Abstract
The potential role of dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN) binding in human immunodeficiency virus transmission across the mucosal barrier was investigated by assessing the ability of simian-human immunodeficiency chimeric viruses (SHIVs) showing varying degrees of mucosal transmissibility to bind the DC-SIGN expressed on the surface of transfected cells. We found that gp120 of the highly transmissible, pathogenic CCR5-tropic SHIVSF162P3 bound human and rhesus DC-SIGN with an efficiency threefold or greater than that of gp120 of the nonpathogenic, poorly transmissible parental SHIVSF162, and this increase in binding to the DC-SIGN of the SHIVSF162P3 envelope gp120 translated into an enhancement of T-cell infection in trans. The presence of an additional glycan at the N-terminal base of the V2 loop of SHIVSF162P3 gp120 compared to that of the parental virus was shown to be responsible for the increase in binding to DC-SIGN. Interestingly, this glycan also conferred escape from autologous neutralization, raising the possibility that the modification occurred as a result of immune selection. Our data suggest that more-efficient binding of envelope gp120 to DC-SIGN could be relevant to the enhanced mucosal transmissibility of SHIVSF162P3 compared to that of parental SHIVSF162.
Infection of macaques with simian-human immunodeficiency virus (SHIV) has provided not only a model system for the evaluation of vaccines and therapies against AIDS but also valuable information on human immunodeficiency virus (HIV) pathogenesis and transmission (6). Through adaptation or serial passaging in vivo, pathogenic variants of CXCR4-tropic (X4) and CCR5-tropic (R5) SHIVs that replicate to high titers, induce CD4+-T-cell depletion in various anatomical compartments, and cause an AIDS-like illness in infected macaques have been derived (27, 33, 40, 52, 57). Furthermore, some of these adapted or passaged viruses are transmitted efficiently across mucosal surfaces (26, 29, 32, 40). Studies conducted in our laboratory have focused on the use of two pathogenic SHIVs, X4-SHIVSF33A and R5-SHIVSF162P3 (26, 27, 29, 40). Atraumatic application of pathogenic X4-SHIVSF33A to the cervico-vaginal mucosa of four rhesus macaques resulted in infection of all four animals that was accompanied by a transient but dramatic loss of peripheral CD4+ T cells at acute viremia (29). In comparison, the parental SHIVSF33 infected two of three animals by the vaginal route but did not induce CD4+-T-cell depletion (summarized in Table 1). In contrast, none of the three animals exposed to R5-SHIVSF162 by the vaginal route showed signs of infection, as indicated by the absence of seroconversion and of cell-associated proviral DNA (Table 1). Mucosal transmission efficiency of the pathogenic R5-SHIVSF162P3, however, was significantly increased. All four macaques exposed to a single inoculum of SHIVSF162P3 were infected, with two animals progressing to simian AIDS (SAIDS) at 24 and 44 weeks postinfection (wpi), respectively (26). The genetic determinants for enhanced pathogenicity of SHIVSF33A in vivo have been mapped to the V1 to V5 region of the external glycoprotein gp120 (28). Sequence changes in this region are associated with several phenotypic characteristics in vitro that could account for the in vivo virulence of the virus (13). Compared to the parental SHIVSF33 envelope glycoprotein, SHIVSF33A gp120 mediates better entry, increases membrane fusogenicity, and confers resistance to neutralization by antibodies. Similar envelope-determined properties have also been shown to be associated with other pathogenic X4- and X4/R5-SHIVs (SHIV-HxBc2P, SHIVKu-1, SHIV-89.6P) (11, 17, 34, 38). However, properties of the virus that could be associated with enhanced transmission across the mucosal barrier are less well defined.
TABLE 1.
Mucosal transmission of SHIVSF33, SHIVSF33A, SHIVSF162, and SHIVSF162P3
| Virus (coreceptor used) | No. infected/no. challenged (route) | Clinical symptoms (reference) |
|---|---|---|
| SHIVSF33 (X4) | 2/3 (intravaginal) | Seroconversion, stable peripheral CD4+-T-cell count, persistent infection with low viral load |
| SHIVSF33A (X4) | 4/4 (intravaginal) | Seroconversion, acute peripheral CD4+-T-cell loss, persistent infection with low viral load (29) |
| 2/2 (oral) | Seroconversion; dramatic CD4+-T-cell loss; SAIDS at 50 wpi in one animal, with remaining animal healthy over 2 yr (40) | |
| SHIVSF162 (R5) | 0/3 (intravaginal) | None (seronegative, cell-associated viral load negative) |
| SHIVSF162P3 (R5) | 4/4 (intravaginal) | Seroconversion; gradual CD4+-T-cell loss; SAIDS in two animals at wk 24 and 44, with remaining two animals healthy over 2 yr (26) |
During sexual transmission, immature dendritic cells (DCs) present in mucosal tissues are among the first cells to be encountered by HIV (19, 20, 30, 59). DCs themselves are either not infected or infected poorly but rather capture and transfer viruses efficiently to target T cells (5, 10, 24, 39, 49-51, 62). This latter property is mediated in part by the DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN) molecule, a 404-amino- acid (aa)-long type II transmembrane mannose binding C-type lectin expressed on the surface of immature DCs that interacts with envelope glycoprotein gp120 (15, 21, 48). It has been suggested that, as a consequence of the normal cellular trafficking of DCs, DC-SIGN plays a major role in HIV transmission and dissemination at the mucosal surfaces by capturing and transporting the virus to peripheral lymph nodes and gut-associated lymphoid tissues (9, 21, 25, 54). The availability of viruses that differ in their transmission efficiency provides the tools necessary to assess the potential role of DC-SIGN binding in infection across the mucosal barrier and to dissect the sites on gp120 that modulate this property. We therefore compared the ability of the envelope glycoproteins of parental SHIVSF162 and the pathogenic, mucosally transmissible variant SHIVSF162P3 to bind DC-SIGN-expressing cells, with gp120s from SHIVSF33 and SHIVSF33A serving as controls.
MATERIALS AND METHODS
Cells.
Human embryonic kidney (HEK-293T) cells used for transfection were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum (FCS) and antibiotics. The CEMx174 5.25 M7 cell line (a generous gift from N. R. Landau, Salk Institute, La Jolla, Calif.) was stably transduced with an HIV-1-LTR-GFP and HIV-1-LTR-Luc reporter construct and with CCR5. These cells were maintained in RPMI 1640 medium supplemented with 10% FCS, 1 μg of puromycin/ml, and 200-μg/ml concentrations each of G418 and hygromycin. Human peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll gradient centrifugation and cultured in RPMI 1640 medium supplemented with 10% FCS and 20 U of recombinant interleukin-2 (Chiron Corp., Emeryville, Calif.)/ml.
PCR and sequencing of viral DNA.
Viral DNA sequences containing the HIV type 1 (HIV-1) env gene were amplified from the lymph node PBMCs of SHIVSF162P3-infected animal T353 at week 20 postinfection by nested PCR with ED3 and ED14 as first-round primers and ED5 and ED12 as second-round primers as described previously (29). The amplified products were cloned into the pCR 2.1 TA vector (Invitrogen, Carlsbad, Calif.), and the consensus sequence for the PCR products was obtained.
Viruses.
Full-length proviral DNAs expressing the envelope glycoproteins of SHIVSF33, SHIVSF33A, SHIVSF162, and SHIVSF162P3 were constructed on the genomic backbone of HIV-1 R7/3, a proviral HXB2 clone in which the nef open reading frame had been restored (a gift from M. Muesing, Aaron Diamond AIDS Research Center, New York, N.Y.). To replace the HIV-1 R7/3 env gene with that of SHIVSF33, the BbsI-BamHI fragment of clone R7/3 (nucleotides 6219 to 8475) was replaced with the corresponding sequences in HIV-1SF33. The BamHI site, which was not conserved in HIV-1SF33, was introduced at position 2720 by site-directed mutagenesis (numbering refers to sequence M38427 in GenBank) using the QuikChange kit (Stratagene, San Diego, Calif.). The resulting proviral construct, HIV-1 R7/3-33, expressed an envelope glycoprotein identical to that of HIV-1SF33 except for the last 106 aa of the cytoplasmic domain, which originated from the R7/3 clone. The similar constructs R7/3-33A and R7/3-162 were obtained by replacing the BbsI-BamHI fragment in R7/3 with sequences from SHIVSF33A2 and HIV-1SF162 (GenBank accession numbers AF373044 and M38428, respectively). The BamHI site was also absent in HIV-1SF162 and was introduced first into the pSM/162 Env expression plasmid (61) by using the complementary oligonucleotides SF162BF (5′-CCA TTA GTG CAT GGA TCC TTA GCA CTC ATC TGG GAC G) and SF162BR to generate pSM/162(B). For construction of the R7/3-162P3 clone, a 1.14-kb DraIII/BsgI fragment containing the V1 to V5 region of pSM/162(B) was replaced with the corresponding sequences amplified from SHIVSF162P3. Viruses were generated by lipofection with 3.0 μg of each proviral DNA construct into HEK-293T cells plated at 4 × 105 per well in 6-well plates. The lipofection was performed with DMRIE-C reagent (Gibco Life Technologies, Gaithersburg, Md.) according to the manufacturer's recommendations. Cell culture supernatants were harvested 48 h posttransfection, centrifuged at 800 × g, filtered through 0.45-μm-pore size filters, and stored at −70°C until use. The viral content was quantified by using a p24 Gag enzyme-linked immunosorbent assay (ELISA) from Abbott Laboratories (Chicago, Ill.).
To generate R7/3-162 glycosylation mutant viruses, site-directed mutagenesis was performed by using the pSM/162(B) Env plasmid and the complementary mutagenic oligonucleotides SF162(g138)F (5′-GCT ACT AAT ACC ACG AGT AGT AAT TGG) and SF162(g138)R and SF162(g158)F (T TGC TCT TTC AAC GTC ACC ACA AGC) and SF162(g158)R to introduce potential glycosylation sites in the V1 region (aa 138) and at the base of the V2 region (aa 158), respectively. The BbsI-BamHI fragments of the mutagenized plasmids were prepared and exchanged with the corresponding region of the R7/3-162 proviral construct. The double glycosylation mutant virus R7/3-162 (g138/158) was generated by introducing the g158 mutation on the backbone of the pSM/162B (g138) plasmid, followed by preparation and substitution of the BbsI-BamHI fragment into the corresponding regions of the proviral R7/3-162 DNA. The presence of the mutations was confirmed by DNA sequencing. Mutant viruses were generated by transfection of HEK-293T cells with proviral DNAs.
Amplification and cloning of human and rhesus DC-SIGN.
Total RNA was extracted from purified DCs with Trizol reagent (Gibco Life Technologies) and treated with 10 U of RNase-free DNase (Promega, Madison, Wis.). cDNA was prepared from 5 μg of RNA by using Superscript II reverse transcriptase (Gibco Life Technologies) and resuspended in 60 μl of TE buffer (10 mM Tris [pH 8.0], 1 mM EDTA). Specific cDNAs were amplified according to the manufacturer's specifications in a 100-μl reaction mixture containing 5 μl of cDNA; 10 mM Tris-HCl (pH 8.5); 50 mM KCl; 1.5 mM MgCl2; 0.1% Triton X-100; 200 μM (each) dATP, dGTP, dCTP, and dTTP; 20 pmol of each primer; and 2.5 U of EXPAND high-fidelity DNA polymerase (Roche Diagnostic Corp., Indianapolis, Ind.). PCR primers hybridizing to the 5′- and 3′-untranslated regions of rhesus and human DC-SIGN were as follows (restriction sites for cloning are underlined): first round, DC-SIGNf1 (5′-TCT GGA CAC TGG GGG AGA GTG G-3′) and DC-SIGNb1 (5′-GGA TGG AGA GAA GGA ACT GTA G-3′); second round, DC-SIGNf2 (5′-TCGAG GGATCCGAATTC GGA GAG TGG GGT GAC ATG AGT G-3′) and DC-SIGNb2 (5′-TCGA GCGGCCGCTCTAGA GCT TAA AAG GGG GTG AAG TTC TG-3′). Amplification cycles were 95°C for 2 min, followed by 35 cycles at 94°C for 15 s, 50°C for 45 s, and 72°C for 1 min and then an incubation at 72°C for 8 min. Products from the second-round amplifications were electrophoresed on a 1% agarose gel and visualized by staining with ethidium bromide. PCR products were purified with the PCR-Prep kit (Promega) and cloned into the expression vector pcDNA4/HisMaxC (Invitrogen). The expression of human and rhesus DC-SIGN was confirmed by Western blot and fluorescence-activated cell sorter (FACS) analysis (data not shown).
FACS analysis of DC-SIGN expression.
HEK-293T cells transfected with the indicated expression plasmids were harvested 48 h posttransfection and washed with phosphate-buffered saline containing 1% FCS and 0.05% sodium azide (FACS buffer). Transfected cells were incubated with a monoclonal antibody specific for human DC-SIGN (Pharmingen, San Diego, Calif.) or with isotypic control antibodies for 15 min at room temperature. Cells were then washed with FACS buffer, fixed in 2% paraformaldehyde, and analyzed by using a FACS apparatus (FACScan; Becton Dickinson, Mountain View, Calif.). Geometrical mean channel fluorescence intensity was determined and used as a measure of relative DC-SIGN expression.
Virus binding and transmission.
Binding of virus particles to DC-SIGN-expressing HEK-293T cells was assessed by measuring cell-associated p24 levels. Briefly, 4 × 105 HEK-293T cells seeded in 6-well plates were transfected with expression vectors encoding human or rhesus DC-SIGN or a pcDNA4 control plasmid by lipofection. Forty-eight hours posttransfection, cells were harvested for FACS analyses of DC-SIGN expression and for virus binding. For binding, 5 or 20 ng of a p24 equivalent of each virus was added in duplicate to 2 × 105 transfected cells in a total volume of 0.5 ml. After 4 to 5 h of incubation at 37°C, the virus inoculum was removed and the cells were extensively washed with Hanks' medium. Cells were lysed in 100 μl of 0.5% Triton X-100 in H2O, and the amount of bound virus was determined by p24 ELISA. Binding to pcDNA4-transfected control cells ranged from 0.1 to 0.3% of that of the input viruses, and these values were deducted from the percentage of viruses bound to DC-SIGN-expressing cells in the data presented. For an assessment of DC-SIGN-mediated infection in trans, cells were plated in 48-well plates and 2 × 106 human PBMCs were added. Culture supernatants were collected at 2, 5, and 8 days postcocultivation and p24 Gag antigen content was determined by ELISA.
To generate cells expressing various amounts of DC-SIGN, HEK-293T cells plated at 4 × 105 cells per well in 6-well plates were transfected with 0.02 to 2 μg of the human or rhesus DC-SIGN expression vector. Cells were collected 48 h later, DC-SIGN expression was measured by FACS, and the cells were used in virus binding studies.
Immunoblot analysis of envelope glycoproteins.
HEK-293T cells transfected with proviral constructs were harvested 72 h posttransfection and lysed in a solution containing 50 mM Tris-HCl, 50 mM NaCl, 1 mM EDTA, 10 mM MgCl2, and 1% Triton X-100. Cell lysates were denatured by boiling in sample buffer, and the proteins were separated by sodium dodecyl sulfate-4 to 12% polyacrylamide gel electrophoresis (NuPAGE; Invitrogen). Envelope gp120s were detected by immunoblotting with a goat anti-gp120 antibody (Chiron Corp.).
Neutralization assays.
Virus neutralization was performed with CEMx174 5.25 M7 cells in 96-well plates as described previously (14). Briefly, serial dilutions of heat-inactivated serum samples from macaque T353 were incubated in triplicate wells with equal volumes (50 μl) of each virus (5 ng of p24) for 1 h at 37°C. A total of 2 × 104 CEMx174 5.25 M7 cells in a 100-μl volume of medium were then added to the virus-serum mixture and cultured for 4 days at 37°C. Control cultures received virus incubated in the absence of antisera. At the end of the incubation period, cells were harvested, lysed, and incubated with the luciferase assay reagents according to the manufacturer's instructions (Promega). The luciferase activity was measured in an MLX microtiter plate luminometer (Dynex Technologies, Inc., Chantilly, Va.). A neutralization curve was generated by plotting the percent neutralization versus serum dilution. The dilution of antiserum that resulted in 90% inhibition was then interpolated from this curve.
RESULTS
SHIVSF162P3 envelope glycoproteins confer increased binding to DC-SIGN.
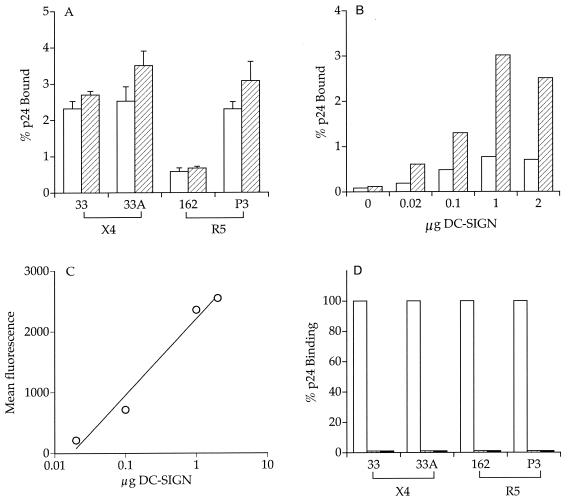
It was previously reported that the pathogenic isolate SHIVSF162P3 is transmitted efficiently across mucosal barriers (26) (summarized in Table 1). To assess whether interaction with the DC-SIGN molecule could contribute to HIV-1 transmission efficiency, the ability of the SHIVSF162 and SHIVSF162P3 envelope glycoprotein gp120 to bind human and rhesus DC-SIGN transiently expressed on HEK-293T cells was examined. Envelope gp120s from SHIVSF33 and SHIVSF33A served as controls in these experiments since these viruses did not differ substantially in their mucosal transmissibility. For these studies, chimeric viruses expressing the various envelope gp120s were constructed in the genomic backbone of the proviral HIV-1 R7/3 clone. The resulting R7/3-33 and R7/3-33A viruses are isogenic except for 25 aa residues in the V1 to V5 regions of envelope gp120 (13). Similarly, R7/3-162 and R7/3-162P3 are isogenic, with only 14 aa differences in the V1 to V5 domains of gp120 (unpublished data). We found that, whereas R7/3-33 and R7/3-33A displayed no significant difference in their binding to 293T cells expressing human DC-SIGN, a threefold or more increase in the binding of R7/3-162P3 compared to that of R7/3-162 was consistently observed (Fig. 1A). Similar results were obtained with 293T cells expressing rhesus DC-SIGN (Fig. 1A). This differential binding of R7/3-162 and R7/3-162P3 viruses was observed over a wide range of DC-SIGN expression levels (Fig. 1B). We verified that transfecting increasing amounts of the DC-SIGN plasmid resulted in a concomitant increase in DC-SIGN surface expression (Fig. 1C). In agreement with previous reports (21, 46), binding of the viruses to DC-SIGN was dose dependent (data not shown) and was eliminated in the presence of EGTA and mannan (Fig. 1D).
FIG. 1.
Increased binding of SHIVSF162P3 envelope gp120 to DC-SIGN-expressing cells. (A) HEK-293T cells transiently expressing human (□) and rhesus (▨) DC-SIGN were incubated with equal amounts (5 ng of p24) of R7/3-33, R7/3-33A, R7/3-162, and R7/3-162P3 viruses, washed, and lysed in 0.5% Triton X-100. The amount of p24 bound was quantified by ELISA and expressed as a percentage of total antigen. Values represent the standard error of the mean from four to five independent experiments. (B) HEK-293T cells were transfected with increasing amounts of the human DC-SIGN plasmid. Transfected cells were incubated with R7/3-162 (□) and R7/3-162P3 (▨) viruses, and the amount of bound p24 was determined. Results are representative of two independent experiments. (C) FACScan analysis of DC-SIGN expression. The mean fluorescence of DC-SIGN monoclonal antibody staining for the transfected cells used in panel B is shown. (D) The binding was performed as described above except that the cells were incubated with mannan (▥, 20 μg/ml) or EGTA (▪, 5 mM) prior to the addition of virus.
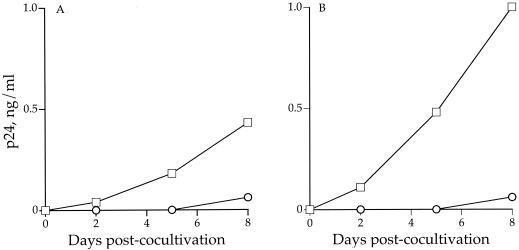
Increased binding to DC-SIGN correlates with enhanced transmission of the virus in trans.
Since binding of the virus to DC-SIGN and its subsequent transfer to receptor-positive cells can be uncoupled (47), we examined whether the increased binding of SHIVSF162P3 envelope gp120 to the surface of DC-SIGN-expressing cells led to increased transmission of the virus to activated PBMCs in trans (Fig. 2). 293T cells expressing DC-SIGN or vector alone were incubated with the R7/3-162 and R7/3-162P3 viruses, extensively washed, and subsequently cocultivated with phytohemagglutinin-stimulated PBMCs. Virus replication was monitored for several days postcocultivation. We found that both the human and rhesus DC-SIGN-transfected cells pulsed with R7/3-162P3 transmitted the virus more efficiently than did cells pulsed with the R7/3-162 virus (Fig. 2). Thus, increased binding to DC-SIGN-expressing cells correlates with enhanced transmission in trans of the R7/3-162P3 virus to PBMCs.
FIG. 2.
Increased binding of SHIVSF162P3 envelope gp120 to DC-SIGN correlates with enhanced transmission to PBMCs in trans. A total of 2 × 105 HEK-293T cells transiently expressing human (A) or rhesus (B) DC-SIGN were incubated with equal amounts of the R7/3-162 or R7/3-162P3 viruses, extensively washed, and cocultivated with 2 × 106 phytohemagglutinin-stimulated PBMCs. p24 Gag antigen in culture supernatant was determined at 2, 5, and 8 days cocultivation. ○, R7/3-162; □, R7/3-162P3.
DC-SIGN-virus interaction is modulated by gp120 glycosylation.
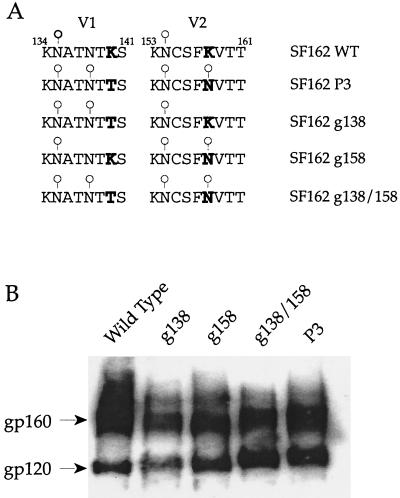
Binding of HIV-1 Env to DC-SIGN is dependent on the C-terminal lectin domain of DC-SIGN and carbohydrate structures on the viral envelope that remain unidentified (21, 46). Consistent with a potential role of glycan moieties in modulating Env/DC-SIGN interaction, comparison of the V1 to V5 sequence of envelope gp120 revealed no difference in the extent of glycosylation of the R7/3-33 and R7/3-33A viruses whereas two additional potential glycosylation sites were present in R7/3-162P3 Env gp120 compared to R7/3-162 Env. These sites were located within the V1 domain (aa 138) and at the N-terminal base of the V2 loop (aa 158) (Fig. 3A). To assess the contribution of these carbohydrate modifications to the differences observed in binding to DC-SIGN, these V1 and V2 glycosylation sites were introduced in the backbone of the R7/3-162 virus to generate the R7/3-162 (g138) and R7/3-162 (g158) viruses, respectively. A mutant virus containing both changes, designated R7/3-162 (g138/158), was also constructed (Fig. 3A). Biochemical analyses revealed a lower mobility of the envelope gp120 of R7/3-162P3 compared to wild-type R7/3-162 gp120, indicating that the additional potential glycosylation sites were utilized. The gp120s of the single mutants g138 and g158 migrated with an apparent molecular mass that was an intermediate of wild-type R7/3-162 and variant R7/3-162P3 envelope gp120s, whereas the mobility of double-mutant R7/3-162 (g138/158) gp120 was similar to that of R7/3-162P3. These findings strongly suggest that the genetic changes introduced resulted in the anticipated carbohydrate modifications (Fig. 3B).
FIG. 3.
(A) Amino acid alignment of the V1 and V2 regions of wild-type, variant, and mutant envelope glycoproteins. Bold letters designate amino acid changes. ○, presence of glycosylation site. WT, wild type. (B) Immunoblot analysis of wild-type, P3 variant, and mutant envelope glycoproteins. HEK-293T cells were transfected with proviral DNAs. Forty-eight hours posttransfection, cells were harvested and lysed and proteins were separated by sodium dodecyl sulfate-4 to 12% polyacrylamide gel electrophoresis. Envelope gp120s were detected by immunoblotting with a polyclonal goat anti-gp120 antibody. Positions of gp160 and gp120 are denoted.
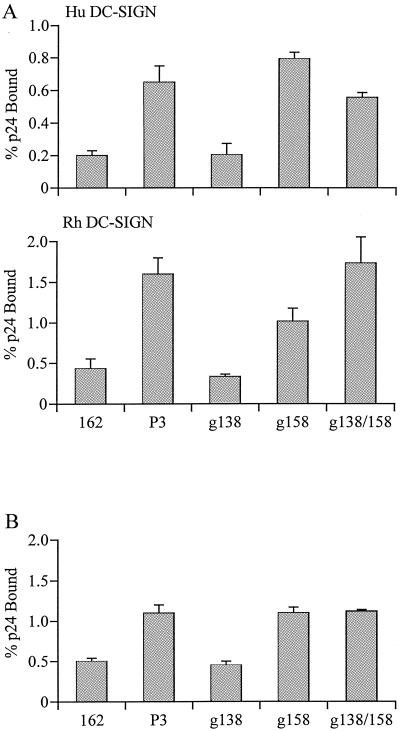
We found that the glycosylation site mutations did not affect replication of the viruses; similar kinetics of replication in human PBMCs were observed for the mutant and wild-type R7/3-162 viruses (data not shown). In virus binding assays, R7/3-162 (g138) gp120 bound to human or rhesus DC-SIGN-expressing cells with an efficiency similar to that of the wild-type R7/3-162 virus (Fig. 4A). In contrast, the binding efficiency of R7/3-162 (g158) and the double-mutant R7/3-162 (g138/158) virus to DC-SIGN was comparable to that of the R7/3-162P3 virus. Since glycosylation can differ between cell types, wild-type and mutant viruses propagated in human PBMCs were examined for their binding to DC-SIGN to ensure that the differences observed were not due to carbohydrate modifications that are unique to 293T cells. We found that viruses propagated in human PBMCs displayed a pattern of binding to rhesus DC-SIGN-expressing cells similar to that of viruses derived from 293T cells (Fig. 4A and B). Thus, the additional carbohydrate side chain at the base of the V2 domain of HIV-1SF162 Env gp120 is responsible for the enhanced binding of the R7/3-162P3 virus to DC-SIGN.
FIG. 4.
Binding of glycan mutant gp120s to human (Hu) and rhesus (Rh) DC-SIGN-expressing cells. DC-SIGN-expressing cells were incubated with 293T-cell derived (A) or human PBMC-propagated (B) wild-type, P3 variant, and gp120 glycan mutant viruses, and the amounts of bound p24 were determined. Values presented in panels A and B are the standard error of the mean from three and two independent experiments, respectively.
The glycosylation change in gp120 that mediates more efficient binding to DC-SIGN also confers escape from immune recognition.
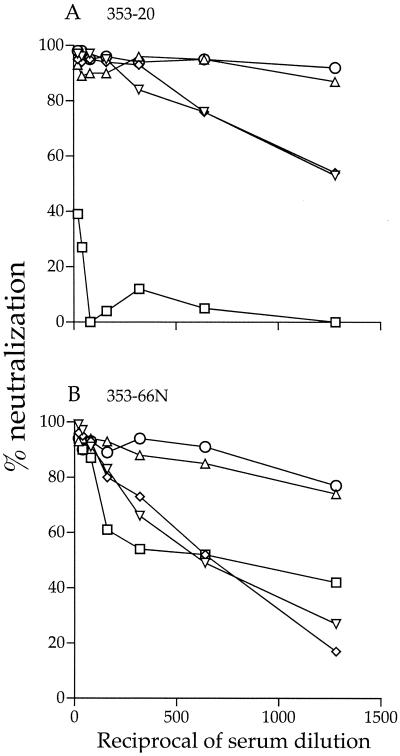
In addition to maintaining the proper folding and function of envelope gp120, N-glycans have been shown to play a critical role in the shielding of neutralization epitopes of both HIV-1 and simian immunodeficiency virus (4, 12, 14, 23, 41, 42, 53, 55, 56). We therefore determined the ability of sera obtained from macaque T353, the animal from which SHIVSF162P3 was isolated (26), to neutralize wild-type, mutant, and variant viruses. Sera collected at 20 wpi, the time SHIVSF162P3 was isolated from T353 lymph node PBMCs, and at necropsy (66 wpi) were used. We found that the variant virus R7/3-162P3 was highly resistant to neutralization by sera from macaque T353, which was indicative of immune escape. A 90% neutralization of the virus was attained with a 1:50 dilution of week 66 serum but not with the serum collected at the time of virus isolation at the lowest dilution tested (1:20). In contrast, greater than 90% neutralization of the wild-type R7/3-162 and R7/3-162 (g138) mutant viruses was achieved with week 20 serum at 1:1,500 and 1:700 serum dilutions, respectively, but neutralizing activity against these viruses was noticeably lower in the week 66 serum (90% inhibitory concentrations of 1:600 and 1:300, respectively). The single R7/3-162 (g158) and double R7/3-162 (g138/158) mutant viruses displayed an intermediate phenotype. Compared to the wild-type R7/3-162 virus, both viruses were five- to sevenfold more resistant to neutralization; 90% neutralization required a 1:300 dilution of the week 20 serum and a 1:100 dilution of the week 66 serum. Collectively, our findings are in support of a temporal evolution in antibody responses directed at the envelope glycoprotein within the host and are in agreement with previous reports for HIV-1 and simian immunodeficiency virus infections (1, 3, 7, 8, 12, 18, 44, 61). Importantly, these data show that the glycan modification that results in better interaction with DC-SIGN also confers escape from antibody-mediated immune neutralization.
DISCUSSION
In this study, we address the proposed role of DC-SIGN in HIV transmission by comparing the ability of envelope glycoproteins obtained from SHIVs showing varying degrees of mucosal transmissibility to bind DC-SIGN expressed on the surface of transfected cells. The X4-SHIVSF33 and -SHIVSF33A viruses established infection via vaginal transmission with comparable efficiency, but the R5-SHIVSF162P3 virus crossed the mucosal barrier and spread with a significantly greater efficiency than the parental SHIVSF162 (Table 1). We found that, whereas the binding of envelope gp120 from X4-SHIVSF33A to DC-SIGN was comparable to that of the parental SHIVSF33, there were observable differences for the R5 strains. gp120 of the pathogenic, highly mucosally transmissible SHIVSF162P3 variant exhibited a threefold or greater increase in binding to DC-SIGN than that of gp120 from the nonpathogenic, poorly transmissible parental SHIVSF162. This difference in the binding of SHIVSF162 and SHIVSF162P3 was observed with DC-SIGN from both humans and macaques and was consistent over a wide range of DC-SIGN expression levels (Fig. 1). This latter finding is of importance since only moderate levels of DC-SIGN expression by DCs in vaginal mucosal tissues have been reported (21, 31). Thus, the difference seen in in vitro DC-SIGN binding of SHIVSF162 and SHIVSF162P3 envelope gp120s could be relevant to the differential mucosal transmissibility of SHIVSF162 and SHIVSF162P3 in vivo.
The increase in binding to DC-SIGN of SHIVSF162P3 envelope gp120 also translates into an enhancement of T-cell infection in trans. Virus containing the variant gp120 (R7/3-P3) was transferred more efficiently to T cells than virus containing gp120 from parental SHIVSF162 (R7/3-162) (Fig. 2). A novel mechanism by which DC-SIGN might enhance HIV infection has recently been suggested, in which internalization of HIV particles into a nonlysosomal intracellular compartment appears to be critical (37). It is proposed that this internalization increases the amount of virus that can be retained by DC-SIGN-expressing cells for subsequent transport to secondary lymphoid tissues and/or induces conformational changes in the envelope that augment viral infectivity. Regardless of the mechanism by which DC-SIGN might enhance HIV infection, it is reasonable to assume that, since more R7/3-P3 viruses are captured by DC-SIGN-expressing cells, more viruses will be transmitted to T cells in trans. This, in turn, could contribute to the marked increase in transmission and dissemination of SHIVSF162P3 in vivo compared to that of SHIVSF162.
The sites on DC-SIGN to which virus binds have been investigated extensively (21, 46, 47), but less well defined are the DC-SIGN binding sites on gp120. We show here that site-specific gp120 glycosylation, rather than a global increase in gp120 glycosylation, appears to be responsible for the increase in binding of SHIVSF162P3 gp120 to DC-SIGN. Compared to wild-type SHIVSF162 gp120, two additional potential glycosylation sites are present in SHIVSF162P3 envelope gp120 and both appear to be utilized (Fig. 3A and B). However, only the addition of a glycan at the base of the V2 loop (aa 158), and not at aa 138 within the V1 domain of wild-type SF162 Env, confers on the mutant virus a DC-SIGN binding efficiency comparable to that of SHIVSF162P3 gp120 (Fig. 4). This could be due to differences in the type or orientation of the sugars located at aa 138 and 158. Alternatively, the glycan at aa 158 does not mediate DC-SIGN binding but may modify the gp120 tertiary structure that participates in DC-SIGN interaction to increase its affinity. In support of this latter possibility, a recent study suggested that glycosylations are not necessary for the interaction of HIV-1 gp120 with DC-SIGN (22).
Interestingly, the same glycan that confers increased binding to DC-SIGN also confers increased resistance to neutralization by autologous sera (Fig. 5). It is now recognized that sugar moieties contribute to steric masking of neutralization epitopes on HIV envelope glycoproteins. In the context of HIV-1SF162 Env, V2 loop glycosylation has been reported to protect the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies (41). The addition of the glycan at position 158 confers resistance to autologous, but not heterologous, serum neutralization (Fig. 5 and data not shown), suggesting modulation of recognition by strain-specific rather than broadly reactive neutralizing antibodies. Indeed, strain-restricted neutralizing antibodies directed against the V2 and V3 domains of HIV-1 gp120 arise relatively early in infection and are easier to escape from (16, 35, 43-45, 58, 61). The presence of a single glycan at the base of the V2 loop, therefore, can change the position of this loop by restricting its movement or modifying the conformation of the V3 domain. Indeed, functional interactions of the V2 and V3 domains of envelope gp120 had been reported (2, 36, 63, 64).
FIG. 5.
Autologous neutralization of wild-type, P3 variant, and glycan mutant viruses. Sera from macaque T353 at 20 wpi (A) and at necropsy (66 wpi) (B) were used. ○, wild-type R7/3-162; □, variant R7/3-162P3; ▵, R7/3-162 (g138); ◊, R7/3-162 (g158); ▿, R7/3-162 (g138/158).
Macaque T353 seroconverted at 3 wpi, and the sequence change at aa 158 in gp120 was detected as early as 6 wpi, only 3 weeks after seroconversion (S. Shapiro, M. Hsu, J. Harouse, C. Cheng-Mayer, and P. Balfe, 9th Conf. Retrovir. Opportunistic Infect., abstr. 358-M, 2002). Thus, it is tempting to speculate that, as a result of escape from immune recognition, the virus also alters its ability to interact with DC-SIGN, improving its transmissibility across mucosal surfaces. It would be of interest to identify the time of emergence of the autologous neutralizing antibodies that select for the g158 mutation, presumably between week 3 and week 6 postinfection, as well as the temporal changes in gp120 that allow escape of the virus from recognition by these antibodies. These studies should provide insights into the selective forces that drive the virus to change antigenically and, perhaps fortuitously, increase transmission of the virus across mucosal surfaces in vivo.
In summary, our data show a relationship between the more efficient binding of envelope gp120 to DC-SIGN and the enhanced transmission and dissemination of SHIVSF162P3 compared to that of parental SHIVSF162. With regard to the X4 viruses, both SHIVSF33 and SHIVSF33A show mucosal transmissibility (two of three for parental SHIVSF33 and six of six for pathogenic SHIVSF33A [Table 1]). The difference in infection between SHIVSF33 and SHIVSF33A by the mucosal route as well as the intravenous route lies principally in the pathological outcome of the infection (29, 40) and can be explained by the increased replicative and fusogenic properties of the SHIVSF33A envelope glycoproteins (13). Both of these X4 viruses bind DC-SIGN with similar efficiency, consistent with the very modest or absent difference in mucosal transmission of the two viruses in vivo. In contrast, attempts to infect macaques with parental R5-SHIVSF162 by the vaginal route have been unsuccessful, indicating that some intrinsic property of the virus that mediates efficient mucosal infection is lacking in this virus. It will be of interest to determine whether SHIVSF162 containing the single g158 mutation can establish vaginal transmission and dissemination in vivo with greater efficiency.
Acknowledgments
We thank Lisa Chakrabarti and Peter Balfe for critical reading of the manuscript. We acknowledge the generous gift of the CEMx174 5.25 M7 cell line from Nathaniel Landau, the HIV-1 R7/3 proviral DNA plasmid from Mark Muesing and Wendy Chen for help with the graphics.
This work was supported by NIH grants CA72822, AI46980, and AI 41945.
REFERENCES
- 1.Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4:107-112. [DOI] [PubMed] [Google Scholar]
- 2.Andeweg, A. C., P. Leeflang, A. D. Osterhaus, and M. L. Bosch. 1993. Both the V2 and V3 regions of the human immunodeficiency virus type 1 surface glycoprotein functionally interact with other envelope regions in syncytium formation. J. Virol. 67:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arendrup, M., C. Nielsen, J. E. Hansen, C. Pedersen, L. Mathiesen, and J. O. Nielsen. 1992. Autologous HIV-1 neutralizing antibodies: emergence of neutralization-resistant escape virus and subsequent development of escape virus neutralizing antibodies. J. Acquir. Immune Defic. Syndr. 5:303-307. [PubMed] [Google Scholar]
- 4.Back, N. K., L. Smit, J. J. De Jong, W. Keulen, M. Schutten, J. Goudsmit, and M. Tersmette. 1994. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology 199:431-438. [DOI] [PubMed] [Google Scholar]
- 5.Blauvelt, A., H. Asada, M. W. Saville, V. Klaus-Kovtun, D. J. Altman, R. Yarchoan, and S. I. Katz. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Investig. 100:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogers, W. M., C. Cheng-Mayer, and R. C. Montelaro. 2000. Developments in preclinical AIDS vaccine efficacy models. AIDS 14:S141-S151. [PubMed] [Google Scholar]
- 7.Bradney, A. P., S. Scheer, J. M. Crawford, S. P. Buchbinder, and D. C. Montefiori. 1999. Neutralization escape in human immunodeficiency virus type 1-infected long-term nonprogressors. J. Infect. Dis. 179:1264-1267. [DOI] [PubMed] [Google Scholar]
- 8.Burns, D. P., C. Collignon, and R. C. Desrosiers. 1993. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J. Virol. 67:4104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron, P., M. Pope, A. Granelli-Piperno, and R. M. Steinman. 1996. Dendritic cells and the replication of HIV-1. J. Leukoc. Biol. 59:158-171. [DOI] [PubMed] [Google Scholar]
- 10.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 11.Cayabyab, M., G. B. Karlsson, B. A. Etemad-Moghadam, W. Hofmann, T. Steenbeke, M. Halloran, J. W. Fanton, M. K. Axthelm, N. L. Letvin, and J. G. Sodroski. 1999. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2). J. Virol. 73:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chackerian, B., L. M. Rudensey, and J. Overbaugh. 1997. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 71:7719-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabarti, L. A., T. Ivanovic, and C. Cheng-Mayer. 2002. Properties of the surface envelope glycoprotein associated with virulence of simian-human immunodeficiency virus SHIVSF33A molecular clones. J. Virol. 76:1588-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng-Mayer, C., A. Brown, J. Harouse, P. A. Luciw, and A. J. Mayer. 1999. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J. Virol. 73:5294-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etemad-Moghadam, B., G. B. Karlsson, M. Halloran, Y. Sun, D. Schenten, M. Fernandes, N. L. Letvin, and J. Sodroski. 1998. Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected monkeys. J. Virol. 72:8437-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etemad-Moghadam, B., Y. Sun, E. K. Nicholson, M. Fernandes, K. Liou, R. Gomila, J. Lee, and J. Sodroski. 2000. Envelope glycoprotein determinants of increased fusogenicity in a pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo. J. Virol. 74:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenyo, E. M., J. Albert, and J. McKeating. 1996. The role of the humoral immune response in HIV infection. AIDS 10:S97-S106. [DOI] [PubMed] [Google Scholar]
- 19.Frankel, S. S., K. Tenner-Racz, P. Racz, B. M. Wenig, C. H. Hansen, D. Heffner, A. M. Nelson, M. Pope, and R. M. Steinman. 1997. Active replication of HIV-1 at the lymphoepithelial surface of the tonsil. Am. J. Pathol. 151:89-96. [PMC free article] [PubMed] [Google Scholar]
- 20.Frankel, S. S., B. M. Wenig, A. P. Burke, P. Mannan, L. D. Thompson, S. L. Abbondanzo, A. M. Nelson, M. Pope, and R. M. Steinman. 1996. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science 272:115-117. [DOI] [PubMed] [Google Scholar]
- 21.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 22.Geijtenbeek, T. B., G. C. van Duijnhoven, S. J. van Vliet, E. Krieger, G. Vriend, C. G. Figdor, and Y. van Kooyk. 2002. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 277:11314-11320. [DOI] [PubMed] [Google Scholar]
- 23.Gram, G. J., A. Hemming, A. Bolmstedt, B. Jansson, S. Olofsson, L. Akerblom, J. O. Nielsen, and J. E. Hansen. 1994. Identification of an N-linked glycan in the V1-loop of HIV-1 gp120 influencing neutralization by anti-V3 antibodies and soluble CD4. Arch. Virol. 139:253-261. [DOI] [PubMed] [Google Scholar]
- 24.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grouard, G., and E. A. Clark. 1997. Role of dendritic and follicular dendritic cells in HIV infection and pathogenesis. Curr. Opin. Immunol. 9:563-567. [DOI] [PubMed] [Google Scholar]
- 26.Harouse, J. M., A. Gettie, T. Eshetu, R. C. Tan, R. Bohm, J. Blanchard, G. Baskin, and C. Cheng-Mayer. 2001. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3). J. Virol. 75:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harouse, J. M., A. Gettie, R. C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816-819. [DOI] [PubMed] [Google Scholar]
- 28.Harouse, J. M., A. Gettie, R. C. Tan, T. Eshetu, M. Ratterree, J. Blanchard, and C. Cheng-Mayer. 2001. Pathogenic determinants of the mucosally transmissible CXCR4-specific SHIV(SF33A2) map to env region. J. Acquir. Immune Defic. Syndr. 27:222-228. [DOI] [PubMed] [Google Scholar]
- 29.Harouse, J. M., R. C. Tan, A. Gettie, P. Dailey, P. A. Marx, P. A. Luciw, and C. Cheng-Mayer. 1998. Mucosal transmission of pathogenic CXCR4-utilizing SHIVSF33A variants in rhesus macaques. Virology 248:95-107. [DOI] [PubMed] [Google Scholar]
- 30.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jameson, B., F. Baribaud, S. Pohlmann, D. Ghavimi, F. Mortari, R. W. Doms, and A. Iwasaki. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joag, S. V., I. Adany, Z. Li, L. Foresman, D. M. Pinson, C. Wang, E. B. Stephens, R. Raghavan, and O. Narayan. 1997. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J. Virol. 71:4016-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joag, S. V., Z. Li, L. Foresman, E. B. Stephens, L. J. Zhao, I. Adany, D. M. Pinson, H. M. McClure, and O. Narayan. 1996. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J. Virol. 70:3189-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson, G. B., M. Halloran, D. Schenten, J. Lee, P. Racz, K. Tenner-Racz, J. Manola, R. Gelman, B. Etemad-Moghadam, E. Desjardins, R. Wyatt, N. P. Gerard, L. Marcon, D. Margolin, J. Fanton, M. K. Axthelm, N. L. Letvin, and J. Sodroski. 1998. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J. Exp. Med. 188:1159-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenealy, W. R., T. J. Matthews, M. C. Ganfield, A. J. Langlois, D. M. Waselefsky, and S. R. Petteway, Jr. 1989. Antibodies from human immunodeficiency virus-infected individuals bind to a short amino acid sequence that elicits neutralizing antibodies in animals. AIDS Res. Hum. Retrovir. 5:173-182. [DOI] [PubMed] [Google Scholar]
- 36.Koito, A., G. Harrowe, J. A. Levy, and C. Cheng-Mayer. 1994. Functional role of the V1/V2 region of human immunodeficiency virus type 1 envelope glycoprotein gp120 in infection of primary macrophages and soluble CD4 neutralization. J. Virol. 68:2253-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 38.LaBonte, J. A., T. Patel, W. Hofmann, and J. Sodroski. 2000. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J. Virol. 74:10690-10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langhoff, E., K. H. Kalland, and W. A. Haseltine. 1993. Early molecular replication of human immunodeficiency virus type 1 in cultured-blood-derived T helper dendritic cells. J. Clin. Investig. 91:2721-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luciw, P. A., C. P. Mandell, S. Himathongkham, J. Li, T. A. Low, K. A. Schmidt, K. E. Shaw, and C. Cheng-Mayer. 1999. Fatal immunopathogenesis by SIV/HIV-1 (SHIV) containing a variant form of the HIV-1SF33 env gene in juvenile and newborn rhesus macaques. Virology 263:112-127. [DOI] [PubMed] [Google Scholar]
- 41.Ly, A., and L. Stamatatos. 2000. V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J. Virol. 74:6769-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malenbaum, S. E., D. Yang, L. Cavacini, M. Posner, J. Robinson, and C. Cheng-Mayer. 2000. The N-terminal V3 loop glycan modulates the interaction of clade A and B human immunodeficiency virus type 1 envelopes with CD4 and chemokine receptors. J. Virol. 74:11008-11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montefiori, D. C., K. A. Reimann, M. S. Wyand, K. Manson, M. G. Lewis, R. G. Collman, J. G. Sodroski, D. P. Bolognesi, and N. L. Letvin. 1998. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J. Virol. 72:3427-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moog, C., H. J. Fleury, I. Pellegrin, A. Kirn, and A. M. Aubertin. 1997. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J. Virol. 71:3734-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13:S137-S162. [PubMed] [Google Scholar]
- 46.Pöhlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Münch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pöhlmann, S., G. J. Leslie, T. G. Edwards, T. Macfarlan, J. D. Reeves, K. Hiebenthal-Millow, F. Kirchhoff, F. Baribaud, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus: virus binding and transfer are dissociable functions. J. Virol. 75:10523-10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pohlmann, S., E. J. Soilleux, F. Baribaud, G. J. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 50.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 182:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reece, J. C., A. J. Handley, E. J. Anstee, W. A. Morrison, S. M. Crowe, and P. U. Cameron. 1998. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 187:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 54.Rowland-Jones, S. L. 1999. HIV: the deadly passenger in dendritic cells. Curr. Biol. 9:R248-R250. [DOI] [PubMed] [Google Scholar]
- 55.Rudensey, L. M., J. T. Kimata, E. M. Long, B. Chackerian, and J. Overbaugh. 1998. Changes in the extracellular envelope glycoprotein of variants that evolve during the course of simian immunodeficiency virus SIVMne infection affect neutralizing antibody recognition, syncytium formation, and macrophage tropism but not replication, cytopathicity, or CCR-5 coreceptor recognition. J. Virol. 72:209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schonning, K., B. Jansson, S. Olofsson, J. O. Nielsen, and J. S. Hansen. 1996. Resistance to V3-directed neutralization caused by an N-linked oligosaccharide depends on the quaternary structure of the HIV-1 envelope oligomer. Virology 218:134-140. [DOI] [PubMed] [Google Scholar]
- 57.Shibata, R., F. Maldarelli, C. Siemon, T. Matano, M. Parta, G. Miller, T. Fredrickson, and M. A. Martin. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176:362-373. [DOI] [PubMed] [Google Scholar]
- 58.Shotton, C., C. Arnold, Q. Sattentau, J. Sodroski, and J. A. McKeating. 1995. Identification and characterization of monoclonal antibodies specific for polymorphic antigenic determinants within the V2 region of the human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 69:222-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stamatatos, L., A. Werner, and C. Cheng-Mayer. 1994. Differential regulation of cellular tropism and sensitivity to soluble CD4 neutralization by the envelope gp120 of human immunodeficiency virus type 1. J. Virol. 68:4973-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsang, M. L., L. A. Evans, P. McQueen, L. Hurren, C. Byrne, R. Penny, B. Tindall, and D. A. Cooper. 1994. Neutralizing antibodies against sequential autologous human immunodeficiency virus type 1 isolates after seroconversion. J. Infect. Dis. 170:1141-1147. [DOI] [PubMed] [Google Scholar]
- 62.Weissman, D., Y. Li, J. M. Orenstein, and A. S. Fauci. 1995. Both a precursor and a mature population of dendritic cells can bind HIV. However, only the mature population that expresses CD80 can pass infection to unstimulated CD4+ T cells. J. Immunol. 155:4111-4117. [PubMed] [Google Scholar]
- 63.Westervelt, P., D. B. Trowbridge, L. G. Epstein, B. M. Blumberg, Y. Li, B. H. Hahn, G. M. Shaw, R. W. Price, and L. Ratner. 1992. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J. Virol. 66:2577-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye, Y., Z. H. Si, J. P. Moore, and J. Sodroski. 2000. Association of structural changes in the V2 and V3 loops of the gp120 envelope glycoprotein with acquisition of neutralization resistance in a simian-human immunodeficiency virus passaged in vivo. J. Virol. 74:11955-11962. [DOI] [PMC free article] [PubMed] [Google Scholar]