Abstract
Vesicular stomatitis virus (VSV) modulates protein synthesis in infected cells in a way that allows the translation of its own 5′-capped mRNA but inhibits the translation of host mRNA. Previous data have shown that inactivation of eIF2α is important for VSV-induced inhibition of host protein synthesis. We tested whether there is a role for eIF4F in this inhibition. The multisubunit eIF4F complex is involved in the regulation of protein synthesis via phosphorylation of cap-binding protein eIF4E, a subunit of eIF4F. Translation of host mRNA is significantly reduced under conditions in which eIF4E is dephosphorylated. To determine whether VSV infection alters the eIF4F complex, we analyzed eIF4E phosphorylation and the association of eIF4E with other translation initiation factors, such as eIF4G and the translation inhibitor 4E-BP1. VSV infection of HeLa cells resulted in the dephosphorylation of eIF4E at serine 209 between 3 and 6 h postinfection. This time course corresponded well to that of the inhibition of host protein synthesis induced by VSV infection. Cells infected with a VSV mutant that is delayed in the ability to inhibit host protein synthesis were also delayed in dephosphorylation of eIF4E. In addition to decreasing eIF4E phosphorylation, VSV infection also resulted in the dephosphorylation and activation of eIF4E-binding protein 4E-BP1 between 3 and 6 h postinfection. Analysis of cap-binding complexes showed that VSV infection reduced the association of eIF4E with the eIF4G scaffolding subunit at the same time as its association with 4E-BP1 increased and that these time courses correlated with the dephosphorylation of eIF4E. These changes in the eIF4F complex occurred over the same time period as the onset of viral protein synthesis, suggesting that activation of 4E-BP1 does not inhibit translation of viral mRNAs. In support of this idea, VSV protein synthesis was not affected by the presence of rapamycin, a drug that blocks 4E-BP1 phosphorylation. These data show that VSV infection results in modifications of the eIF4F complex that are correlated with the inhibition of host protein synthesis and that translation of VSV mRNAs occurs despite lowered concentrations of the active cap-binding eIF4F complex. This is the first noted modification of both eIF4E and 4E-BP1 phosphorylation levels among viruses that produce capped mRNA for protein translation.
Many viruses alter the translation apparatus such that they effectively block translation of host mRNAs and use the host cell machinery to translate their own mRNAs (16, 27). The inhibition of host protein synthesis is an important step in the suppression of the host antiviral response (27), while the continued translation of viral proteins is important for virus propagation. The prototype rhabdovirus vesicular stomatitis virus (VSV) is a classic example of a virus that rapidly inhibits protein synthesis from host mRNA and, at the same time, promotes protein synthesis from its own mRNA (32, 47).
The inhibition of host protein synthesis in VSV-infected cells is part of a program of virus-induced changes that result in the inhibition of host gene expression. In addition to inhibiting host protein synthesis, VSV infection also inhibits the transcription of host mRNAs and their nuclear-cytoplasmic transport (27, 35, 43, 48). However, the block of host protein synthesis does not result from these inhibitory effects on the production of host mRNAs, since the host mRNA pool that exists prior to infection is not depleted (26). It has been proposed that the inhibition of host protein synthesis in VSV-infected cells is a result of competition for ribosomes by an “overwhelming abundance of viral mRNAs” (16, 26). This hypothesis was disproved by demonstrating that the inhibition of host protein synthesis was unaffected by a 10-fold reduction in viral mRNA levels (40). Furthermore, in cells infected with mutants of VSV that are defective in the ability to inhibit host protein synthesis, the synthesis of viral proteins occurs at the same levels as in cells infected with wild-type (wt) VSV (15, 40, 41).
It is now widely appreciated that the inhibition of host protein synthesis by VSV is due primarily to inhibition of the activity of host translation initiation factors (27). Host protein synthesis in extracts from infected cells can be recovered by addition of either eIF2 or the eIF4F complex, leading to the hypothesis that the activity of one or both of these factors is inhibited during VSV infection (10). Subsequent studies have shown that VSV infection results in the inhibition of eIF2 activity as a result of the phosphorylation and inactivation of the eIF2α subunit by protein kinase R (PKR) (1, 2). Since supplying eIF4F could also restore host protein synthesis, the studies presented here address whether eIF4F is modified during VSV infection.
The eIF4F complex is made up of several proteins, including the 7-methyl cap-binding protein eIF4E, its kinase Mnk1, and the scaffolding protein eIF4G, which binds the components of the eIF4F complex together (18). eIF4E has been proposed to be the rate-controlling component of the eIF4F complex, since it is the least abundant protein of those that make up the complex in cells like HeLa cells (11). eIF4E is a phosphoprotein whose phosphorylation is maintained by Mnk1 (45, 46). Cells that are rapidly synthesizing protein have an increased amount of phosphorylated eIF4E (14, 37, 38), while cells that synthesize less protein, such as those under stress, have largely dephosphorylated eIF4E (12, 29, 44). This has led to the idea that the phosphorylated form of eIF4E is important for high levels of host protein synthesis.
The level of eIF4E phosphorylation and the activity of the eIF4F complex can be regulated by changing the activity of the Mnk1 kinase (45, 46). Additionally, eIF4E can be regulated by eIF4F assembly and disassembly. The disassembly of eIF4E from the eIF4F complex is carried out by a family of eIF4E-binding proteins (4E-BPs), such as 4E-BP1 (also known as PHAS1) (25, 34). 4E-BP1 is a phosphoprotein that only binds to eIF4E once it has been dephosphorylated. 4E-BP1 binding to eIF4E occurs at a site that overlaps the eIF4G-binding site (28), and 4E-BP1 binding displaces eIF4E from eIF4G (21). This displacement disrupts the eIF4F complex and removes eIF4E from Mnk1, resulting in dephosphorylation of eIF4E.
The results presented here show that VSV infection induces the dephosphorylation of eIF4E and 4E-BP1. Consistent with these results, there was a reduction in the amount of eIF4E associated with eIF4G. These changes in the eIF4F complex occurred over the same time period as the inhibition of host protein synthesis and the onset of viral protein synthesis. In addition, VSV protein synthesis was not affected by the presence of rapamycin, a drug that blocks 4E-BP1 phosphorylation. These data show that eIF4E dephosphorylation and eIF4F disruption by 4E-BP1 contribute to blocking of host protein translation but do not block viral protein synthesis.
MATERIALS AND METHODS
Chemicals, unless otherwise stated, were purchased from Fisher Scientific. Trizol reagent was purchased from Invitrogen. Rapamycin and antibodies against Mnk1, eIF4E, phospho-eIF4E, and phospho-4E-BP1 were purchased from Cell Signaling Technologies. Antibodies against eIF4G and actin were purchased from Santa Cruz Biotechnology, Inc. m7-GTP Sepharose, secondary antibodies, and [35S]methionine were purchased from Amersham-Pharmacia. Four to 20% precast gels were purchased from Bio-Rad Laboratories, Inc. Okadaic acid and microcystin were purchased from Alexis Pharmaceuticals.
Virus infections.
HeLa cells were cultured in Dulbecco's modified Eagle medium (DMEM; Gibco-BRL) containing 10% fetal bovine serum (FBS) and 2 mM glutamine. Cells were grown to 80 to 90% confluency and infected with wt VSV (Indiana serotype, Orsay strain) or the tsO82 mutant virus in DMEM with 2% FBS at a multiplicity of 20 PFU/cell in a small volume (500 μl per well of a six-well dish). At 1 h postinfection, the culture volume was doubled by addition of DMEM plus 2% FBS.
Northern blotting.
Infected or mock-infected cells were lysed in six-well dishes with 1 ml of Trizol reagent (Invitrogen), and RNA was purified in accordance with the manufacturer's instructions. Five micrograms of total RNA from each sample was glyoxylated and separated on a 1% agarose gel. Following acridine orange staining, to check for loading, RNA was transferred onto nitrocellulose (GeneScreen Plus; NEN) and probed with an M protein cDNA probe generated with the Prime-A-Gene labeling system (Promega). Results of the Northern procedure were determined by phosphorescence imaging and analyzed with ImageQuant software.
Immunoblotting.
Infected or mock-infected cells were lysed in six-well dishes with 400 μl of EBC buffer (39) containing 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 100 nM okadaic acid, and 100 nM microcystin. Lysates were spun at 10,000 × g for 8 min in a refrigerated centrifuge, and 360 μl of the supernatant was added to 40 μl of 10× sample buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Equal volumes of lysate were subjected to SDS-12% PAGE. Following electrophoresis, gels were electroblotted onto nitrocellulose and blocked in Tris-buffered saline (pH 7.5) plus 5% dry milk. Antibodies were diluted as recommended by the manufacturers. Band intensities were quantitated by scanning and analysis with Quantity One software (Bio-Rad).
Cap-binding protein enrichment assays.
For isolation of the cap-binding protein eIF4E and its associated proteins, cells were lysed in 500 μl of EBC buffer containing 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 100 nM okadaic acid, and 100 nM microcystin. Lysates were spun at 10,000 × g for 8 min, and 400μl of supernatant was incubated with a 30-μl bed volume of m7-GTP Sepharose with shaking for 1 h. Beads were spun for 3 min at 300 × g. The supernatant was removed, and beads were washed once with 1 ml of TENS buffer (39) and three times with 1 ml of TSE buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA). Following the last wash, the supernatant was removed and 25 μl of 2× SDS-PAGE sample buffer was added.
Metabolic labeling.
HeLa cells were mock infected or infected with VSV and then labeled with [35S]methionine for 10 min at 1, 2, 3, 4, and 6 h postinfection as previously described (24). Following labeling, cells were washed three times with phosphate buffered saline and lysed in 400 μl of radioimmunoprecipitation assay buffer (39) for 10 min at 4°C. Lysates were spun at 10,000 × g for 8 min. Three hundred sixty microliters of supernatant was added to 40 μl of 10× SDS-PAGE sample buffer, and 10 μl was subjected to SDS-12% PAGE. Gels were stained with Coomassie blue to determine their protein content and then analyzed by phosphorescence imaging (Molecular Dynamics, Inc.). Quantitation of phosphorescence intensities was done with ImageQuant software.
RESULTS
VSV infection results in the dephosphorylation of eIF4E concurrent with inhibition of host protein synthesis.
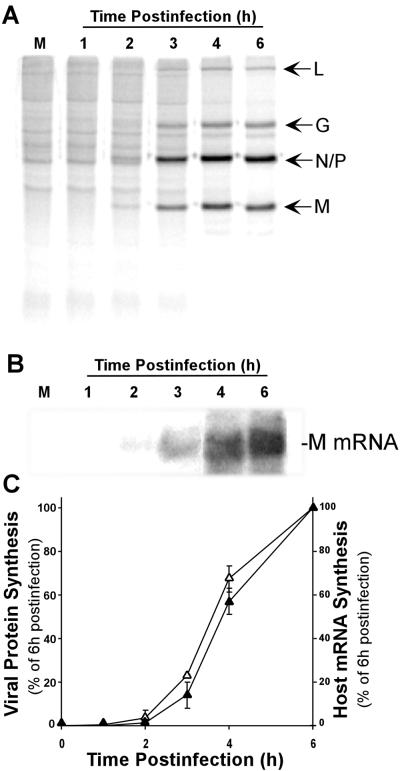
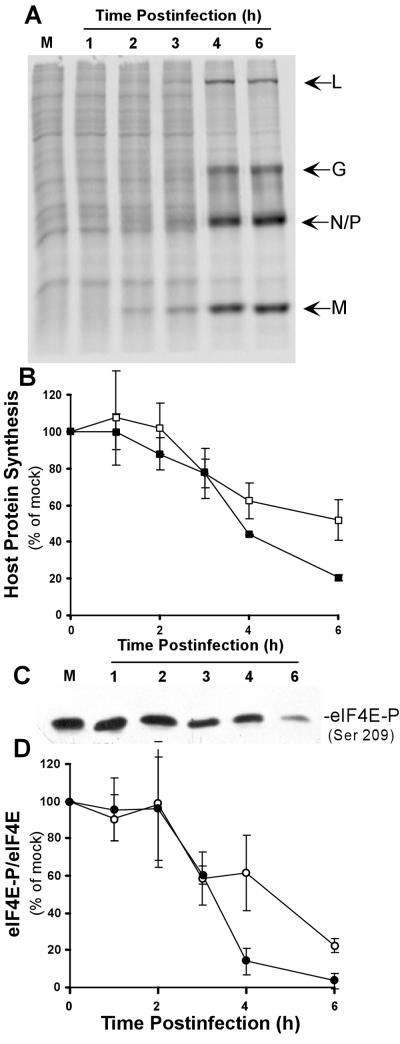
VSV infection results in changes in the host protein synthetic machinery so that viral mRNAs are translated and host protein synthesis is inhibited. Figure 1A shows the changes in protein synthesis in HeLa cells at early times postinfection with VSV. Cells were pulse-labeled with [35S]methionine and analyzed by SDS-PAGE and phosphorescence imaging. The synthesis of host proteins was apparent as a ladder of dark bands in the mock-infected control lane and from 1 to 3 h postinfection with VSV. Between 3 and 6 h postinfection, there was rapid inhibition of host protein synthesis. This inhibition can be seen most clearly in regions of the gel devoid of viral proteins. Synthesis of five VSV proteins, L, G, N, P, and M, began in approximately the same time frame as the inhibition of protein synthesis, and these became the predominant translation products at 6 h postinfection.
FIG. 1.
Viral and host protein synthesis following VSV infection. (A) HeLa cells were mock infected (M) or infected with VSV for the indicated times and then labeled with [35S]methionine for 10 min. Protein lysates (10 μl) were electrophoresed on a 12% gel, a phosphorescence image of which is shown. Viral proteins are indicated to the right of the image. (B) Total RNA was prepared from HeLa cells mock infected (M) or infected with VSV for the indicated times. RNA was separated on a 1% gel and transferred to nitrocellulose, and viral mRNA was detected with a 32P-labeled probe against the M protein mRNA. A phosphorescence image of a representative gel is shown. (C) Quantification of viral protein synthesis inhibition (closed triangles) and M protein mRNA (open triangles). The rate of viral protein synthesis was determined from experiments like that shown in panel A by quantitation of the radioactivity in the viral L and G bands and the N/P and M bands. The amount of mRNA present was determined by densitometry of M protein mRNA in Northern blots like that in panel B. The data shown are means ± standard deviations of three separate experiments
We were interested in determining whether the protein synthesis inhibition resulting from VSV infection was a specific inhibition of host mRNA translation or whether it represented a global inhibition of protein synthesis that VSV overcomes by overproduction of viral mRNA during this time period. VSV mRNAs were analyzed by Northern blot assays, and their levels were correlated with the rates of viral protein synthesis. Northern analysis of viral M mRNA production (Fig. 1B) showed that there was a steadily increasing amount of M mRNA in VSV-infected HeLa cells between 3 and 6 h postinfection. A comparison of M mRNA levels and M protein translation (Fig. 1C) showed that both mRNA production and protein translation followed the same time course, supporting the hypothesis that viral mRNAs are translated during the time of inhibition of host mRNA translation.
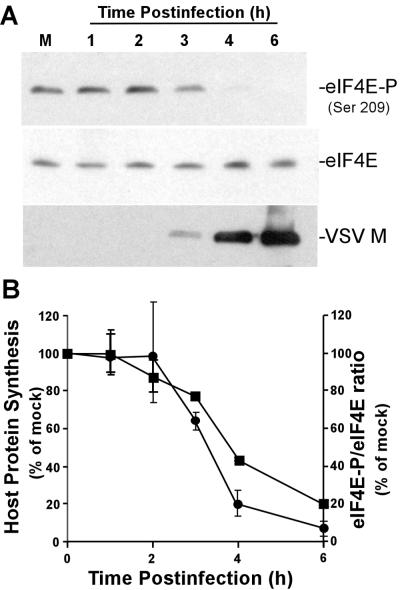
Previous data have demonstrated that two initiation factors, eIF2α and the multisubunit eIF4F complex, are important for the control of translation in VSV-infected cells (10). The inactivation of eIF2α has been shown to be due to phosphorylation by PKR (1, 2). To test for modification of the eIF4F complex, we determined whether phosphorylation of cap-binding protein eIF4E was changed in VSV-infected cells over the time period in which host protein synthesis is inhibited. Phosphorylation of eIF4E was assayed in VSV-infected cells and mock-infected controls by Western blot analysis with an antibody specific for eIF4E phosphorylated at serine 209, as shown in Fig. 2A. There was a decrease in eIF4E phosphorylation in VSV-infected cells beginning at 3 h postinfection, so that by 6 h postinfection, the phosphorylated form of eIF4E was barely detectable. This decrease was not due to an effect on levels of total eIF4E, as shown by Western blot analysis with an antibody that reacts with both the phosphorylated and dephosphorylated forms of eIF4E. The phosphorylation of eIF4E was inversely correlated with the translation of VSV mRNAs, as shown by the accumulation of the viral M protein (Fig. 2A, anti-VSV M-protein Western blot). These data indicated that viral protein synthesis occurred despite the dephosphorylation of eIF4E.
FIG. 2.
(A) Extracts from mock- and VSV-infected cells were resolved by SDS-PAGE, transferred to nitrocellulose, and analyzed by Western blotting with antibodies against eIF4E, eIF4E-P phosphorylated at serine 209 [eIF4E-P (Ser 209)], or the VSV M protein. Shown is a single blot reprobed with the three different antibodies. M, mock infection. (B) Quantification of host protein synthesis inhibition (closed squares) and eIF4E phosphorylation (closed circles). The rate of host protein synthesis was determined from experiments like that shown in Fig. 1 by quantitation of the radioactivity between the viral L and G bands, between the P and M bands, and in the region below the M band. The levels of eIF4E phosphorylation were determined by densitometry of phospho-eIF4E-specific Western blots and quantitated as a ratio to total eIF4E. The data shown are means ± standard deviations of three experiments.
Figure 2B shows a quantitative comparison of the inhibition of host protein synthesis and the dephosphorylation of eIF4E from three independent experiments. The rate of host protein synthesis was determined from experiments like that shown in Fig. 1A by quantitation of the sum of the radioactivity between the viral L and G bands, between the P and M bands, and in the region below the M band. The levels of eIF4E phosphorylation were determined by densitometry of phospho-eIF4E-specific Western blots and normalized to the total eIF4E. There was a close correlation between the onset of host protein synthesis inhibition and the dephosphorylation of eIF4E, and both eIF4E dephosphorylation and inhibition of host protein synthesis were almost complete by 6 h postinfection. These data are consistent with a role for eIF4E dephosphorylation in the inhibition of host protein synthesis by VSV.
VSV does not alter the concentration of eIF4F protein complex members.
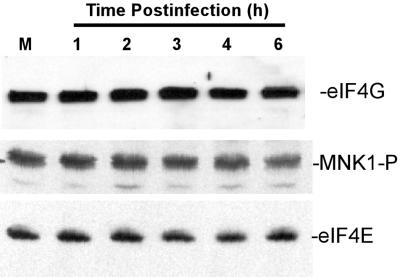
Dephosphorylation of eIF4E could result from a decrease in the cellular levels of the eIF4E-phosphorylating protein kinase Mnk1 or the eIF4G scaffolding subunit that binds eIF4E and Mnk1 together. To test whether the level of either of these two proteins changed during VSV infection, we used antibodies against eIF4G and an antibody-activated Mnk1 phosphorylated at Thr 197 and Thr 202, the antibody against active Mnk1 phosphorylated by kinases such as p38 mitogen-activated protein kinase. Western blot analysis showed little, if any, change in eIF4G protein levels throughout the first 6 h of infection (Fig. 3, top). Similarly, the level of phosphorylated Mnk1 also did not appear to change during VSV infection (Fig. 3, middle). As shown in Fig. 2, there was no change in eIF4E in these experiments (Fig. 3, bottom). These results indicated that the dephosphorylation of eIF4E in VSV-infected cells was not due to a reduction of these components of the eIF4F complex.
FIG. 3.
VSV infection does not decrease the levels of eIF4G or phosphorylated Mnk1. Extracts from HeLa cells that were mock infected (M) or infected with VSV for the indicated time were separated on a 6% gel (for eIF4G) or a 12% gel and then analyzed by Western blotting with antibodies against eIF4G, Mnk1 phosphorylated at Thr 197 and Thr 202, and eIF4E.
VSV infection results in a shift of eIF4E binding from eIF4G to 4E-BP1.
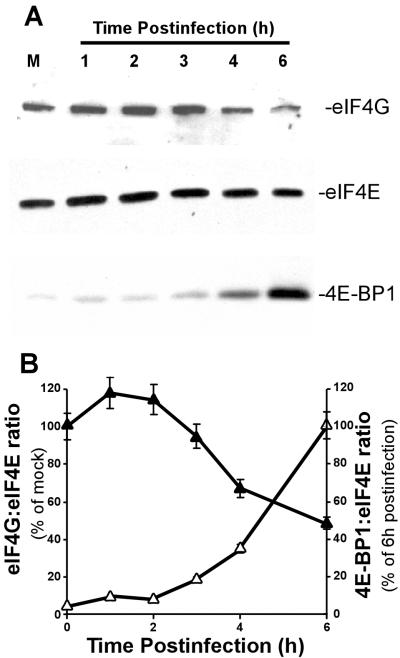
Because the dephosphorylation of eIF4E was not due to a change in the level of eIF4G or Mnk1, we determined whether eIF4E was associated with 4E-BP1 rather than eIF4G in virus-infected cells. Cap-binding complexes composed of eIF4E and its associated proteins were isolated from VSV-infected or mock-infected cells with a cap analog affinity matrix, m7-GTP Sepharose. Proteins bound to eIF4E were detected by Western blotting following the separation of the isolated complexes by SDS-PAGE. In mock-infected cells, eIF4E bound to m7-GTP Sepharose was associated with eIF4G (Fig. 4A, top). Between 3 and 6 h postinfection, eIF4G association with eIF4E was reduced. As a control for the efficiency of complex isolation, the amount of eIF4E bound to m7-GTP Sepharose remained constant for each sample (Fig. 4A, middle). In contrast to eIF4G, there was little, if any, 4E-BP1 associated with eIF4E in mock-infected cells (Fig. 4A, bottom). However, between 3 and 6 h postinfection, the amount of eIF4E associated with 4E-BP1 increased significantly.
FIG. 4.
Isolation of eIF4E and eIF4E-binding proteins from VSV-infected cells. (A) HeLa cells were mock infected (M) or infected with VSV for the indicated times. Cell extracts were then incubated with m7-GTP Sepharose to enrich for eIF4E and eIF4E-binding proteins. Proteins bound to eIF4E were determined by Western blotting following electrophoresis on a 4 to 20% gradient gel with antibodies against eIF4E, eIF4G, and 4E-BP1. Shown is a single blot reprobed with the three different antibodies. (B) Quantitation of eIF4G (closed triangles) and 4E-BP1 (open triangles) bound to eIF4E during VSV infection. eIF4G was quantitated as a ratio of eIF4G signal to eIF4E signal and expressed as a percentage of the mock-infected control. eIF4E-BP1 was also quantitated as a ratio to eIF4E and was expressed as a percentage of the value at 6 h postinfection. The data shown are averages ± standard deviations of three separate experiments.
To compare the time courses of the decrease in eIF4G and the increase in 4E-BP1 associated with eIF4E, the results of three experiments were quantitated by densitometry and are shown in Fig. 4B. Levels of eIF4G were expressed as a percentage of mock-infected controls, while 4E-BP1 levels were expressed as a percentage of the level at 6 h postinfection. By 6 h postinfection, eIF4G association with eIF4E decreased to about 40% of that of the mock-infected control, indicating that a majority of eIF4F complexes were disrupted but that some of these complexes remained assembled. The increase in association of 4E-BP1 with eIF4E occurred on the same time scale as the decrease in eIF4G binding, suggesting that 4E-BP1 was responsible for the dissociation of eIF4E from eIF4G.
VSV infection results in dephosphorylation of 4E-BP1.
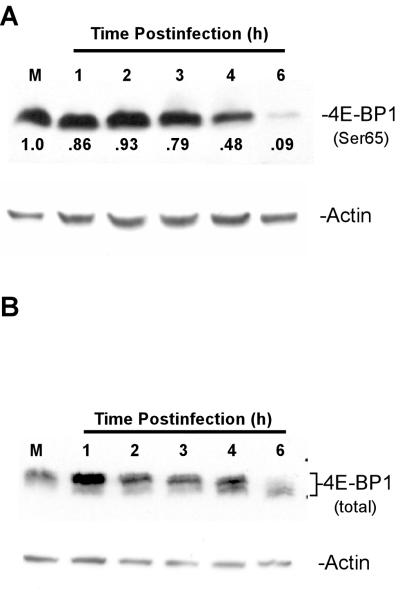
The association of 4E-BP1 with eIF4E is regulated by 4E-BP1 phosphorylation. 4E-BP1 can be phosphorylated at multiple sites (17, 31). Phosphorylated 4E-BP1 does not bind to eIF4E (25, 34), while unphosphorylated 4E-BP1 binds tightly to eIF4E. The increase in 4E-BP1 binding by eIF4E shown in Fig. 3 suggested that 4E-BP1 was being activated by dephosphorylation during VSV infection. To test this hypothesis, whole-cell lysates were analyzed by Western blot assays with an antibody specific for 4E-BP1 phosphorylated at serine 65. Phosphorylated 4E-BP1 was readily detectible in mock-infected cells (Fig. 5A, top), but between 4 and 6 h postinfection, there was a 90% loss of immunoreactivity with the phospho-4E-BP1 antibody.
FIG. 5.
4E-BP1 phosphorylation in VSV-infected cells. Cells were mock infected (M) or infected with VSV; cell extracts were separated on a 15% gel and analyzed by Western blotting with antibodies to 4E-BP1 phosphorylated at Ser 65 (A) or total 4E-BP1 (B). Protein loading was analyzed by blotting with an antibody against actin. Quantitation of the relative amounts of phospho 4E-BP1 (compared to mock infection) is shown directly below the bands.
This result was confirmed by analysis of total 4E-BP1. When separated by SDS-PAGE, the different phosphorylated forms of 4E-BP1 show a laddered appearance, with the more heavily phosphorylated forms of the protein migrating more slowly and the unphosphorylated form migrating more quickly. Consistent with the results in Fig. 5A, Western analysis of total 4E-BP1 in cells infected with VSV (Fig. 5B, top) showed that 4E-BP1 was hyperphosphorylated in mock-infected cells and that dephosphorylation of 4E-BP1 occurred between 4 and 6 h postinfection, when the faster-migrating dephosphorylated bands began to appear in virus-infected cells. By 6 h postinfection, a substantial amount of 4E-BP1 was converted to the faster-migrating dephosphorylated form.
The data in Fig. 2 to 5 are consistent with regulation of eIF4F in VSV-infected cells by mechanisms that are known to disrupt the eIF4F complex. 4E-BP1 was activated by dephosphorylation (Fig. 5). Dephosphorylated 4E-BP1 bound to eIF4E and dissociated eIF4E from eIF4G (Fig. 4). This suggests that the dephosphorylation of eIF4E (Fig. 2) is due, in part, to the separation of eIF4E from the other components of the eIF4F complex, including the protein kinase Mnk1.
A VSV mutant that is delayed in inhibition of protein synthesis is delayed in dephosphorylation of eIF4E.
To further test the hypothesis that the dephosphorylation of eIF4E plays a role in VSV-induced inhibition of host protein synthesis, the phosphorylation of eIF4E was analyzed in cells infected with the tsO82 mutant of VSV. tsO82 virus contains the M51R point mutation in its M protein (8), which renders this virus defective in inhibition of host protein synthesis (24). This is apparent in Fig. 6A as a delay in the inhibition of protein synthesis such that synthesis of host proteins continued through 6 h postinfection. Viral protein synthesis in cells infected with the tsO82 virus proceeded at the same rate as in cells infected with wt VSV, beginning at around 3 h and reaching high levels by 6 h (Fig. 6A; compare to 4- and 6-h postinfection lanes in Fig. 1A)). Quantitation of the rate of host protein synthesis in three separate experiments showed that there was almost twice as much host protein synthesis at 6 h postinfection in cells infected with tsO82 virus as in cells infected with wt VSV (Fig. 6B).
FIG. 6.
Effect of tsO82 mutant virus on host cell protein synthesis and eIF4E phosphorylation. (A) HeLa cells were mock infected (M) or infected with tsO82 virus for the indicated times and then labeled with [35S]methionine for 10 min. Lysates (10 μl) were electrophoresed on a 12% gel, a phosphorescence image of which is shown. Viral proteins are indicated to the right of the image. (B) Quantification of host protein synthesis inhibition following tsO82 virus or VSV infection. The rate of host protein synthesis was determined from experiments like that shown in panel A by quantitation of the radioactivity between the viral L and G bands, between the P and M bands, and in the region below the M band. Cells infected with wt VSV are represented by filled squares, and cells infected with tsO82 virus are represented by open squares. (C) Extracts from mock- and VSV-infected cells were resolved by SDS-PAGE, transferred to nitrocellulose, and analyzed by Western blotting with antibodies to eIF4E phosphorylated at serine 209 [eIF4E-P (Ser 209)]. (D) Quantification of eIF4E phosphorylation following VSV or tsO82 infection. The levels of eIF4E phosphorylation were determined by densitometry of phospho-eIF4E-specific Western blots and normalized to total eIF4E. Cells infected with wt VSV are represented by filled circles, and cells infected with tsO82 virus are represented by open circles. The data shown are averages ± standard deviations of three separate experiments.
Analysis of eIF4E phosphorylation following infection with tsO82 virus showed that eIF4E was still dephosphorylated (Fig. 6C) but that the dephosphorylation was not as rapid as that observed with wt VSV (Fig. 6D). When tsO82 virus was compared to wt VSV, the delay in host protein synthesis inhibition (Fig. 6B) was similar to the delay in eIF4E dephosphorylation (Fig. 6D), lending further support to the idea that eIF4E dephosphorylation plays a role in the inhibition of host protein synthesis. However, the delays in these two processes were slightly different.
In the case of eIF4E dephosphorylation, the greatest difference between the tsO82 and wt viruses was observed 4 h postinfection. This difference was highly reproducible. The error bars seen in Fig. 6D represent the day-to-day variation between experiments. However, in each paired experiment, the dephosphorylation of eIF4E in the wt VSV-infected cells was greater than that in the tsO82-infected cells at 4 h postinfection. This difference in eIF4E dephosphorylation supports the idea that the dephosphorylation of eIF4E accelerates the shutoff of host protein synthesis.
The greatest difference between the ts082 and wt viruses in their inhibition of host protein synthesis was observed at 6 h postinfection (Fig. 6B). The difference in eIF4E dephosphorylation at this time point, while still apparent, was less than that seen at the 4-h time point. These results suggest that the dephosphorylation of eIF4E is not solely responsible for the inhibition of host protein synthesis and that the tsO82 virus may also be defective in the inhibition of other translation initiation factors, such as eIF2α.
Both wt VSV and the tsO82 mutant recruit eIF4E into an eIF4E/4EBP1 complex.
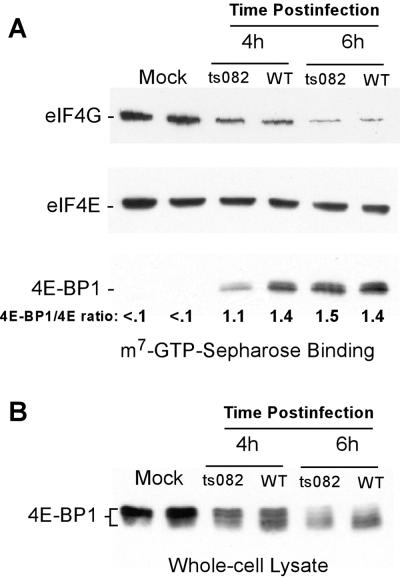
Cap-binding complexes in cells infected with tsO82 virus were analyzed to determine whether the delay in eIF4E dephosphorylation was accompanied by a delay of 4E-BP1 binding to eIF4E. Cells were infected with tsO82 virus or wt VSV as a control and analyzed at either 4 or 6 h postinfection. Cap-binding complexes were isolated on m7-GTP Sepharose, and the amount of 4E-BP1 and eIF4G bound to eIF4E was determined by Western blotting. At 4 h postinfection, a smaller amount of 4E-BP1 was associated with eIF4E in tsO82 virus-infected cells than in wt VSV-infected cells (eIF4E-BP1/eIF4E ratio shown at the bottom of Fig. 7A), but by 6 h, similar amounts of 4E-BP1 were associated with eIF4E in cells infected with either virus (Fig. 7A). The difference between the results obtained with wt VSV versus tsO82 was reproducible in four separate experiments. These results are consistent with the timing of eIF4E dephosphorylation, in which there was a marked difference between the tsO82 and wt viruses at 4 h postinfection, but this difference was much smaller by 6 h postinfection. Despite the difference in 4E-BP1 binding at 4 h postinfection, the levels of eIF4G bound to eIF4E appeared to be similar in cells infected with both viruses. The reason for this discrepancy is not known, but it may mean that there are other events that reduce the binding of eIF4E to eIF4G, perhaps through protein-protein interactions with stress-activated factors. There was noticeably less eIF4G bound to eIF4E at 6 h postinfection than at 4 h postinfection in cells infected with either virus.
FIG. 7.
4E-BP1 phosphorylation and isolation of eIF4E-binding proteins in tsO82 virus-infected cells. (A) Extracts from mock-, wt VSV-, or tsO82 virus-infected cells were incubated with m7-GTP Sepharose to enrich for eIF4E and eIF4E-binding proteins. Proteins bound to eIF4E were detected by Western blotting following separation on a 4 to 20% gel. The ratio of eIF4E to eIF4E-BP1 was quantified as described in Materials and Methods. (B) Extracts from mock-, wt VSV- or tsO82 virus-infected cells were separated on a 15% gel and analyzed by Western blotting with antibodies to total eIF4E-BP1.
The difference in the phosphorylation of 4E-BP1 was also seen when whole-cell lysates were analyzed for total 4E-BP1. In wt VSV-infected cells, 4E-BP1 began to be dephosphorylated at 4 h and was largely dephosphorylated by 6 h. In tsO82 virus-infected cells, 4E-BP1 was dephosphorylated to a lesser extent at 4 h but was dephosphorylated to the same extent as in wt VSV-infected cells by 6 h (Fig. 7B). These experiments showed that the dephosphorylation of 4E-BP1 was delayed in cells infected with the tsO82 mutant. This supports the idea that dephosphorylation of 4E-BP1 plays a role in the dephosphorylation of eIF4E and the inhibition of host protein synthesis.
Rapamycin does not block VSV protein synthesis.
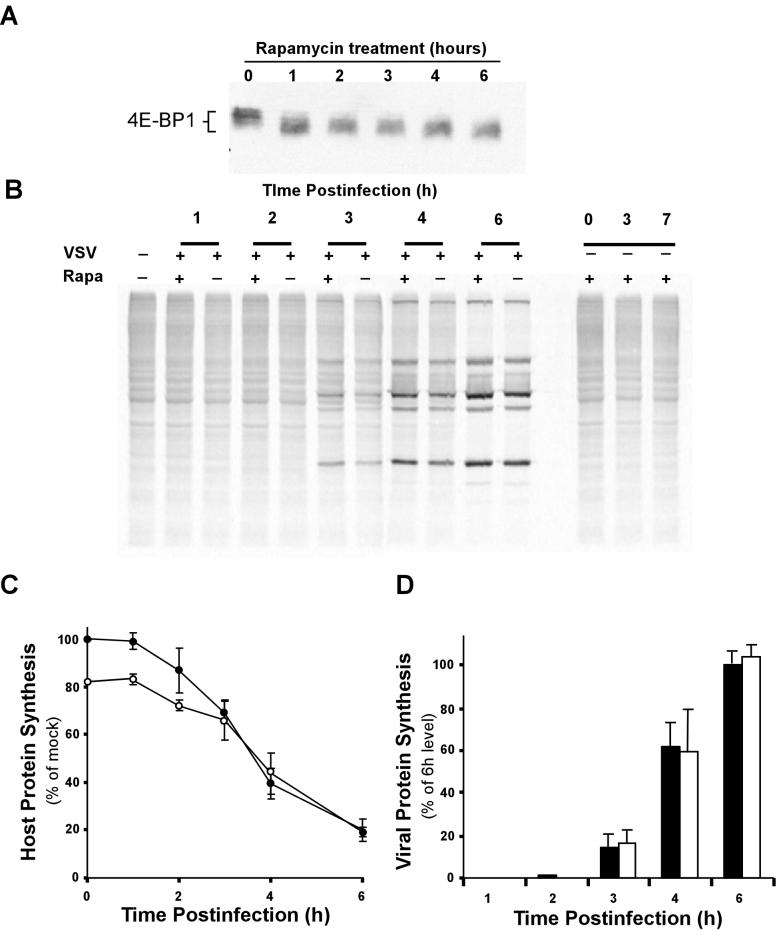
Previous work showed that the synthesis of viral proteins is decreased in VSV-infected cells treated with the immunosuppressant drug rapamycin (3). In contrast, our data showing that VSV translation occurs in the presence of 4E-BP1 phosphorylation suggested that rapamycin should not affect the synthesis of VSV proteins. Rapamycin interferes with mTOR, a kinase that maintains 4E-BP1 in its phosphorylated form (6, 19). Addition of rapamycin to cells blocks mTOR function and induces the dephosphorylation of 4E-BP1 (17, 31). Our results show an activation of 4E-BP1 dephosphorylation in VSV-infected cells that is similar to the effect of rapamycin. This suggested that while 4E-BP1 activation might play a role in VSV's inhibition of host protein synthesis, viral protein synthesis is not affected by activation of 4E-BP1, since viral mRNAs are translated at high rates in infected cells despite the activation of 4E-BP1. To test the influence of 4E-BP1 preactivation on VSV's inhibition of protein synthesis and viral protein synthesis, we determined the effect of pretreatment of cells with rapamycin on viral and host protein synthesis. Pretreatment of HeLa cells for 1 h with 25 nM rapamycin was sufficient to induce a conversion of 4E-BP1 from its phosphorylated, slow-migrating form (Fig. 8A, 0 h, lane 1) to its unphosphorylated, faster-migrating form (lane 2). This unphosphorylated form of 4E-BP1 was stable for the 6-h time course of the experiment (lanes 2 to 6). Treatment of mock-infected cells with rapamycin for 3 or 7 h resulted in a similar change in the synthesis of host proteins (Fig. 8B, far right). Quantitation of radioactivity in gels like that shown in Fig. 8B showed that cellular protein synthesis was decreased by approximately 20% by the addition of rapamycin. This is consistent with the results of previous experiments showing that activation of 4E-BP1 by treatment with rapamycin only modestly affects host protein syntheses in the absence of other inhibitory mechanisms (3).
FIG. 8.
Effect of rapamycin on host and viral protein synthesis in VSV-infected cells. (A) Western blot analysis of total 4E-BP1 following treatment of HeLa cells with 25 nM rapamycin for the indicated times. (B) HeLa cells were either left untreated (−) or treated (+) with 25 nM rapamycin (Rapa) for 1 h. These cells were then mock infected (−) or infected with VSV (+) for the indicated times and labeled with [35S]methionine for 10 min. Lysates were electrophoresed on a 12% gel, a phosphorescence image of which is shown. (C) Quantitation of host protein synthesis in untreated (closed circles) or rapamycin-treated (open circles) cells infected with VSV. Rapamycin inhibition of protein synthesis is indicated as a dotted line. Results from four independent experiments were quantified and are expressed as a percentage of the untreated, mock-infected control. (D) VSV protein synthesis in rapamycin-treated (dark bars) or untreated (white bars) cells. Results from four independent experiments were quantified and expressed as a percentage of the level at 6 h postinfection in untreated cells.
Figure 8B also shows the effect of rapamycin pretreatment on viral protein synthesis. Over the course of a 6-h infection, pretreatment with rapamycin had little effect on the synthesis of viral proteins. Protein synthesis following VSV infection in the presence or absence of rapamycin was quantified in three separate experiments. Host protein synthesis is shown in Fig. 8C, and viral protein synthesis is shown in Fig. 8D. Because of the inhibitory effect of the drug, host protein synthesis in rapamycin-treated cells was initially lower than in mock-infected cells that were not treated with rapamycin (Fig. 8C). However, by 3 to 4 h postinfection, host protein synthesis in both treated and untreated cells was inhibited to the same extent by VSV. This is the expected result for a situation in which 4E-BP1 activation plays an active role in VSV's inhibition of host protein synthesis. If 4E-BP1 activation was not a factor in VSV's inhibition of host protein synthesis, the addition of rapamycin should have resulted in two nonintersecting curves. Thus, this result supports the hypothesis that 4E-BP1 activation plays a role in VSV's inhibition of host protein synthesis. The levels of viral protein synthesis were nearly identical in treated and untreated cells (Fig. 8D), indicating that VSV protein synthesis was not affected by preactivation of 4E-BP1. In additional experiments, viral protein synthesis was not affected by the pretreatment of HeLa or BHK cells with concentrations of rapamycin as high as 250 nM (data not shown). These data indicate that the translation of VSV mRNAs is not significantly affected by activation of 4E-BP1 cells at the initial stages of viral infection.
DISCUSSION
One of the most striking changes in gene expression in VSV-infected cells is the nearly complete inhibition of translation of host mRNAs and the effective translation of viral mRNAs. This is a result of at least two changes in the host translation machinery. Earlier data had shown that one of these changes is the inactivation of the translation initiation factor eIF2 (7, 10) due to activation of PKR, which phosphorylates and inactivates the α subunit of eIF2 (2). The data presented here show that a second translation initiation factor, the eIF4F complex, is also altered in VSV-infected cells. The alteration in the eIF4F complex involves dephosphorylation of the cap-binding protein eIF4E (Fig. 2) and dissociation of eIF4E from the eIF4F complex (Fig. 4). The dissociation of eIF4E is a result of dephosphorylation of 4E-BP1 and subsequent formation of the eIF4E/4E-BP1 complex (Fig. 5). These changes in the eIF4F complex are consistent with previous experiments showing that host protein synthesis in extracts from VSV-infected cells could be restored by addition of purified eIF4F, as well as eIF2 (10).
The dephosphorylation of eIF4E in VSV-infected cells is due, in part, to its dissociation from the eIF4F complex as a result of binding to 4E-BP1. This has the effect of separating eIF4E from its protein kinase, Mnk1. However, analysis of cap-binding complexes in infected cells showed that approximately 40% of eIF4E remained bound to eIF4G at 6 h postinfection (Fig. 4). This suggests that additional mechanisms contribute to eIF4E dephosphorylation, since nearly all of the eIF4E in infected cells was dephosphorylated by this time (Fig. 2). The dephosphorylation of eIF4E that remained bound to eIF4G could occur through inhibition of a protein kinase that normally maintains eIF4E phosphorylation. However, if an eIF4E kinase is inhibited, it is likely to be a kinase other than Mnk1, such as Mnk2, since the levels of the active form of Mnk1 were not changed in VSV-infected cells and overexpression of Mnk1, a high-activity mutant, or an inactive mutant had little effect on host protein synthesis inhibition (Fig. 3 and data not shown). A second hypothesis is that VSV infection results in activation of an eIF4E phosphatase. Protein phosphatase 2A has been proposed to mediate dephosphorylation of both eIF4E and 4E-BP1 (22, 23, 36). Thus, activation of this phosphatase might account for both the dissociation of most eIF4F complexes by 4E-BP1 and the dephosphorylation of eIF4E in the remaining undissociated complexes.
Both the phosphorylation of eIF2α shown previously and the dephosphorylation of eIF4E shown here are inhibitory mechanisms that contribute to the inhibition of host protein synthesis in VSV-infected cells. However, viral mRNAs are effectively translated at the same time that translation of host mRNAs is inhibited (Fig. 1). The ability of VSV mRNAs to be translated under conditions of reduced eIF4F activity was further demonstrated by using rapamycin to induce dephosphorylation of 4E-BP1 (Fig. 8). Treatment of mock-infected cells with rapamycin inhibited host protein synthesis, although not as effectively as the inhibition observed in VSV-infected cells. This is consistent with the idea that the inactivation of two translation initiation factors, both eIF2 and eIF4F, is required to fully inhibit host protein synthesis in VSV-infected cells. While treatment with rapamycin partially inhibited host protein synthesis, it did not inhibit the synthesis of viral proteins. This supports the idea that translation of VSV mRNA is not affected by a decrease in the amount of functional eIF4F complexes.
VSV mRNAs have 5′ caps and are believed to be translated by a cap-dependent process. This is likely to be mediated by the 40% of eIF4E that remains bound to eIF4G in VSV-infected cells. The reason that viral proteins are synthesized in the presence of these alterations in the eIF4F complex may lie in the structural features of the viral mRNAs. The 5′ untranslated regions (UTRs) of VSV mRNAs are short, unlike those of host mRNAs, which tend to be at least 70 nucleotides (nt) in length and can be longer than 500 nt. In contrast, the longest 5′ UTR in VSV mRNA is 49 nt long and three of the five are shorter than 14 nt. These short 5′ UTRs, which probably lack secondary structure, may allow efficient translation under conditions in which there are few functioning eIF4F complexes. A role for the 5′ UTR of VSV mRNA was suggested by one report showing that a gene fragment containing the UTR from the VSV N gene confers extended translation of a β-globin reporter gene in VSV-infected cells (4).
An interesting aspect of the changes in the translation apparatus following VSV infection is the similarity of these changes to the host cell's natural response to environmental stress. Both heat shock and ischemic stress lead to the phosphorylation of eIF2α and the dephosphorylation of 4E-BP1 in mammalian cells (12, 29, 33, 44). In both cases, this results in inhibition of translation. However, the mRNAs for some proteins, such as heat shock proteins and hypoxia-induced factors, contain sequences that enhance their translation under these conditions (30, 42). Because VSV mRNAs are translated in cells under conditions that resemble these stress responses, this suggests that VSV has adopted strategies similar to those used to translate host stress response mRNAs to translate viral messages under similar conditions.
The similarity to the effects of stress responses suggests that the inhibition of translation of host mRNAs in VSV-infected cells is the result of the activation of a host response system and not the direct effect of a VSV protein or RNA. This raises the question of how the products of virus infection activate a “stress” response. The available evidence indicates that viral double-stranded RNA is responsible for activation of PKR in VSV-infected cells (27). However, it is possible that other viral products contribute to PKR activation. It is also likely that there are several viral products whose activity leads to the dephosphorylation of eIF4E and 4E-BP1. One of these products is likely to be the viral M protein, as the tsO82 virus contains the M51R point mutation in its M protein, which renders this virus defective in the ability to inhibit both host RNA synthesis and host protein synthesis (5, 8, 24). The dephosphorylation of eIF4E and 4E-BP1 is delayed in cells infected with tsO82 virus versus wt VSV (Fig. 6 and 7). However, by 6 h postinfection, the dephosphorylation of eIF4E in tsO82 virus-infected cells is nearly complete (Fig. 6) and there is little, if any, difference compared to cells infected with wt VSV in the amount of 4E-BP1 associated with eIF4E (Fig. 7). These results suggest that a viral product other than M protein is primarily responsible for inducing dephosphorylation of eIF4E and 4E-BP1 and that the effect of M protein is to accelerate this process. This idea is further supported by the observation that expression of M protein in transfected cells in the absence of other viral components does not inhibit translation (5). Instead, expression of M protein actually stimulates translation of a cotransfected mRNA (5). Whether this stimulation reflects an effect of M protein on the eIF4F complex is currently being investigated.
The alteration of both eIF4E and 4E-BP1 phosphorylation following VSV infection is the first reported modification of both of these proteins among viruses that produce capped mRNA for protein translation. Analysis of cells infected with picornaviruses, such as poliovirus and the cardiovirus encephalomyocarditis virus, has shown that these RNA viruses also induce the dephosphorylation eIF4E and 4E-BP1 (3, 20). In addition, many picornaviruses induce proteolytic cleavage of eIF4G (16), which also results in the dissociation of eIF4E from other members of the eIF4F complex. However, in contrast to VSV, these viruses produce uncapped mRNA with long 5′ UTRs that are translated through cap-independent mechanisms and therefore benefit from disruption of the eIF4F complex (16). Adenoviruses, which produce 5′-capped mRNA, also direct the dephosphorylation of eIF4E upon infection (9). In contrast to picornavirus and VSV infections, adenovirus infection results in the phosphorylation and inactivation of 4E-BP1 (13). This may be because adenovirus mRNAs require the assembled eIF4F complex to aid in their translation, which would be inhibited by the formation of the eIF4E/4E-BP1 complex.
In summary, the data presented here show that VSV infection results in changes in the eIF4F cap-binding complex that play a role in the inhibition of host protein synthesis but do not prevent viral protein synthesis, which is unusual among viruses that produce capped mRNAs. These changes in the eIF4F complex include both the dissociation of most of the eIF4E and the dephosphorylation of the remaining eIF4E that remains bound to eIF4F complexes. The dissociation of eIF4E is due to dephosphorylation and activation of 4E-BP1. These changes, together with the previously described inactivation of eIF2, resemble the changes in the translation machinery that occur in stress responses. These results support a model for translational control in VSV-infected cells in which activation of signal transduction pathways involved in stress responses inhibits translation of host mRNAs and favors translation of viral mRNAs. Future studies will address how VSV controls the phosphorylation state of eIF4E and 4E-BP1 and the aspects of VSV mRNAs that make them particularly suited for translation under these conditions.
Acknowledgments
We thank Griffith Parks, Maryam Ahmed, and Sarah Kopecky for helpful advice and comments on the manuscript. We also acknowledge the use of the Comprehensive Cancer Center Analytical Imaging Facility for phosphorimaging.
This work was supported by Public Health Service grants AI15892 and AI32983 from the National Institute of Allergy and Infectious Diseases (D.S.L.). J.H.C. was supported by Signal Transduction Mechanisms and Cell Function training program grant CA-09422 from the National Cancer Institute.
REFERENCES
- 1.Balachandran, S., M. Porosnicu, and G. N. Barber. 2001. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J. Virol. 75:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 3.Beretta, L., Y. V. Svitkin, and N. Sonenberg. 1996. Rapamycin stimulates viral protein synthesis and augments the shutoff of host protein synthesis upon picornavirus infection. J. Virol. 70:8993-8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, D. T., and B. W. Grinnell. 1992. 5′ sequence of vesicular stomatitis virus N-gene confers selective translation of mRNA. Biochem. Biophys. Res. Commun. 189:1585-1590. [DOI] [PubMed] [Google Scholar]
- 5.Black, B. L., G. Brewer, and D. S. Lyles. 1994. Effect of vesicular stomatitis virus matrix protein on host-directed translation in vivo. J. Virol. 68:555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunn, G. J., C. C. Hudson, A. Sekulic, J. M. Williams, H. Hosoi, P. J. Houghton, J. C. Lawrence, Jr., and R. T. Abraham. 1997. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277:99-101. [DOI] [PubMed] [Google Scholar]
- 7.Centrella, M., and J. Lucas-Lenard. 1982. Regulation of protein synthesis in vesicular stomatitis virus-infected mouse L-929 cells by decreased protein synthesis initiation factor 2 activity. J. Virol. 41:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulon, P., V. Deutsch, F. Lafay, C. Martinet-Edelist, F. Wyers, R. C. Herman, and A. Flamand. 1990. Genetic evidence for multiple functions of the matrix protein of vesicular stomatitis virus. J. Gen. Virol. 71:991-996. [DOI] [PubMed] [Google Scholar]
- 9.Cuesta, R., Q. Xi, and R. J. Schneider. 2000. Adenovirus-specific translation by displacement of kinase Mnk1 from cap-initiation complex eIF4F. EMBO J. 19:3465-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dratewka-Kos, E., I. Kiss, J. Lucas-Lenard, H. B. Mehta, C. L. Woodley, and A. J. Wahba. 1984. Catalytic utilization of eIF-2 and mRNA binding proteins are limiting in lysates from vesicular stomatitis virus infected L cells. Biochemistry 23:6184-6190. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, R., S. C. Milburn, and J. W. Hershey. 1987. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J. Biol. Chem. 262:380-388. [PubMed] [Google Scholar]
- 12.Feigenblum, D., and R. J. Schneider. 1996. Cap-binding protein (eukaryotic initiation factor 4E) and 4E-inactivating protein BP-1 independently regulate cap-dependent translation. Mol. Cell. Biol. 16:5450-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feigenblum, D., and R. J. Schneider. 1993. Modification of eukaryotic initiation factor 4F during infection by influenza virus. J. Virol. 67:3027-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn, A., and C. G. Proud. 1995. Serine 209, not serine 53, is the major site of phosphorylation in initiation factor eIF-4E in serum-treated Chinese hamster ovary cells. J. Biol. Chem. 270:21684-21688. [DOI] [PubMed] [Google Scholar]
- 15.Francoeur, A. M., L. Poliquin, and C. P. Stanners. 1987. The isolation of interferon-inducing mutants of vesicular stomatitis virus with altered viral P function for the inhibition of total protein synthesis. Virology 160:236-245. [DOI] [PubMed] [Google Scholar]
- 16.Gale, M., Jr., S. L. Tan, and M. G. Katze. 2000. Translational control of viral gene expression in eukaryotes. Microbiol. Mol. Biol. Rev. 64:239-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gingras, A. C., B. Raught, S. P. Gygi, A. Niedzwiecka, M. Miron, S. K. Burley, R. D. Polakiewicz, A. Wyslouch-Cieszynska, R. Aebersold, and N. Sonenberg. 2001. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 15:2852-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gingras, A. C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 19.Gingras, A. C., B. Raught, and N. Sonenberg. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15:807-826. [DOI] [PubMed] [Google Scholar]
- 20.Gingras, A. C., Y. Svitkin, G. J. Belsham, A. Pause, and N. Sonenberg. 1996. Activation of the translational suppressor 4E-BP1 following infection with encephalomyocarditis virus and poliovirus. Proc. Natl. Acad. Sci. USA 93:5578-5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haghighat, A., S. Mader, A. Pause, and N. Sonenberg. 1995. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 14:5701-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang, Y., and J. R. Broach. 1999. Tor proteins and protein phosphatase 2A reciprocally regulate Tap42 in controlling cell growth in yeast. EMBO J. 18:2782-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleijn, M., C. L. Vrins, H. O. Voorma, and A. A. Thomas. 1996. Phosphorylation state of the cap-binding protein eIF4E during viral infection. Virology 217:486-494. [DOI] [PubMed] [Google Scholar]
- 24.Kopecky, S. A., M. C. Willingham, and D. S. Lyles. 2001. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 75:12169-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, T. A., X. Kong, T. A. Haystead, A. Pause, G. Belsham, N. Sonenberg, and J. C. Lawrence, Jr. 1994. PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science 266:653-656. [DOI] [PubMed] [Google Scholar]
- 26.Lodish, H. F., and M. Porter. 1980. Translational control of protein synthesis after infection by vesicular stomatitis virus. J. Virol. 36:719-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyles, D. S. 2000. Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol. Mol. Biol. Rev. 64:709-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mader, S., H. Lee, A. Pause, and N. Sonenberg. 1995. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4γ and the translational repressors 4E-binding proteins. Mol. Cell. Biol. 15:4990-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin, M. E., F. M. Munoz, M. Salinas, and J. L. Fando. 2000. Ischaemia induces changes in the association of the binding protein 4E-BP1 and eukaryotic initiation factor (eIF) 4G to eIF4E in differentiated PC12 cells. Biochem. J. 351(Pt. 2):327-334. [PMC free article] [PubMed] [Google Scholar]
- 30.McGarry, T. J., and S. Lindquist. 1985. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell 42:903-911. [DOI] [PubMed] [Google Scholar]
- 31.Mothe-Satney, I., D. Yang, P. Fadden, T. A. Haystead, and J. C. Lawrence, Jr. 2000. Multiple mechanisms control phosphorylation of PHAS-I in five (S/T)P sites that govern translational repression. Mol. Cell. Biol. 20:3558-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mudd, J. A., and D. F. Summers. 1970. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology 42:328-340. [DOI] [PubMed] [Google Scholar]
- 33.Munoz, F., M. E. Martin, J. Manso-Tomico, J. Berlanga, M. Salinas, and J. L. Fando. 2000. Ischemia-induced phosphorylation of initiation factor 2 in differentiated PC12 cells: role for initiation factor 2 phosphatase. J. Neurochem. 75:2335-2345. [DOI] [PubMed] [Google Scholar]
- 34.Pause, A., G. J. Belsham, A. C. Gingras, O. Donze, T. A. Lin, J. C. Lawrence, Jr., and N. Sonenberg. 1994. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371:762-767. [DOI] [PubMed] [Google Scholar]
- 35.Petersen, J. M., L. S. Her, V. Varvel, E. Lund, and J. E. Dahlberg. 2000. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Biol. 20:8590-8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson, R. T., B. N. Desai, J. S. Hardwick, and S. L. Schreiber. 1999. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycin associated protein. Proc. Natl. Acad. Sci. USA 96:4438-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rinker-Schaeffer, C. W., V. Austin, S. Zimmer, and R. E. Rhoads. 1992. Ras transformation of cloned rat embryo fibroblasts results in increased rates of protein synthesis and phosphorylation of eukaryotic initiation factor 4E. J. Biol. Chem. 267:10659-10664. [PubMed] [Google Scholar]
- 38.Rychlik, W., J. S. Rush, R. E. Rhoads, and C. J. Waechter. 1990. Increased rate of phosphorylation-dephosphorylation of the translational initiation factor eIF-4E correlates with the induction of protein and glycoprotein biosynthesis in activated B lymphocytes. J. Biol. Chem. 265:19467-19471. [PubMed] [Google Scholar]
- 39.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Schnitzlein, W. M., M. K. O'Banion, M. K. Poirot, and M. E. Reichmann. 1983. Effect of intracellular vesicular stomatitis virus mRNA concentration on the inhibition of host cell protein synthesis. J. Virol. 45:206-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanners, C. P., A. M. Francoeur, and T. Lam. 1977. Analysis of VSV mutant with attenuated cytopathogenicity: mutation in viral function, P, for inhibition of protein synthesis. Cell 11:273-281. [DOI] [PubMed] [Google Scholar]
- 42.Stein, I., A. Itin, P. Einat, R. Skaliter, Z. Grossman, and E. Keshet. 1998. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol. Cell. Biol. 18:3112-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Kobbe, C., J. M. van Deursen, J. P. Rodrigues, D. Sitterlin, A. Bachi, X. Wu, M. Wilm, M. Carmo-Fonseca, and E. Izaurralde. 2000. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin nup98. Mol. Cell 6:1243-1252. [DOI] [PubMed] [Google Scholar]
- 44.Vries, R. G., A. Flynn, J. C. Patel, X. Wang, R. M. Denton, and C. G. Proud. 1997. Heat shock increases the association of binding protein-1 with initiation factor 4E. J. Biol. Chem. 272:32779-32784. [DOI] [PubMed] [Google Scholar]
- 45.Waskiewicz, A. J., A. Flynn, C. G. Proud, and J. A. Cooper. 1997. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 16:1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waskiewicz, A. J., J. C. Johnson, B. Penn, M. Mahalingam, S. R. Kimball, and J. A. Cooper. 1999. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol. Cell. Biol. 19:1871-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wertz, G. W., and J. S. Youngner. 1970. Interferon production and inhibition of host synthesis in cells infected with vesicular stomatitis virus. J. Virol. 6:476-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan, H., S. Puckett, and D. S. Lyles. 2001. Inhibition of host transcription by vesicular stomatitis virus involves a novel mechanism that is independent of phosphorylation of TATA-binding protein (TBP) or association of TBP with TBP-associated factor subunits. J. Virol. 75:4453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]