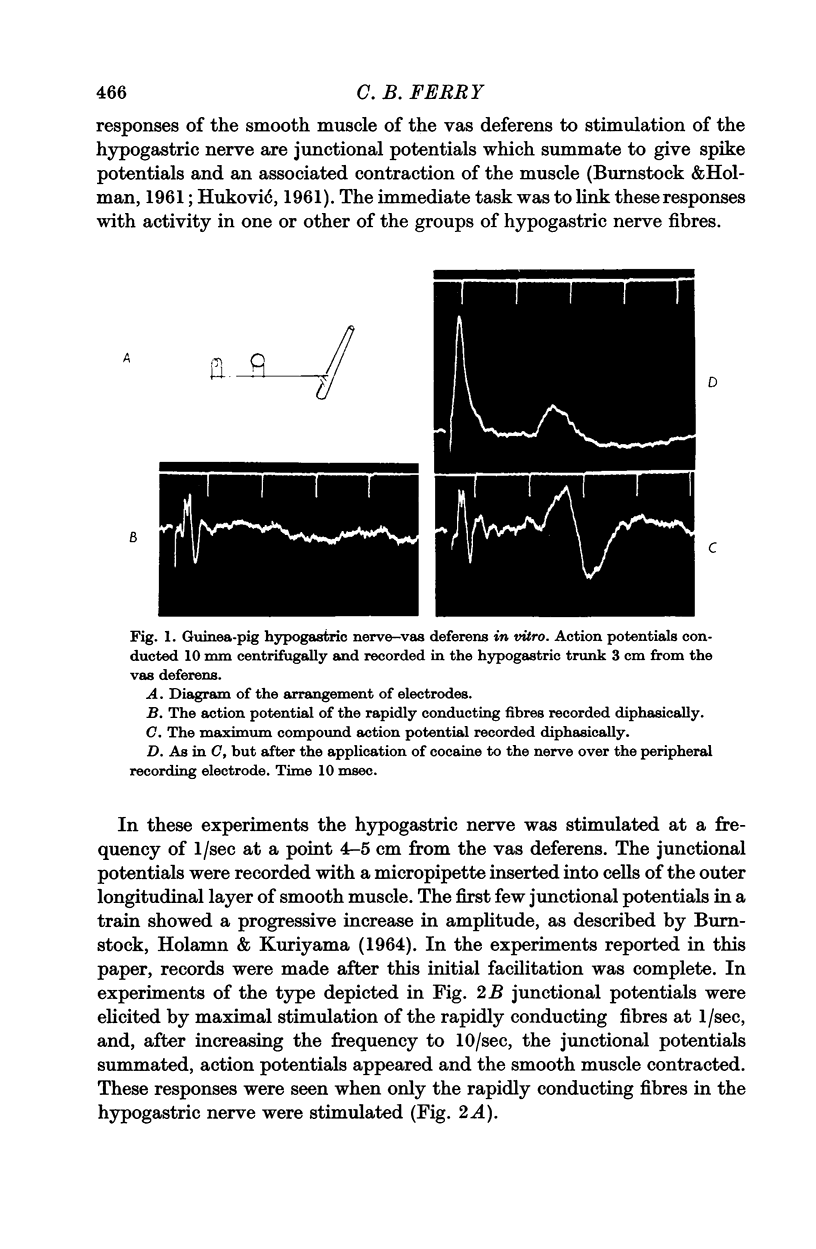
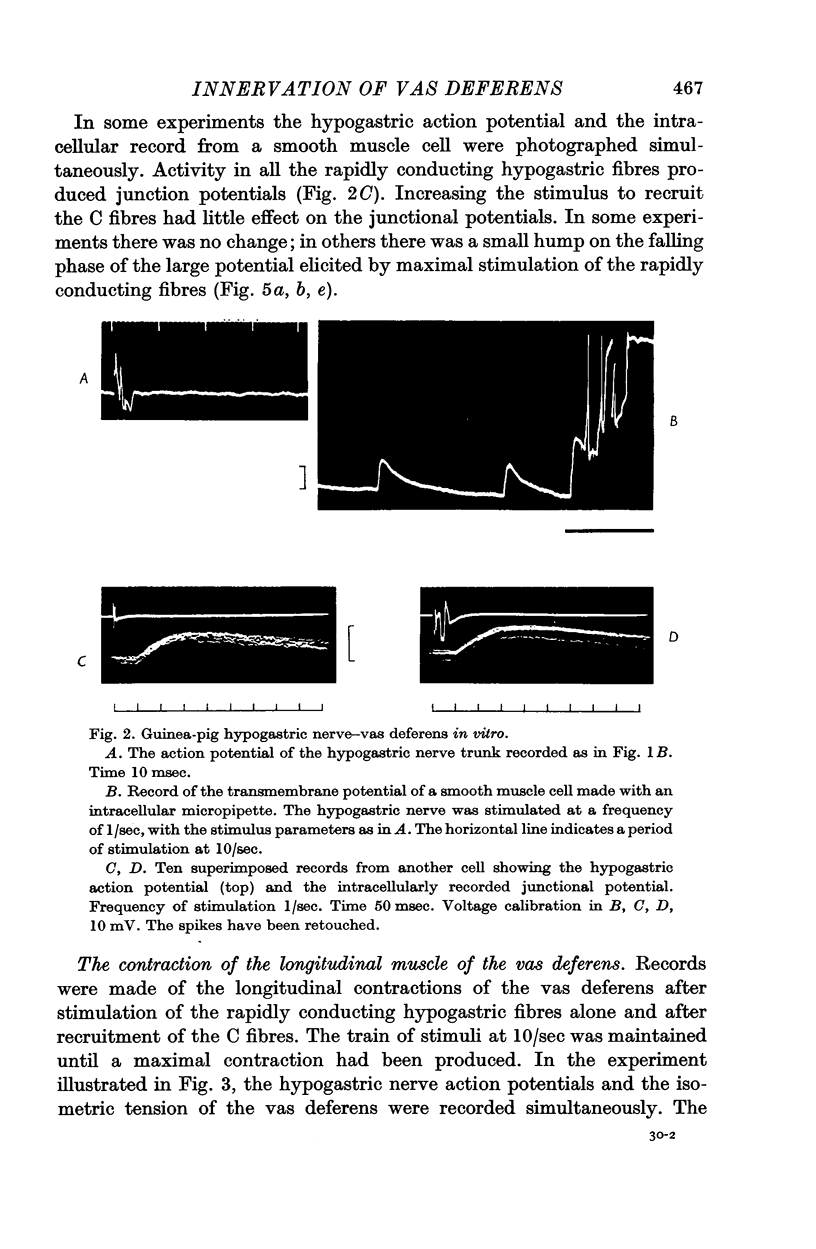
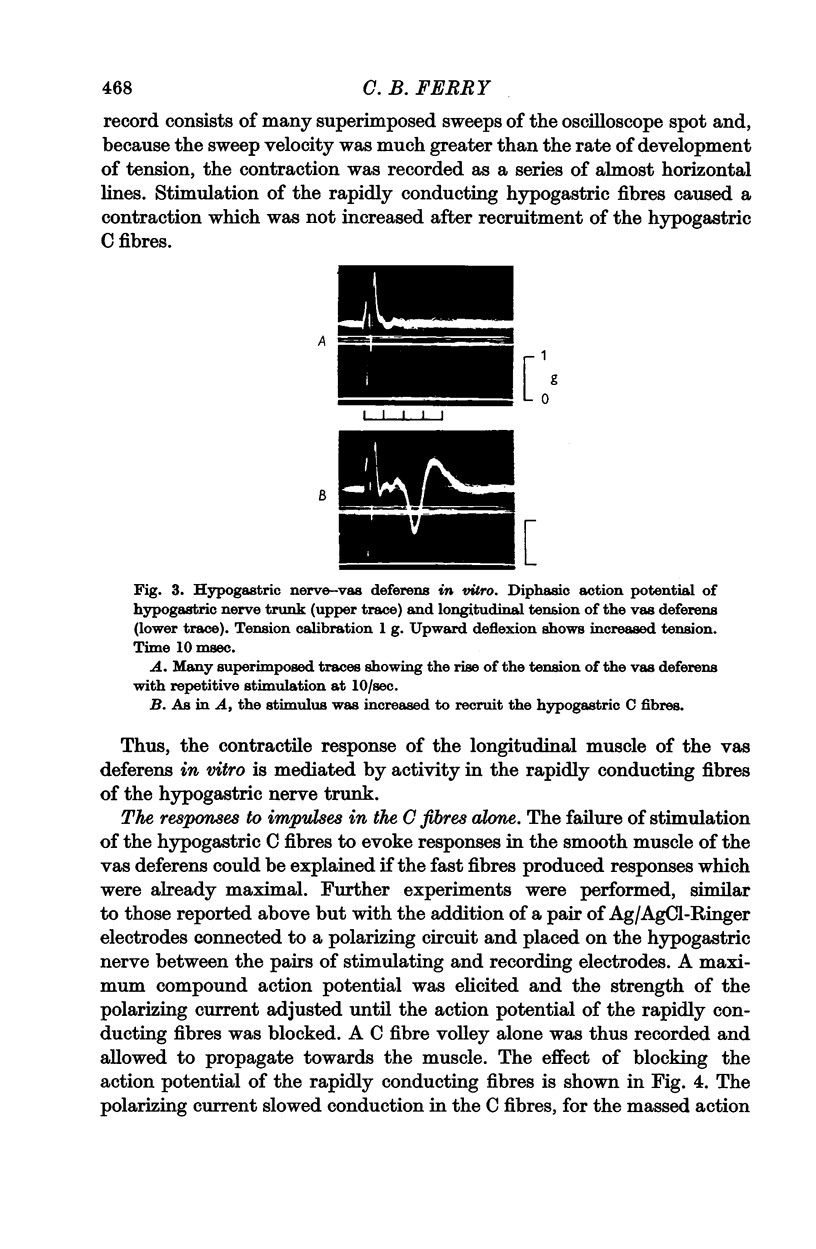
Abstract
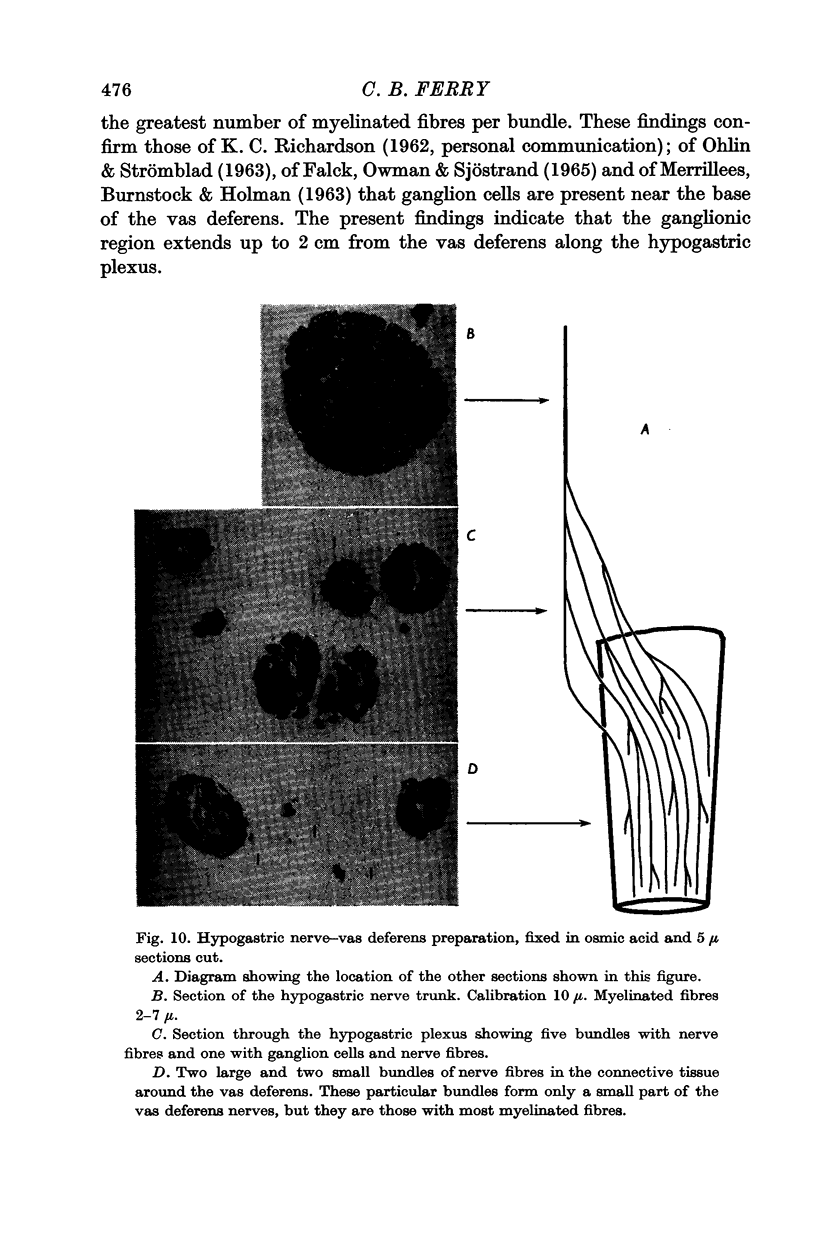
1. The compound action potential of the hypogastric nerve of the guinea-pig contained two main elevations. The low-threshold fibres had a range of conduction velocities from 1·5 to 10 m/sec. The high threshold fibres conducted action potentials at less than 1 m/sec. The hypogastric nerve contained small myelinated fibres and non-myelinated fibres.
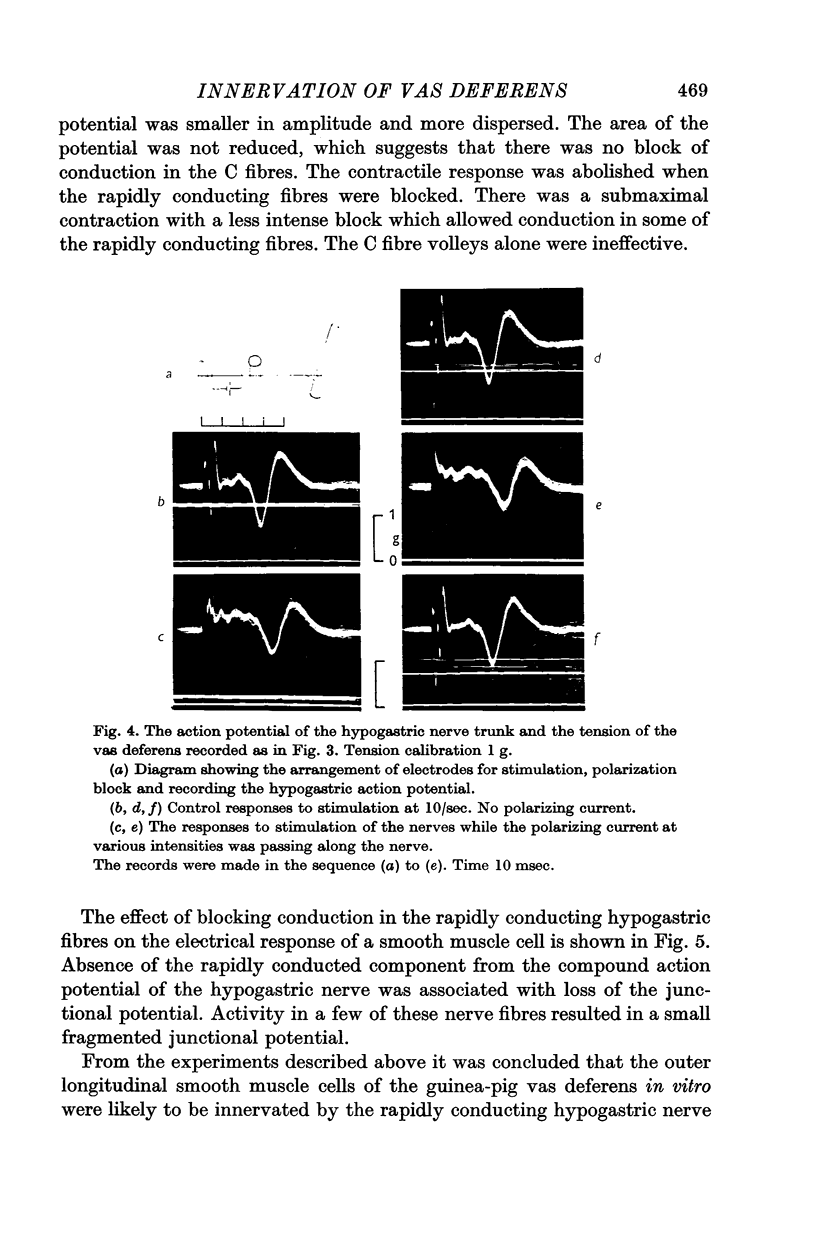
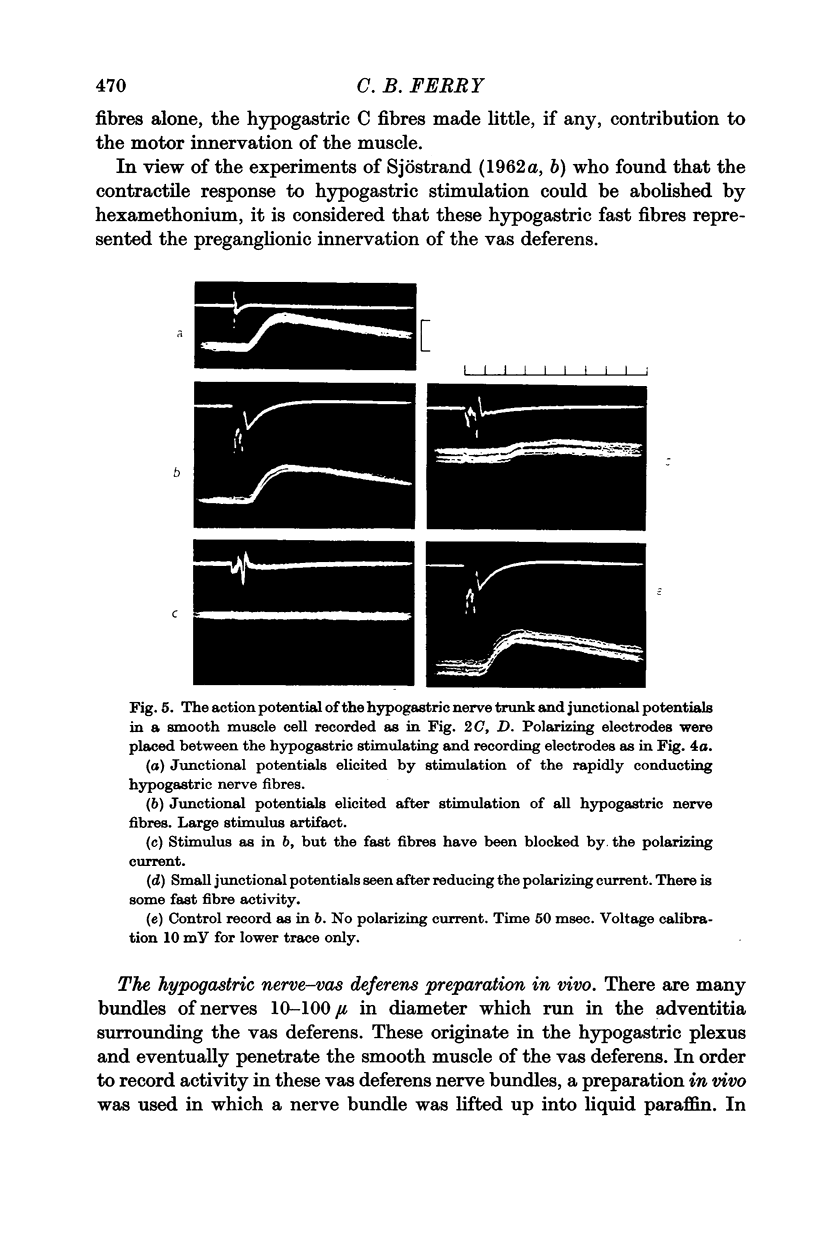
2. In the preparation in vitro, junctional potentials and contractions were elicited by stimulation of the rapidly conducting fibres alone. Trains of C fibre volleys were ineffective.
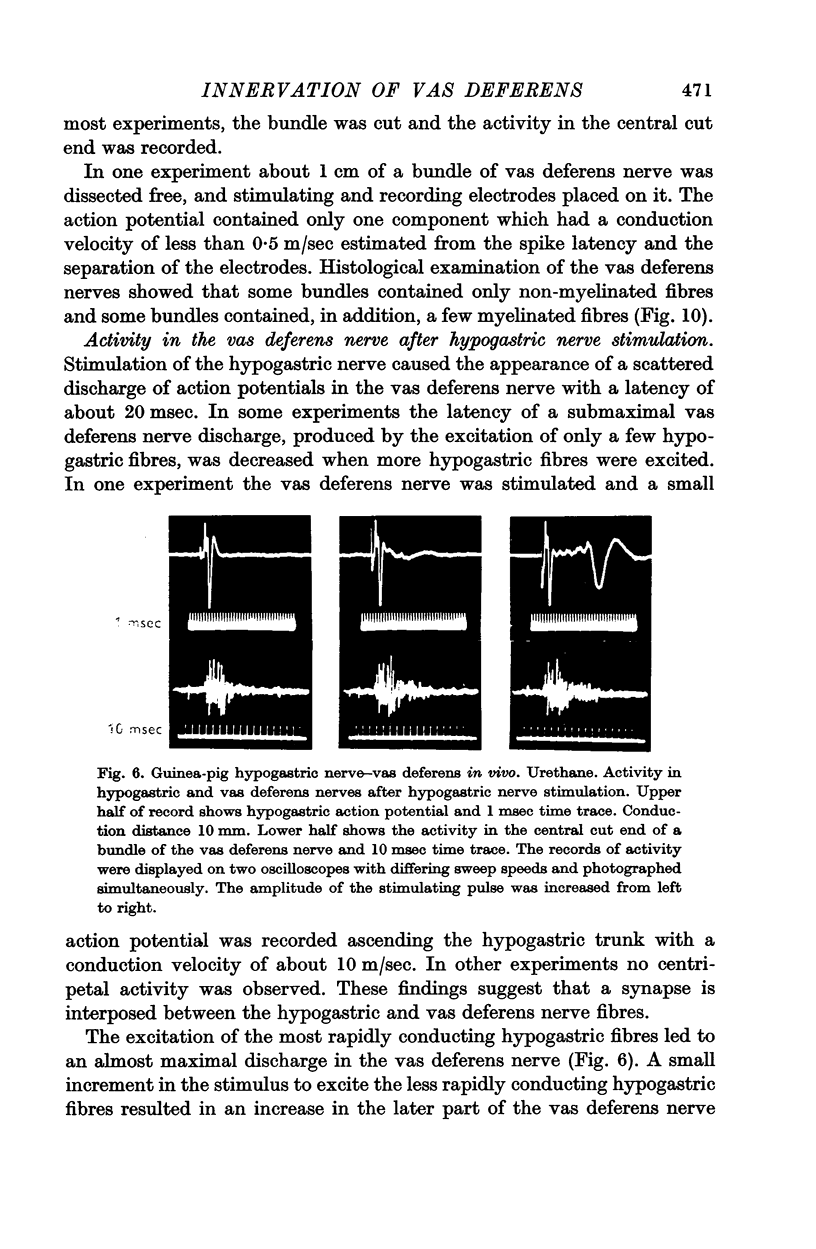
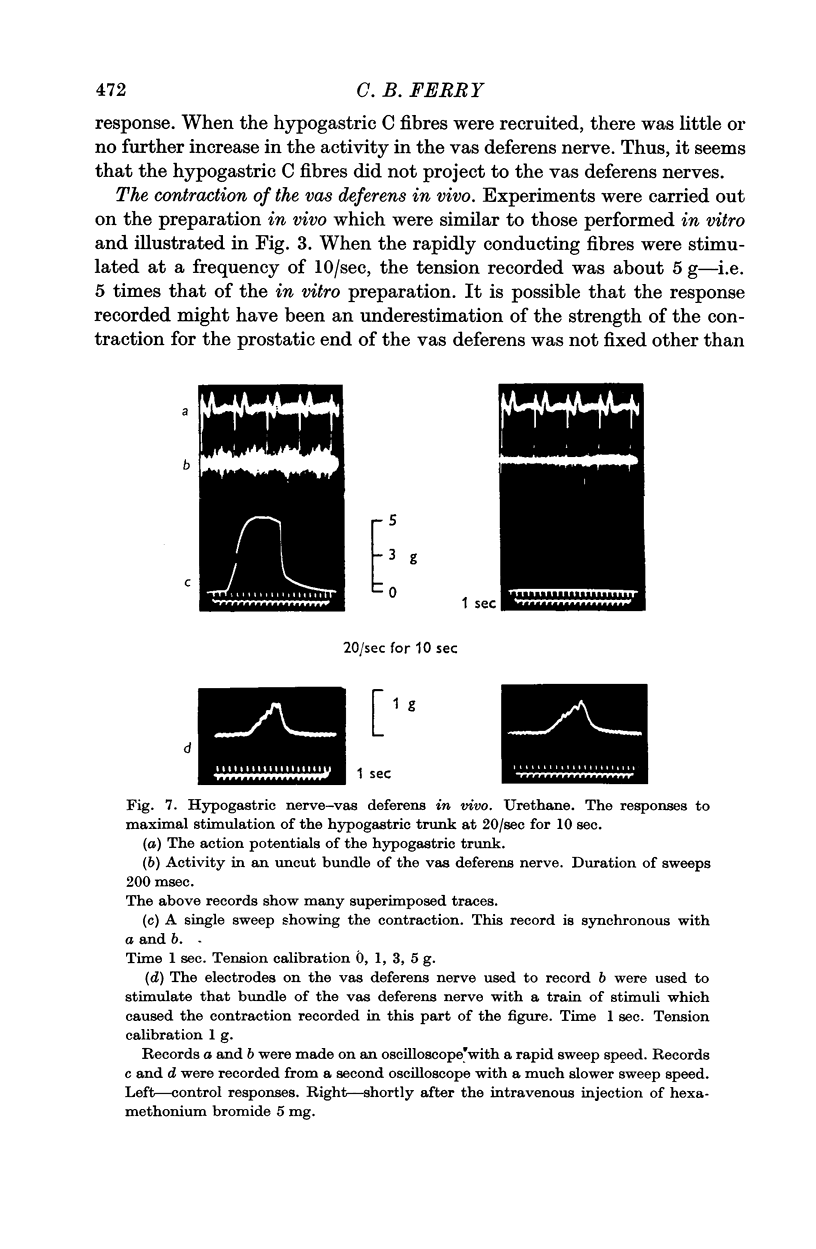
3. In the preparation in vivo, conduction from the hypogastric nerve to the vas deferens nerve was unidirectional and abolished by hexamethonium. After the administration of hexamethonium, the contraction produced by stimulation of the vas deferens nerve was unaffected.
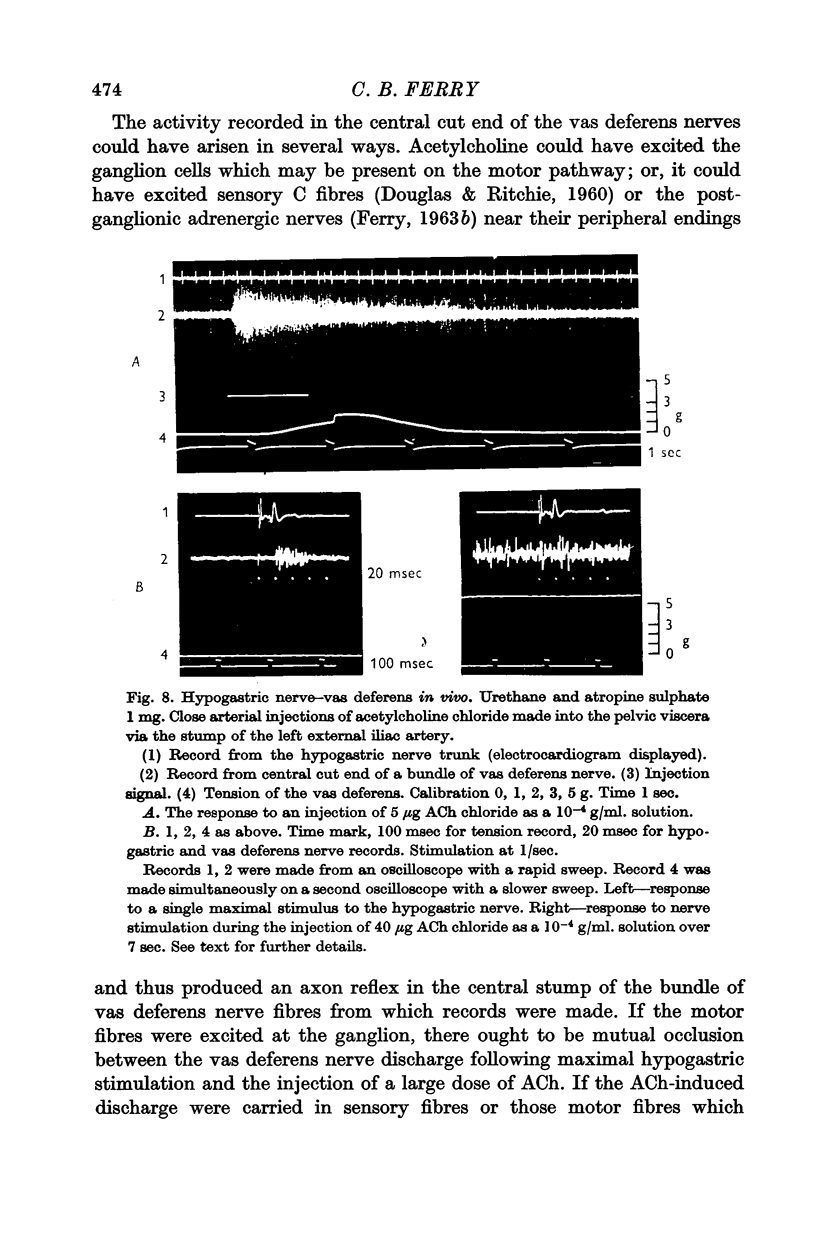
4. The close arterial injection of acetylcholine (ACh) into the pelvic viscera caused centrifugal activity in the motor fibres of the vas deferens nerve, but no impulses were detected in the hypogastric nerve.
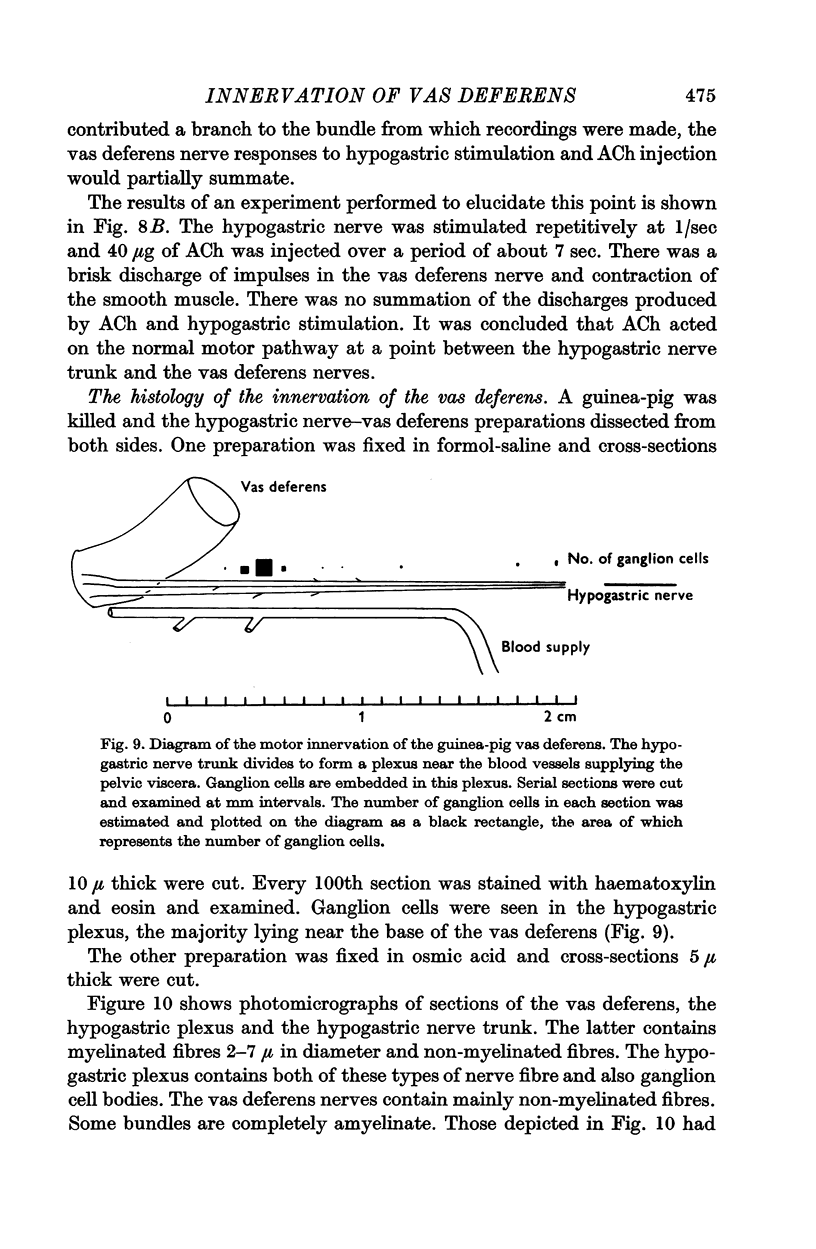
5. Ganglion cells are present in the last 2 cm of the hypogastric nerve.
6. It is concluded that there is a ganglionic relay between the hypogastric and vas deferens nerves.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENTLEY G. A., SABINE J. R. THE EFFECTS OF GANGLION-BLOCKING AND POSTGANGLIONIC SYMPATHOLYTIC DRUGS ON PREPARATIONS OF THE GUINEA-PIG VAS DEFERENS. Br J Pharmacol Chemother. 1963 Aug;21:190–201. doi: 10.1111/j.1476-5381.1963.tb01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRMINGHAM A. T., WILSON A. B. PREGANGLIONIC AND POSTGANGLIONIC STIMULATION OF THE GUINEA-PIG ISOLATED VAS DEFERENS PREPARATION. Br J Pharmacol Chemother. 1963 Dec;21:569–580. doi: 10.1111/j.1476-5381.1963.tb02024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. Effect of denervation and of reserpine treatment on transmission at sympathetic nerve endings. J Physiol. 1962 Mar;160:461–469. doi: 10.1113/jphysiol.1962.sp006859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E., KURIYAMA H. FACILITATION OF TRANSMISSION FROM AUTONOMIC NERVE TO SMOOTH MUSCLE OF GUINEA-PIG VAS DEFERENS. J Physiol. 1964 Jul;172:31–49. doi: 10.1113/jphysiol.1964.sp007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RITCHIE J. M. The excitatory action of acetylcholine on cutaneous non-myelinated fibres. J Physiol. 1960 Mar;150:501–514. doi: 10.1113/jphysiol.1960.sp006401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALCK B., OWMAN C., SJOSTRAND N. O. PERIPHERALLY LOCATED ADRENERGIC NEURONS INNERVATING THE VAS DEFERENS AND THE SEMINAL VESICLE OF THE GUINEA-PIG. Experientia. 1965 Feb 15;21:98–100. doi: 10.1007/BF02144766. [DOI] [PubMed] [Google Scholar]
- FERRY C. B. The synpathomimetic effect of acetylcholine on the spleen of the cat. J Physiol. 1963 Jul;167:487–504. doi: 10.1113/jphysiol.1963.sp007164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUKOVIC S. Responses of the isolated sympathetic nerveductus deferens preparation of the guinea-pig. Br J Pharmacol Chemother. 1961 Apr;16:188–194. doi: 10.1111/j.1476-5381.1961.tb00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H. ELECTROPHYSIOLOGICAL OBSERVATIONS ON THE MOTOR INNERVATION OF THE SMOOTH MUSCLE CELLS IN THE GUINEA-PIG VAS DEFERENS. J Physiol. 1963 Nov;169:213–228. doi: 10.1113/jphysiol.1963.sp007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRILLEES N. C., BURNSTOCK G., HOLMAN M. E. CORRELATION OF FINE STRUCTURE AND PHYSIOLOGY OF THE INNERVATION OF SMOOTH MUSCLE IN THE GUINEA PIG VAS DEFERENS. J Cell Biol. 1963 Dec;19:529–550. doi: 10.1083/jcb.19.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHLIN P., STROMBLAD B. C. Observations on the isolated vas deferens. Br J Pharmacol Chemother. 1963 Apr;20:299–306. doi: 10.1111/j.1476-5381.1963.tb01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJOSTRAND N. O. Effect of reserpine and hypogastric denervation on the noradrenaline content of the vas deferens and the seminal vesicle of the guinea-pig. Acta Physiol Scand. 1962 Nov-Dec;56:376–380. doi: 10.1111/j.1748-1716.1962.tb02513.x. [DOI] [PubMed] [Google Scholar]