Abstract
1. In cats anaesthetized with N2O-halothane acetylcholine (ACh) release from the parietal cortex was measured. In addition, low and high-frequency electroencephalographic (e.e.g.) activity was recorded quantitatively.
2. Stimulation of the mesencephalic reticular formation at 30, 60 and 100/sec produced an identical increase in cortical ACh output, while 300/sec stimulation was about ⅓ as effective and 10/sec stimulation failed to increase ACh output.
3. Reticular formation stimulation at 60 and 100/sec reduced the low-frequency and increased the high-frequency e.e.g. activity. Stimulation at 30 and 300/sec was less effective, while 10/sec stimulation had no effect on e.e.g.
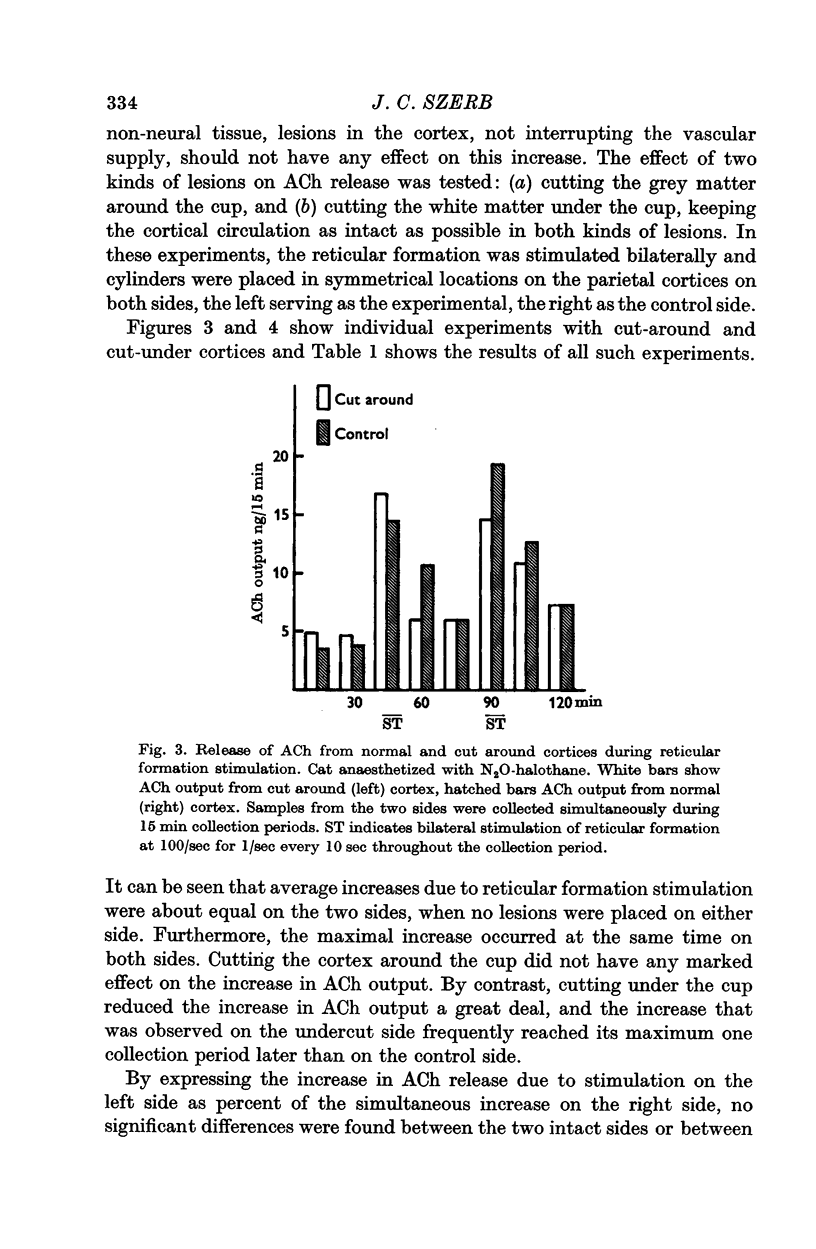
4. Acute undercutting of the cortex did not affect the resting output of ACh but greatly reduced the increase due to reticular formation stimulation as compared to the contralateral intact side. Cutting the cortex around the collection area did not affect the increase in ACh output due to reticular formation stimulation.
5. Stimulation of the hypothalamus, medial thalamus and septum at 100/sec also increased cortical ACh output while stimulation of the dorsal hippocampus and caudate nucleus failed to do so.
6. Low frequency cortical e.e.g. activity was reduced by stimulating the reticular formation, the hypothalamus, the medial thalamus and slightly by septum stimulation. High-frequency e.e.g. activity was increased by stimulating the reticular formation and the hypothalamus.
7. It is concluded that the ACh measured originates from the neural tissue underlying the collection area. The increased release is concomitant to e.e.g. activation but the pathways involved in cortical e.e.g. activation and increased ACh release are distinct, since the two phenomena do not vary in a parallel fashion when the reticular formation is stimulated at different frequencies or when different subcortical areas are stimulated.
8. The effectiveness of septal stimulation in increasing ACh release indicates that at least part of the cortical cholinergic fibres traverse this area on their way to the cortex.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADEY W. R., MERRILLEES N. C., SUNDERLAND S. The entorhinal area; behavioural, evoked potential, and histological studies of its interrelationships with brain-stem regions. Brain. 1956 Sep;79(3):414–439. doi: 10.1093/brain/79.3.414. [DOI] [PubMed] [Google Scholar]
- Celesia G. G., Jasper H. H. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966 Nov;16(11):1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- Collier B., Mitchell J. F. The central release of acetylcholine during consciousness and after brain lesions. J Physiol. 1967 Jan;188(1):83–98. doi: 10.1113/jphysiol.1967.sp008125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B., Mitchell J. F. The central release of acetylcholine during stimulation of the visual pathway. J Physiol. 1966 May;184(1):239–254. doi: 10.1113/jphysiol.1966.sp007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT K. A. C., SWANK R. L., HENDERSON N. Effects of anesthetics and convulsants on acetylcholine content of brain. Am J Physiol. 1950 Aug 1;162(2):469–474. doi: 10.1152/ajplegacy.1950.162.2.469. [DOI] [PubMed] [Google Scholar]
- GREEN J. D., MORIN F. Hypothalamic electrical activity and hypothalamo-cortical relationships. Am J Physiol. 1953 Jan;172(1):175–186. doi: 10.1152/ajplegacy.1952.172.1.175. [DOI] [PubMed] [Google Scholar]
- GREEN J. D., SHIMAMOTO T. Hippocampal seizures and their propagation. AMA Arch Neurol Psychiatry. 1953 Dec;70(6):687–702. doi: 10.1001/archneurpsyc.1953.02320360002001. [DOI] [PubMed] [Google Scholar]
- HEUSER G., BUCHWALD N. A., WYERS E. J. The "caudatespindle". II. Facilitatory and inhibitory caudate-cortical pathways. Electroencephalogr Clin Neurophysiol. 1961 Aug;13:519–524. doi: 10.1016/0013-4694(61)90165-1. [DOI] [PubMed] [Google Scholar]
- INGVAR D. H. Extraneuronal influences upon the electrical activity of isolated cortex following stimulation of the reticular activating system. Acta Physiol Scand. 1955;33(2-3):169–193. doi: 10.1111/j.1748-1716.1955.tb01202.x. [DOI] [PubMed] [Google Scholar]
- KANAI T., SZERB J. C. MESENCEPHALIC RETICULAR ACTIVATING SYSTEM AND CORTICAL ACETYLCHOLINE OUTPUT. Nature. 1965 Jan 2;205:80–82. doi: 10.1038/205080b0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Silver A. A histochemical study of cholinergic fibres in the cerebral cortex. J Anat. 1965 Oct;99(Pt 4):711–759. [PMC free article] [PubMed] [Google Scholar]
- LAURSEN A. M. Caudate nucleus and electrocortical activation in cats. Acta Physiol Scand. 1961 Nov-Dec;53:233–238. doi: 10.1111/j.1748-1716.1961.tb02281.x. [DOI] [PubMed] [Google Scholar]
- LONGO V. G. Effects of scopolamine and atropine electroencephalographic and behavioral reactions due to hypothalamic stimulation. J Pharmacol Exp Ther. 1956 Feb;116(2):198–208. [PubMed] [Google Scholar]
- MOTOKIZAWA F., FUJIMORI B. FAST ACTIVITIES AND DC POTENTIAL CHANGES OF THE CEREBRAL CORTEX DURING EEG AROUSAL RESPONSE. Electroencephalogr Clin Neurophysiol. 1964 Dec;17:630–637. doi: 10.1016/0013-4694(64)90230-5. [DOI] [PubMed] [Google Scholar]
- PURPURA D. P. A neurohumoral mechanism of reticulo-cortical activation. Am J Physiol. 1956 Aug;186(2):250–254. doi: 10.1152/ajplegacy.1956.186.2.250. [DOI] [PubMed] [Google Scholar]
- SCHLAG J. D., CHAILLET F. Thalamic mechanisms involved in cortical desynchronization and recruiting responses. Electroencephalogr Clin Neurophysiol. 1963 Feb;15:39–62. doi: 10.1016/0013-4694(63)90038-5. [DOI] [PubMed] [Google Scholar]
- SZERB J. C. Nature of acetylcholine-like activity released from brain in vivo. Nature. 1963 Mar 9;197:1016–1017. doi: 10.1038/1971016a0. [DOI] [PubMed] [Google Scholar]
- SZERB J. THE EFFECT OF TERTIARY AND QUATERNARY ATROPINE ON CORTICAL ACETYLCHOLINE OUTPUT AND ON THE ELECTROENCEPHALOGRAM IN CATS. Can J Physiol Pharmacol. 1964 May;42:303–314. doi: 10.1139/y64-036. [DOI] [PubMed] [Google Scholar]
- Torii S., Wikler A. Effects of atropine on electrical activity of hippocampus and cerebral cortex in cat. Psychopharmacologia. 1966;9(3):189–204. doi: 10.1007/BF02198479. [DOI] [PubMed] [Google Scholar]
- WEINBERGER N. M., VELASCO M., LINDSLEY D. B. EFFECTS OF LESIONS UPON THALAMICALLY INDUCED ELECTROCORTICAL DESYNCHRONIZATION AND RECRUITING. Electroencephalogr Clin Neurophysiol. 1965 Mar;18:369–377. doi: 10.1016/0013-4694(65)90056-8. [DOI] [PubMed] [Google Scholar]



