Abstract
1. The smooth muscle of guinea-pig taenia coli was used to investigate the relation between metabolic and physiological effects of adrenaline. Electrical and mechanical activity was recorded with the sucrose gap technique and, in parallel experiments, the concentration of energy-rich phosphate compounds, adenosinetriphosphate and creatine phosphate, (ATP and CP) in the tissue was determined.
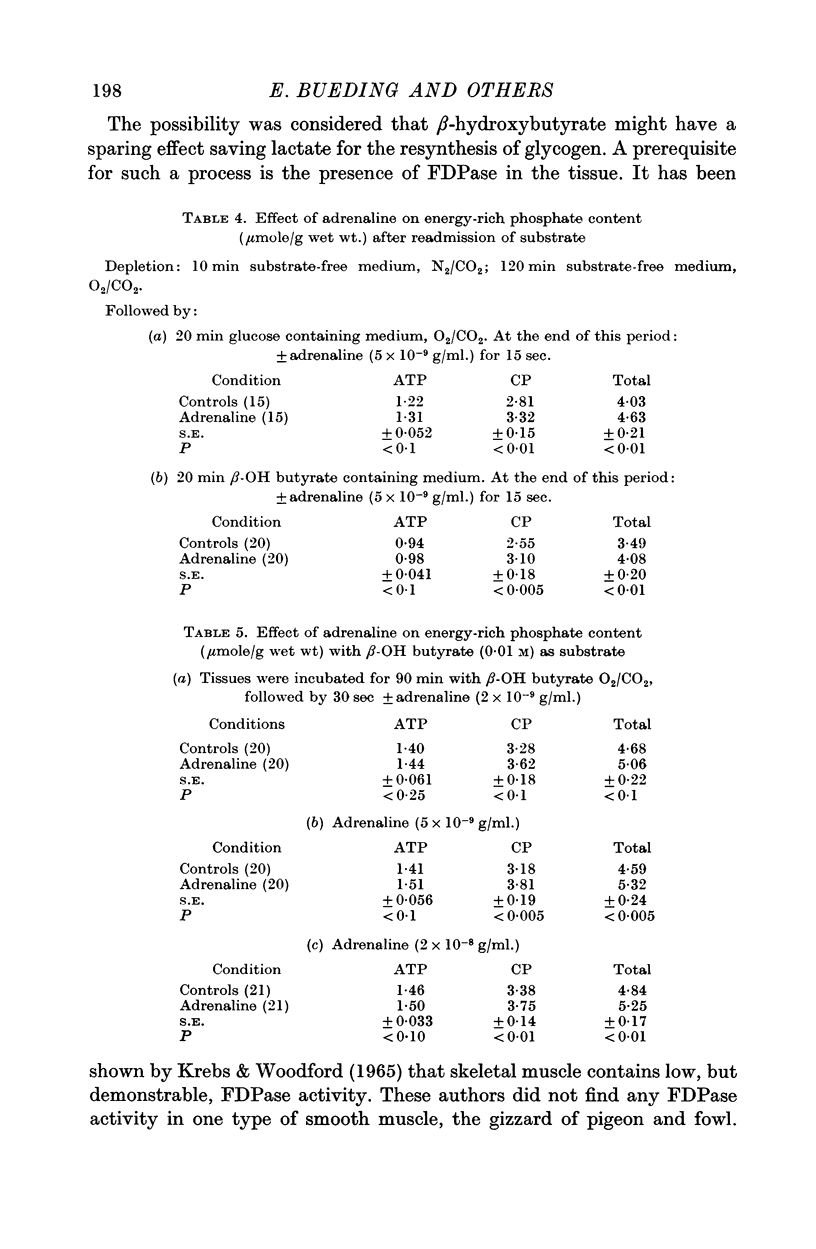
2. Adrenaline (in concentrations from 2 × 10-9 g/ml. to 5 × 10-8 g/ml.) increased the tissue content of energy-rich phosphate compounds. This effect was coincident with the physiological, inhibitory effect on electrical and mechanical activity.
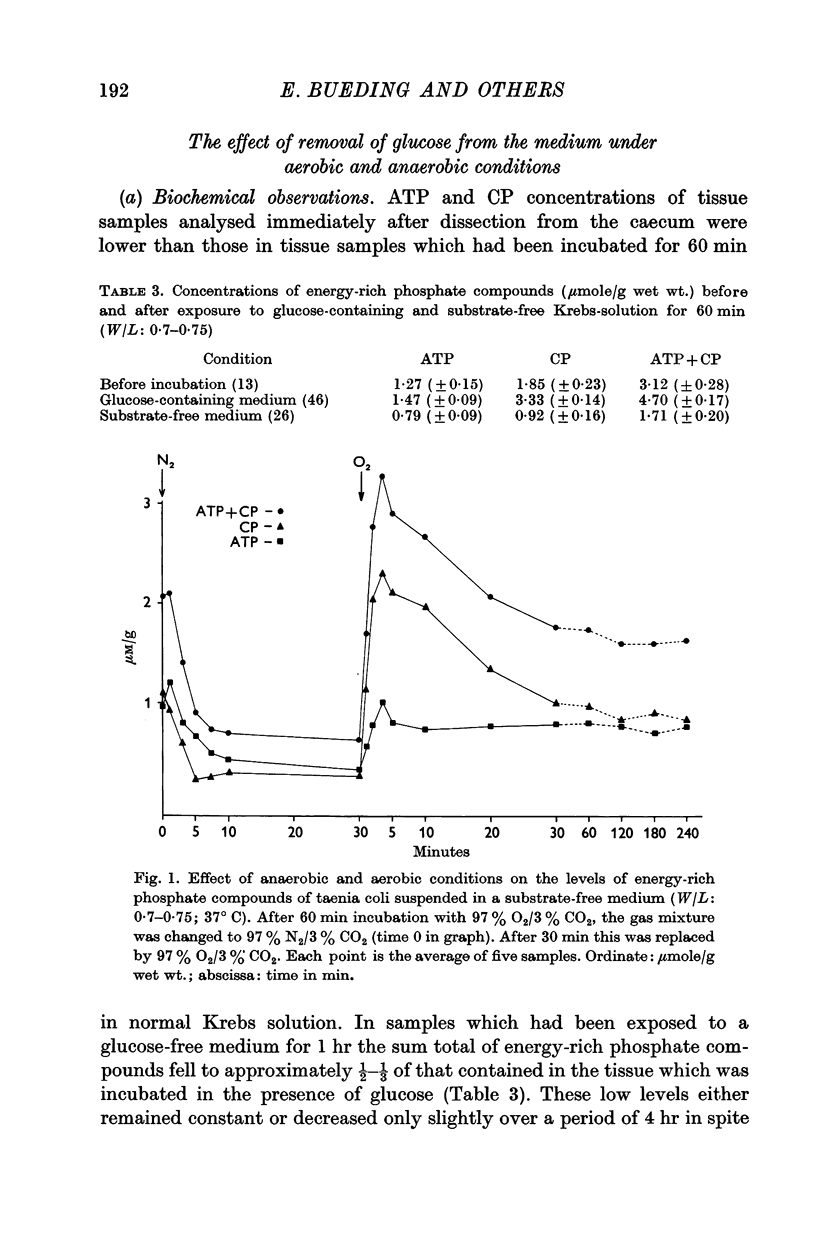
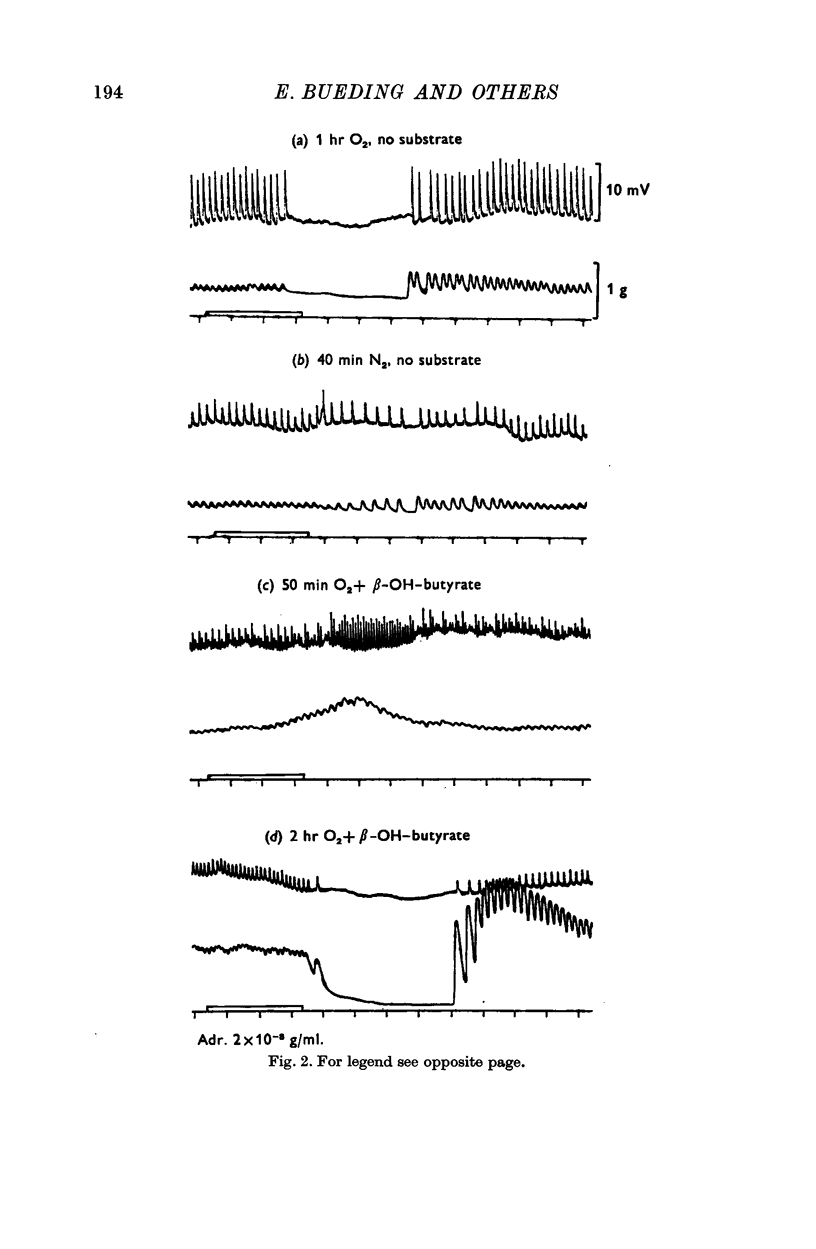
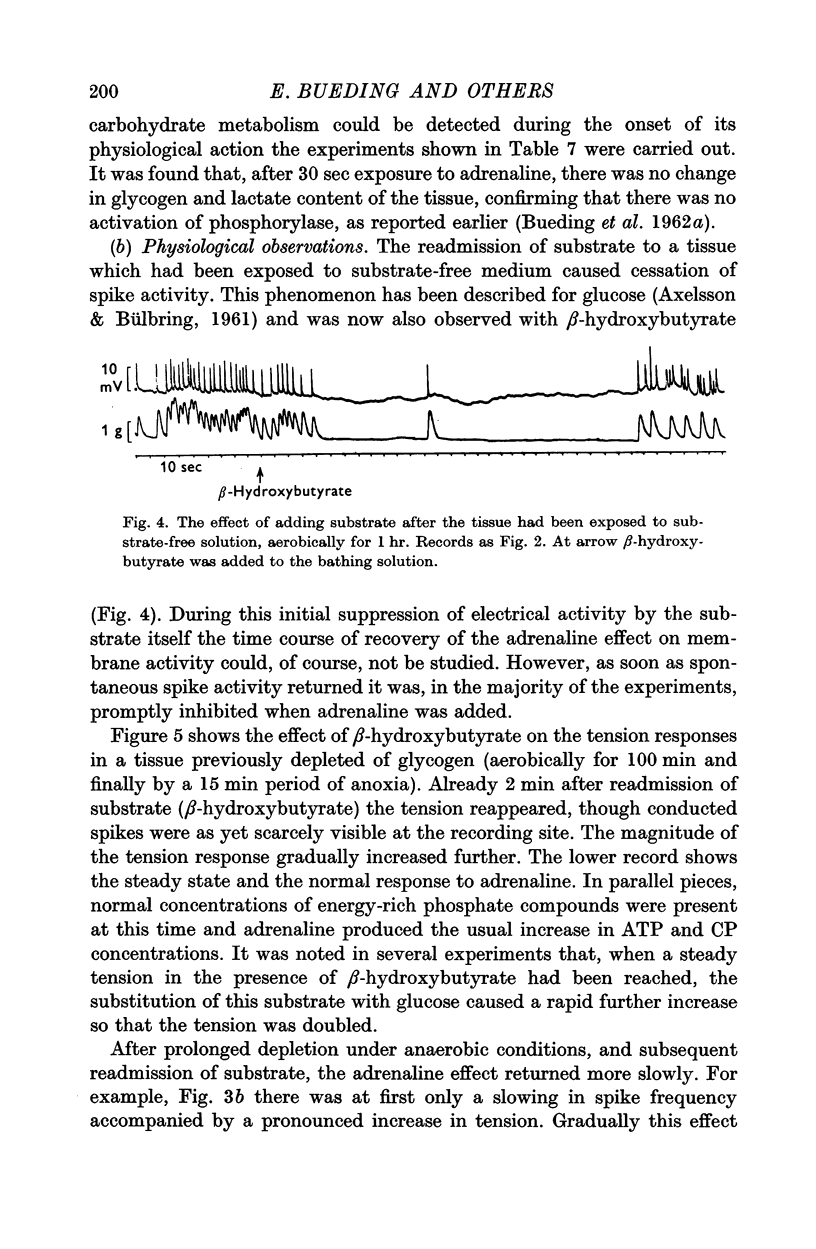
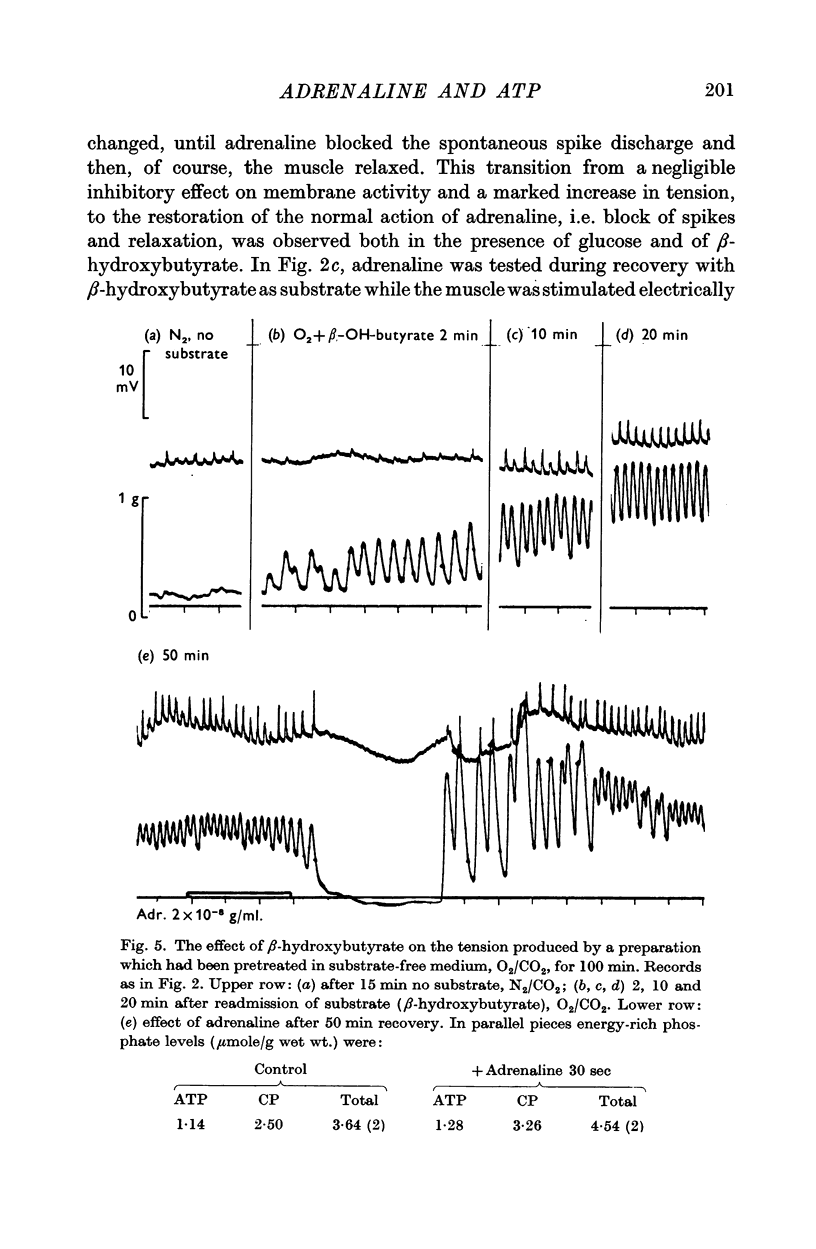
3. After anaerobic exposure of the tissue to substrate-free medium, the biochemical and the physiological effects of adrenaline were both abolished; both recovered after readmission of oxygen and/or substrate.
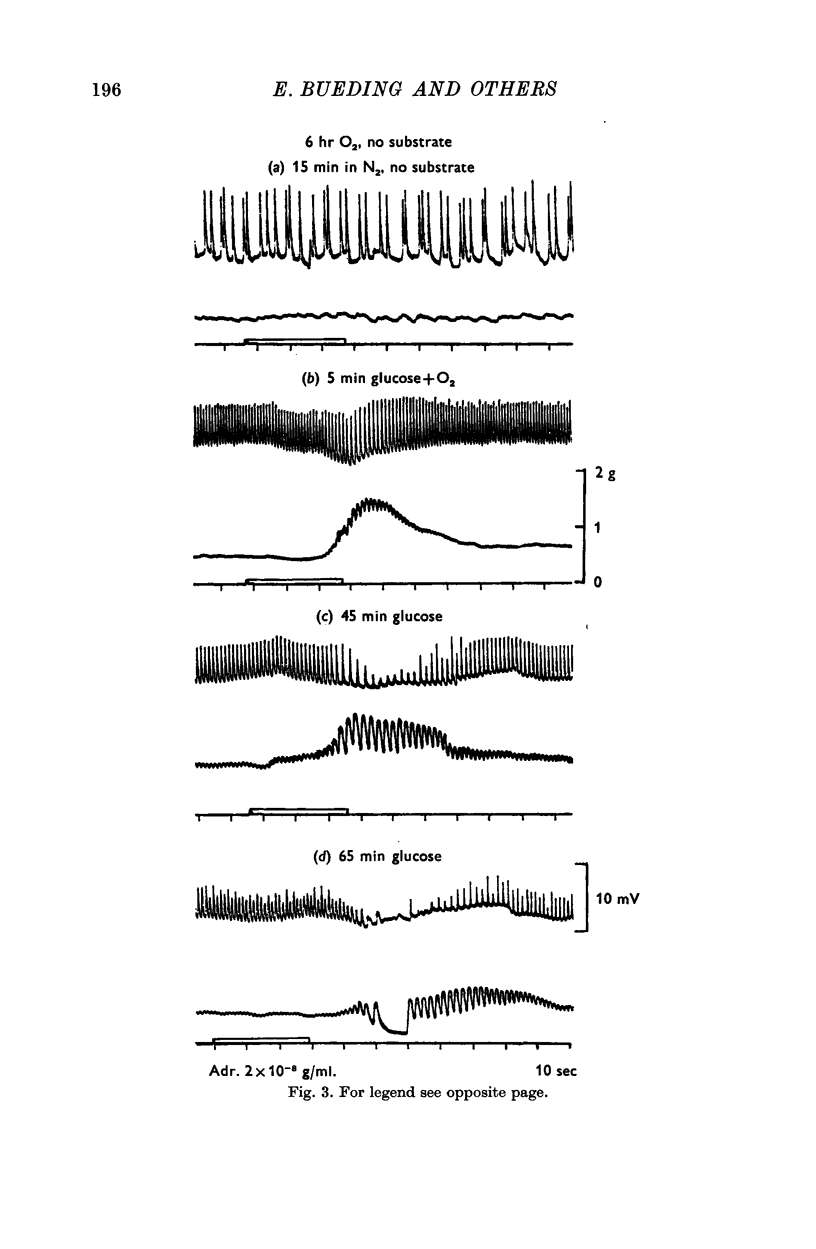
4. In muscles depleted of glycogen in substrate-free medium, either by anoxia or by high temperature, adrenaline produced its stabilizing effect on the cell membrane and the increase in ATP and CP content when β-hydroxybutyrate was the substrate, i.e. in the complete absence of carbohydrate from the medium and from the tissue.
5. When adrenaline was applied simultaneously with the readmission of substrate, the ATP and CP content of pieces treated with adrenaline was greater than of control pieces, though the tension of both was zero. This indicates that the effect was not secondary to the muscle relaxation but was the result of increased ATP synthesis.
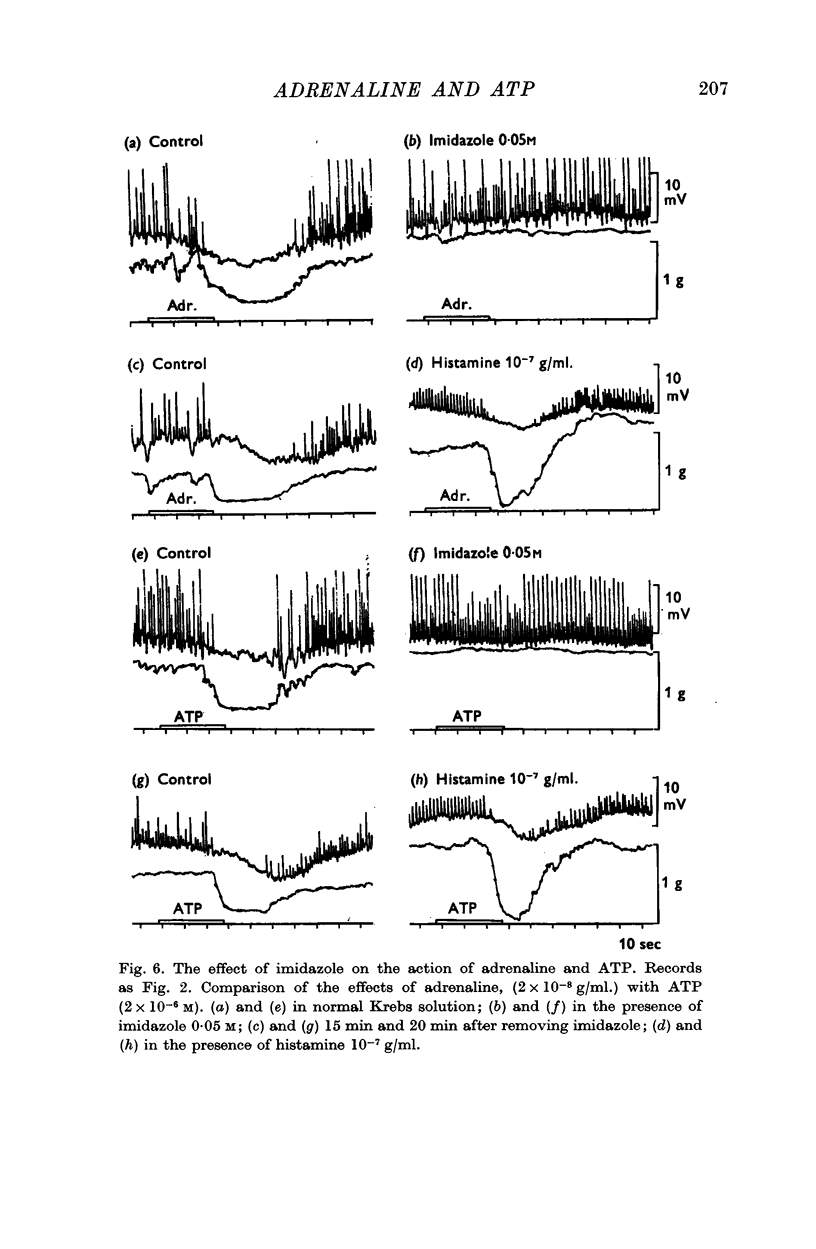
6. The physiological and biochemical effects of adrenaline were both abolished by the same concentration of imidazole (0·05 M).
7. Low concentrations of ATP (1 × 106-5 × 10-6 M)—like adrenaline—inhibited electrical and mechanical activity of the taenia. This effect was also abolished by imidazole.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., BUEDING E., BULBRING E. The inhibitory action of adrenaline on intestinal smooth muscle in relation to its action on phosphorylase activity. J Physiol. 1961 Apr;156:357–374. doi: 10.1113/jphysiol.1961.sp006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AXELSSON J., BULBRING E. Metabolic factors affecting the electrical activity of intestinal smooth muscle. J Physiol. 1961 Apr;156:344–356. doi: 10.1113/jphysiol.1961.sp006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G. V., BULBRING E. The effect of 2:4-dinitrophenol (DNP) on the smooth muscle of the guinea-pig's taenia coli. J Physiol. 1955 Mar 28;127(3):626–635. doi: 10.1113/jphysiol.1955.sp005283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G. V. The relation between the tension and the high-energy phosphate content of smooth muscle. J Physiol. 1956 Mar 28;131(3):704–711. doi: 10.1113/jphysiol.1956.sp005495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUEDING E., BULBRING E., KURIYAMA H., GERCKEN G. Lack of activation of phosphorylase by adrenaline during its physiological effect on intestinal smooth muscle. Nature. 1962 Dec 8;196:944–946. doi: 10.1038/196944b0. [DOI] [PubMed] [Google Scholar]
- BUEDING E., HAWKINS J. T. ENZYMIC DEGRADATION AND MICRODETERMINATION OF GLYCOGEN. Anal Biochem. 1964 Jan;7:26–36. doi: 10.1016/0003-2697(64)90116-2. [DOI] [PubMed] [Google Scholar]
- BUELBRING E., KURIYAMA H. THE EFFECT OF ADRENALINE ON THE SMOOTH MUSCLE OF GUINEA-PIG TAENIA COLI IN RELATION TO THE DEGREE OF STRETCH. J Physiol. 1963 Nov;169:198–212. doi: 10.1113/jphysiol.1963.sp007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUELDING E., HAWKINS J. T. NOTE ON THE ENZYMIC DEGRADATION AND DETERMINATION OF GLYCOGEN. Anal Biochem. 1964 Sep;9:115–116. doi: 10.1016/0003-2697(64)90089-2. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., STRAUB R. W. A method for studying the effects of ions and drugs on the resting and action potentials in smooth muscle with external electrodes. J Physiol. 1958 Jan 23;140(1):156–167. doi: 10.1113/jphysiol.1958.sp005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Bartelstone H. J., Nasmyth P. A., Telford J. M. The significance of adenosine cyclic 3',5'-monophosphate for the contraction of smooth muscle. J Physiol. 1967 Jan;188(2):159–176. doi: 10.1113/jphysiol.1967.sp008131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueding E., Butcher R. W., Hawkins J., Timms A. R., Sutherland E. W., Jr Effect of epinephrine on cyclic adenosine 3',5'-phosphate and hexose phosphates in intestinal smooth muscle. Biochim Biophys Acta. 1966 Jan 25;115(1):173–178. doi: 10.1016/0304-4165(66)90061-4. [DOI] [PubMed] [Google Scholar]
- Bueding E., Bülbring E. Relationship between energy metabolism of intestinal smooth muscle and the physiological actions of epinephrine. Ann N Y Acad Sci. 1967 Feb 10;139(3):758–761. doi: 10.1111/j.1749-6632.1967.tb41243.x. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Golenhofen K. Oxygen consumption by the isolated smooth muscle of guinea-pig taenia coli. J Physiol. 1967 Nov;193(1):213–224. doi: 10.1113/jphysiol.1967.sp008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUMMOND G. I., VALADARES J. R., DUNCAN L. EFFECT OF EPINEPHRINE ON CONTRACTILE TENSION AND PHOSPHORYLASE ACTIVATION IN RAT AND DOG HEARTS. Proc Soc Exp Biol Med. 1964 Oct;117:307–309. doi: 10.3181/00379727-117-29566. [DOI] [PubMed] [Google Scholar]
- ELLIS S., BECKETT S. B. The action of epinephrine on the anaerobic or the iodoacetate-treated rat's diaphragm. J Pharmacol Exp Ther. 1954 Oct;112(2):202–209. [PubMed] [Google Scholar]
- Fraenkel D. G., Horecker B. L. Fructose-1, 6-diphosphatase and acid hexose phosphatase of Escherichia coli. J Bacteriol. 1965 Oct;90(4):837–842. doi: 10.1128/jb.90.4.837-842.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gercken G., Hürter P. Stationäre Metabolitkonzentrationen im insuffizienten Säugetierherzen nach Monojodacetat- und Natriumfluoridvergiftung. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(2):100–117. [PubMed] [Google Scholar]
- Gillespie J. H. The biological significance of the linkages in adenosine triphosphoric acid. J Physiol. 1934 Feb 28;80(4):345–359. doi: 10.1113/jphysiol.1934.sp003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenhofen K. Untersuchungen zur Minuten-Rhythmik der glatten Muskulatur an der isolierten Taenia coli des Meerschweinchens. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(1):34–45. [PubMed] [Google Scholar]
- KREBS H. A., WOODFORD M. FRUCTOSE 1, 6-DIPHOSPHATASE IN STRIATED MUSCLE. Biochem J. 1965 Feb;94:436–445. doi: 10.1042/bj0940436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- MAYER S. E., de COTTEN M. V., MORAN N. C. Dissociation of the augmentation of cardiac contractile force from the activation of myocardial phosphorylase by catecholamines. J Pharmacol Exp Ther. 1963 Mar;139:275–282. [PubMed] [Google Scholar]
- UTTER M. F., KURAHASHI K. Mechanism of action of oxalacetic carboxylase. J Biol Chem. 1954 Apr;207(2):821–841. [PubMed] [Google Scholar]



