of MIP-1α, MIP-1β, MIP-2, and MCP-1 may be due to release of chemokines from infected cells and may contribute to the recruitment of inflammatory cells to the site of infection. Most studies of chemokines induced following infection of lungs with viruses have focused on early times postinfection, when viral replication is highest (7, 8, 14, 32). Therefore, it is unknown whether prolonged chemokine production is unique to MHV-68 infection or characteristic of other viral infections of the lungs. We observed continued chemokine expression after virus gene expression or infectious virus could no longer be detected in the lungs. Viral DNA was still detectable, although at a very low level, shortly after the disappearance of infectious virus as measured by plaque assay. While this may indicate the presence of a small amount of infectious virus undetectable by plaque assay, the absence of viral gene expression at these time points suggests that it may also represent the presence of latent virus in the lung. These data are consistent with a report that pulmonary epithelial cells may act as a nonlymphoid reservoir for viral persistence (26) and suggest that a low-level viral persistence may serve as an inflammatory stimulus.
We observed very high levels of RANTES that were maintained until the experiment was terminated at 29 dpi. Interestingly, elevated RANTES expression was also observed in the spleens of MHV-68-infected mice at 45 dpi (9). Sarawar and colleagues also demonstrated elevated RANTES mRNA extracted from whole lungs isolated from MHV-68-infected mice at 25 dpi; surprisingly, they did not observe elevated RANTES protein levels at 25 dpi (21). They also did not observe MIP-2 protein at 15 dpi. One possible explanation for these differences is that Sarawar et al. measured RANTES in cells isolated from bronchoalveolar lavage specimens (21) rather than from whole-lung homogenates as we did in this study. The measurement of chemokines in bronchoalveolar lavage specimens may preferentially measure chemokines produced by cells recruited to the sites of infection rather than the cells infected with the virus.
The chemokines which had a slower response and delayed peaks of expression relative to those of viral genes are chemokines that have been shown to be important for the recruitment of T cells (13). Thus, the continued expression of RANTES and TCA-3 may contribute to the recruitment of CD8+ T cells necessary for the generation of a memory immune response to viral infection. In contrast, many of the chemokines expressed to higher levels earlier in infection (for example, MIP-1α, MIP-1β, and MCP-1) are important in dendritic cell maturation and migration to the lymph nodes (13). An early chemokine response may induce the migration of dendritic cells out of the lung and into the lymph nodes to initiate a specific immune response, and a later chemokine response may be necessary for the recruitment of T cells into sites of infection.
Effect of M3 gene deletion on the host chemokine response.
MHV-68 encodes two proteins that can potentially modulate the host response to MHV-68 by modulating chemokines: the M3 protein and open reading frame 74, a CXCR2 chemokine receptor homologue (34, 35). The M3 protein binds both CC and CXC chemokines in vitro (16, 33). Interestingly, despite high levels of expression of M3 in the lungs during acute infection (19), M3 deficiency has little effect on lytic virus replication in the respiratory tract or on the initial spread of virus to lymphoid tissue (4).
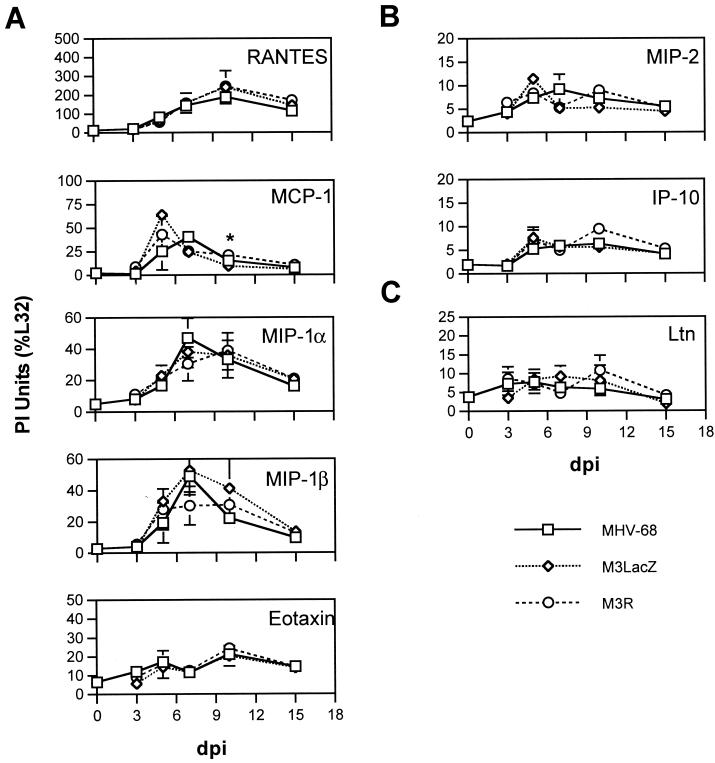
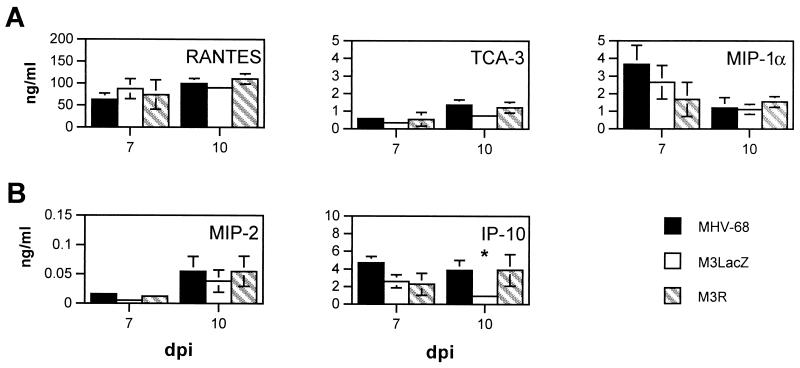
To test whether expression of M3 alters the chemokine response in lungs, between three and eight mice per time point were infected with MHV-68, with a virus lacking the M3 coding sequence (M3LacZ) (4), or with a revertant virus that restores the M3 coding sequence (M3R) (4). Chemokine gene transcripts were detected in the lungs of mice infected with MHV-68, M3LacZ, or M3R by RPA with the m-CK5 riboprobe template (Fig. 3). The overall kinetics of chemokine gene expression were similar following infection with MHV-68, M3LacZ, and M3R. To test the possibility that M3 might bind chemokine proteins and thus decrease overall levels of chemokine protein expression in the lungs, levels of selected chemokine proteins were measured at 7 and 10 dpi following infection with MHV-68, M3LacZ, or M3R (Fig. 4). These time points correspond to the time of peak viral replication in the lungs (7 dpi) and a time shortly following the clearance of detectable replicating virus from the lung (10 dpi). We observed no significant differences other than a small but statistically significant decrease in IP-10 protein levels following M3LacZ infection compared to levels after MHV-68 or M3R infection (P < 0.05). These data suggest that M3 does not modulate the levels of chemokines expressed in the lungs of MHV-68-infected mice.
FIG. 3.
Effect of M3 gene deletion on host chemokine gene expression. RNA extracted from the lungs of mice infected with MHV-68 (□), M3LacZ (◊), or M3R (○) was analyzed by RPA with the mCK-5 riboprobe template. PI units were obtained for each protected probe fragment, and levels of expression of CC (A), CXC (B), and C (C) chemokine genes are presented as percentages of the internal housekeeping signal L32. Means ± SEM are shown for each time point. Comparison by Student's t test indicated statistically significant differences between mice infected with MHV-68 and mice infected with M3LacZ (*, P < 0.05).
FIG. 4.
Effect of M3 viral gene deletion on host chemokine protein production. Lungs of mice infected with MHV-68, M3LacZ, or M3R were harvested at 7 and 10 dpi. Lung homogenates were analyzed for protein levels of selected CC (A) and CXC (B) chemokines by ELISA. Means ± SEM are shown for each time point. Comparison by Student's t test indicated statistically significant differences between mice infected with MHV-68 and mice infected with M3LacZ (*, P < 0.05).
Based on the data presented here, the delay in chemokine expression relative to the peak of viral infection is not due a direct modulation of the host chemokine response by the M3 protein. What role then does M3 play, if any, in the acute phase of viral replication in the lungs? M3 may instead significantly inhibit the effector functions of chemokines in a smaller microenvironment within the lung. This action would provide a selective advantage for the virus by inhibiting the antiviral activity of recruited leukocytes, allowing the virus to replicate at higher levels in the presence of cells serving as potential targets for the establishment of latency. Alternatively, chemokine modulation by M3 may contribute to the evasion of the host response following viral reactivation.
In sum, we have begun to describe the complex chemokine cascade that occurs following MHV-68 infection of the lungs. We found that MHV-68 induces a robust chemokine response and that the M3 chemokine binding protein does not modulate this response. An important next step is to identify which cells within the lungs express the chemokines induced during viral infection. The effects of chemokines depend on both their quantitative and qualitative patterns of expression. Only by describing the coordinated responses of multiple chemokines in conjunction with other arms of the host immune response, can we begin to elucidate the complex role of chemokines in the host response to viral infection.
Acknowledgments
Jason Weinberg was supported by a Pediatric Infectious Diseases Society Fellowship Award sponsored by Merck and Co. (Whitehouse Station, N.J.). This work was funded by NIH grants CA73556 (R.R.) and HL031237 (S.L.K.) and the Wellcome Trust and Medical Research Council, London, United Kingdom (S.E.), as well as the Suzanne and John Munn Foundation IDEA award (R.R.).
We thank Pamela Lincoln for technical assistance with the chemokine ELISAs.
REFERENCES
- 1.Billstrom, M. A., G. L. Johnson, N. J. Avdi, and G. S. Worthen. 1998. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J. Virol. 72:5535-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaskovic, D., M. Stancekova, J. Svobodova, and J. Mistrikova. 1980. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 24:468.. [PubMed] [Google Scholar]
- 3.Blaskovic, D., D. Stanekova, and J. Rajcani. 1984. Experimental pathogenesis of murine herpesvirus in newborn mice. Acta Virol. 28:225-231. [PubMed] [Google Scholar]
- 4.Bridgeman, A., P. G. Stevenson, J. P. Simas, and S. Efstathiou. 2001. A secreted chemokine binding protein encoded by murine gammaherpesvirus-68 is necessary for the establishment of a normal latent load. J. Exp. Med. 194:301-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 7.Domachowske, J. B., C. A. Bonville, K. D. Dyer, A. J. Easton, and H. F. Rosenberg. 2000. Pulmonary eosinophilia and production of MIP-1α are prominent responses to infection with pneumonia virus of mice. Cell. Immunol. 200:98-104. [DOI] [PubMed] [Google Scholar]
- 8.Domachowske, J. B., C. A. Bonville, J. L. Gao, P. M. Murphy, A. J. Easton, and H. F. Rosenberg. 2000. MIP-1 alpha is produced but it does not control pulmonary inflammation in response to respiratory syncytial virus infection in mice. Cell. Immunol. 206:1-6. [DOI] [PubMed] [Google Scholar]
- 9.Ebrahimi, B., B. M. Dutia, D. G. Brownstein, and A. A. Nash. 2001. Murine gammaherpesvirus-68 infection causes multi-organ fibrosis and alters leukocyte trafficking in interferon-gamma receptor knockout mice. Am. J. Pathol. 158:2117-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrod, K. S., A. D. Mounday, B. R. Stripp, and J. A. Whitsett. 1998. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am. J. Physiol. 275:L924-L930. [DOI] [PubMed]
- 11.Hogaboam, C. M., M. L. Steinhauser, H. Schock, N. Lukacs, R. M. Strieter, T. Standiford, and S. L. Kunkel. 1998. Therapeutic effects of nitric oxide inhibition during experimental fecal peritonitis: role of interleukin-10 and monocyte chemoattractant protein 1. Infect. Immun. 66:650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luster, A. D. 1998. Chemokines-chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 13.Matsukawa, A., N. W. Lukacs, C. M. Hogaboam, S. W. Chensue, and S. L. Kunkel. 2001. Chemokines and other mediators. 8. Chemokines and their receptors in cell-mediated immune responses in the lung. Microsc. Res. Technol. 53: 298-306. [DOI] [PubMed] [Google Scholar]
- 14.Miyazato, A., K. Kawakami, Y. Iwakura, and A. Saito. 2000. Chemokine synthesis and cellular inflammatory changes in lungs of mice bearing p40tax of human T-lymphotropic virus type 1. Clin. Exp. Immunol. 120:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore, K. W., P. Vieira, D. F. Fiorentino, M. L. Trounstine, T. A. Khan, and T. R. Mosmann. 1990. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRF1. Science 248:1230-1234. [DOI] [PubMed] [Google Scholar]
- 16.Parry, C. M., J. P. Simas, V. P. Smith, C. A. Stewart, A. C. Minson, S. Efstathiou, and A. Alcami. 2000. A broad spectrum secreted chemokine binding protein encoded by a herpesvirus. J. Exp. Med. 191:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penfold, M. E. T., D. J. Dairaghi, G. M. Duke, N. Saederup, E. S. Mocarski, G. W. Kemble, and T. J. Schall. 1999. Cytomegalovirus encodes a potent α chemokine. Proc. Natl. Acad. Sci. USA 96:9839-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajcani, J., D. Blaskovic, J. Svobodova, F. Ciampor, D. Huckova, and D. Stanekova. 1985. Pathogenesis of acute and persistent murine herpesvirus infection in mice. Acta Virol. 29:51-60. [PubMed] [Google Scholar]
- 19.Rochford, R., M. L. Lutzke, R. S. Alfinito, A. Clavo, and R. D. Cardin. 2001. Kinetics of murine gammaherpesvirus 68 gene expression following infection of murine cells in culture and in mice. J. Virol. 75:4955-4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 21.Sarawar, S. R., B. J. Lee, M. Anderson, Y. C. Teng, R. Zuberi, and S. Von Gesjen. 2002. Chemokine induction and leukocyte trafficking to the lungs during murine gammaherpesvirus 68 (MHV-68) infection. Virology 293:54-62. [DOI] [PubMed] [Google Scholar]
- 22.Schulz, T. F. 1998. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8). J. Gen. Virol. 79:1573-1591. [DOI] [PubMed] [Google Scholar]
- 23.Simas, J. P., and S. Efstathiou. 1998. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 6:276-282. [DOI] [PubMed] [Google Scholar]
- 24.Smith, G. L., J. A. Symons, A. Khanna, A. Vanderplasschen, and A. Alcami. 1997. Vaccinia virus immune evasion. Immunol. Rev. 159:137-154. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson, P. G., and P. C. Doherty. 1998. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J. Virol. 72:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 28.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 29.Svobodova, J., D. Blaskovic, and J. Mistrikova. 1982. Growth characteristics of herpesviruses isolated from free living small rodents. Acta Virol. 26:256-263. [PubMed] [Google Scholar]
- 30.Svobodova, J., M. Stancekova, D. Blaskovic, J. Mistrikova, J. Lesso, G. Russ, and P. Masarova. 1982. Antigenic relatedness of alphaherpesviruses isolated from free-living rodents. Acta Virol. 26:438-443. [PubMed] [Google Scholar]
- 31.Taub, D. D. 1996. Chemokine-leukocyte interactions. The voodoo that they do so well. Cytokine Growth Factor Rev. 7:355-376. [DOI] [PubMed] [Google Scholar]
- 32.Tripp, R. A., L. Jones, and L. J. Anderson. 2000. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J. Virol. 74:6227-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Berkel, V., J. Barrett, H. L. Tiffany, D. H. Fremont, P. M. Murphy, G. McFadden, S. H. Speck, and H. W. Virgin. 2000. Identification of a gammaherpesvirus selective chemokine binding protein that inhibits chemokine action. J. Virol. 74:6741-6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakeling, M. N., D. J. Roy, A. A. Nash, and J. P. Stewart. 2001. Characterization of the murine gammaherpesvirus 68 ORF74 product: a novel oncogenic G protein-coupled receptor. J. Gen. Virol. 82:1187-1197. [DOI] [PubMed] [Google Scholar]
- 36.Walley, K. R., N. W. Lukacs, T. J. Standiford, R. M. Strieter, and S. L. Kunkel. 1997. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect. Immun. 65:3847-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao, Z., W. C. Fanslow, M. F. Seldin, A. M. Rousseau, S. L. Painter, M. R. Comeau, J. I. Cohen, and M. K. Spriggs. 1995. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3:811-821. [DOI] [PubMed] [Google Scholar]