Abstract
CD8+ cytotoxic T lymphocytes (CTLs) are now recognized as important mediators of immunity against intracellular pathogens, including human immunodeficiency virus and tumors. How to efficiently evoke antigen-specific CTL responses in vivo has become a crucial problem in the development of modern vaccines. Here, we developed a completely novel CTL vaccine—mimovirus, which is a kind of virus-size particulate antigen delivery system. It was formed by the self-assembly of a cationic peptide containing 18 lysines and a CTL-epitope peptide of HBsAg28-39, with a plasmid encoding mouse interleukin-12 (IL-12) through electrostatic interactions. We examined the formation of mimovirus by DNA retardation assay, DNase I protection assay, and transmission electron microscopy and demonstrated that mimovirus could efficiently transfer the plasmid encoding IL-12 into mammalian cells such as P815 cells in vitro. Furthermore, it was proved that mimovirus could induce an HBsAg28-39-specific CTL response in vivo. Considering its effectiveness, flexibility, and defined composition, mimovirus is potentially a novel system for vaccination against intracellular pathogens and tumors.
CD8+ cytotoxic T lymphocytes (CTLs) play a key role in the immunity against tumors and intracellular pathogens including human immunodeficiency virus. Recent investigations have indicated that the key to eliciting CTL responses is to efficiently deliver antigens into the major histocompatibility complex class I (MHC-I) processing pathway of professional antigen-presenting cells (APCs), especially dendritic cells (DCs), which can thereby display high numbers of the MHC-I-peptide complexes in a rich costimulatory context to prime the specific naïve CD8+ T cells (25, 26, 30, 33). For this purpose, several particulate antigen delivery systems, such as immune-stimulating complexes (35), liposomes (2), virus-like particles (18), microspheres (22), and phage-displayed particles (8, 38), have been proved to be efficient. In addition, it has been suggested that coadministration of antigen with cytokines or other immunoregulatory molecules, either in the form of recombinant proteins or plasmids, could profoundly enhance immunity (25, 30, 33). Among them, interleukin-12 (IL-12) has been shown to be potent to promote the induction of CTL responses in vivo (6).
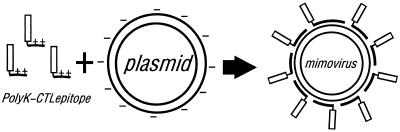
Synthetic peptides corresponding to epitopes recognized by CTLs have been investigated as the basis of safe, effective vaccines (5). The cationic peptides represent a kind of synthetic DNA delivery system that is gaining increasing prominence in gene therapy (11, 19, 24, 37). These cationic peptides (e.g., oligolysine, [K]n) can bind to plasmid DNA through electrostatic interactions between the positively charged lysine residues and the negatively charged phosphate backbone of the DNA. This interaction forms highly condensed particles that protect the packaged DNA from the effects of nucleases and allow internalization by mammalian cells. Here, rationally combining the epitope-based peptide vaccine and the cationic-peptide DNA delivery system of gene therapy, we firstly designed a completely novel CTL vaccine-mimovirus, which is a kind of virus-size particulate antigen delivery system. In the present study, we designed and synthesized a cationic peptide composed of 18 lysines and a CTL epitope corresponding to residues 28 to 39 of HBsAg (H-2Ld restricted) (28, 31). Together with the plasmid containing mouse IL-12 gene, this cationic peptide can spontaneously form particles through electrostatic interactions at an appropriate charge ratio of peptide and DNA (Fig. 1). With a size equivalent to a virus, the particle contains a molecule of DNA literally packaged by presumably several thousands of antigenic peptides. And these particles may simulate the mechanisms of cell entry and DNA delivery used by viruses into mammalian cells including professional APCs such as DC. Therefore, we designated this kind of particle mimovirus, which might also mimic the immunogenicity of a virus in vivo to some extent. In our studies, the H-2d mice immunized with the mimovirus were shown to mount an effective, specific anti-HBsAg28-39 CTL response. Moreover, we demonstrated that both the particulate entity and the IL-12 gene transfer ability of mimovirus contributed to its effect of inducing the effective CTL responses in vivo.
FIG. 1.
Schematic diagram of forming a mimovirus model. A cationic antigenic peptide (PolyK-CTLepitope) was designed and synthesized to contain 18 lysines (K) and a CTL epitope HBsAg28-39 (H-2Ld restricted). Together with the plasmid containing the mouse IL-12 gene, this cationic peptide could spontaneously form particles through electrostatic interactions at an appropriate charge ratio of peptide and DNA. Due to many formal and antigenic similarities with virus, we designated this kind of particle mimovirus. PolyK, polylysine.
MATERIALS AND METHODS
Plasmids, cell lines, and mice.
The plasmid pIL-12 (8,725 bp) was kindly provided by Mi-Hua Tao (Academia Sinica, Taiwan, China), which was constructed by inserting p35 and p40 coding sequences of murine IL-12 into a bicistronic plasmid pTCAE (6). As a control plasmid in our study, pTCAE (8,592 bp) was kindly provided by M. Reff (IDEC Corp., San Diego, Calif.). The DBA/2 mastocytoma cell line P815 was obtained from the American Type Culture Collection (Manassas, Va.) and maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 25 mM HEPES (pH 7.2), 50 μM 2-mercaptoethanol, penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C, 5% CO2 in a humidified incubator. The P815/S cell line is a stable transfectant cell line expressing the HBsAg by transfecting the plasmid pcDNA3/HBsAg into P815 cells and maintained under the same conditions as P815 cells, except with the addition of G418 (200 μg/ml) for selection. Female BALB/c mice (H-2d) were obtained from the animal facility of the Third Military Medicine University and bred under specific-pathogen-free conditions. They were used between 6 and 8 weeks of age.
Peptide synthesis and characterization.
The 30-mer cationic peptide N-[K]18IPQSLDSWWTSL ([K]18S12)and the 12-mer Ld-binding HBsAg28-39 peptide IPQSLDSWWTSL were synthesized in an Applied Biosystems peptide synthesizer model 431A (Perkin-Elmer, Foster City, Calif.), with purification and purity assessment by high-pressure liquid chromatography (HPLC) (Waters, Milford, Mass.). Mass spectrometric analyses of the peptides were performed using an API2000 electrospray ionization mass spectrometer (Perkin-Elmer). Amino acid composition analysis was performed using an ABI amino acid analyzer model 420A (Perkin-Elmer).
Plasmid DNA preparation.
For agarose gel electrophoresis and electron microscopy, plasmids were purified from transformed Escherichia coli strain DH5 α by UNIQ-10 column plasmid minprep kit (Sangon, Shanghai, China). Plasmids used for gene transfer in vitro and for vaccination were prepared with the Qiagen Endofree plasmid maxi kit (Qiagen, Hilden, Germany), giving endotoxin levels less than 0.5 endotoxin unit/μg of DNA, as assessed by Limulus amebocyte assay (Chinese Limulus Reagent Ind., Xiamen, China).
DNA retardation and DNase I protection assay.
DNA-[K]18S12 complexes (mimovirus) were prepared in microcentrifuge tubes by the addition of 1 μg of plasmid pIL-12 to serial dilutions of peptide [K]18S12 in 60 μl of HEPES-buffered saline (HBS) containing 10 mM HEPES and 150 mM NaCl, pH 7.4. To facilitate our calculation of the peptide/DNA charge ratio, we assumed that each lysine in peptide [K]18S12 carries one positive charge while each phosphate in DNA carries one negative charge. The peptide/DNA charge ratios were 0, 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16.0, respectively. The mixtures were vortexed and incubated at room temperature for 1 h. For DNA retardation assay, 15 μl of each complex was analyzed by electrophoresis on 0.8% agarose gels. For the DNase I protection assay, 5 μl of a solution of 10 mM MgCl2 and 10 mM CaCl2 was added to the 45 μl of each complex, followed by 5 μl of 0.5-μg/ml DNase I (Pharmacia Biotech) in water. After 30 min at 37°C, the reaction was stopped by addition of 4 μl of 0.5 M EDTA and heat inactivation at 65°C for 10 min. To dissociate the plasmid DNA from the peptide, 15 μl of 1% sodium dodecyl sulfate was added prior to extraction with Tris-EDTA-saturated phenol-chloroform, followed by ethanol precipitation and electrophoresis on 0.8% agarose gel. The integrity of DNA was compared with that of native DNA.
Transmission electron microscopy analysis.
Samples were prepared by mixing 1 μg of plasmid pIL-12 with the peptide [K]18S12 at various peptide/DNA charge ratios in 50 μl of HBS. The mixtures were vortexed and incubated at room temperature for an hour and then processed for transmission electron microscopy using a negative stain technique (11). Briefly, 15-μl drops of aliquot of the mixtures were placed on glow-discharged carbon-coated 200-mesh copper grids for 3 min. Solution was wicked off with filter paper and replaced with 2% aqueous tungsten phosphate for 30 s. After removal of the solution, grids were rinsed in distilled water and allowed to dry. Grids were imaged in an H-300 transmission electron microscope (Hitachi).
Gene transfer studies.
In a 24-well plate, P815 cells were seeded at a density of 5 × 104cells/well and incubated for approximately 24 h until 50 to 70% confluent. Three micrograms of plasmid pIL-12 was mixed, respectively, with peptide [K]18S12 at various peptide/DNA charge ratios or 15 μl of liposome DOTAP (Roche Molecular Biochemicals) in 50 μl of HBS. The control group included negative controls—no DNA (naked P815 cell), naked pIL-12, and pTCAE mixed with DOTAP—and a positive control: pIL-12 mixed with DOTAP. The cells were washed and incubated for 30 min at 37°C in 1 ml of RPMI 1640 (10% fetal calf serum). Immediately after adding the DNA-peptide or DNA-liposome complexes to the cells, the cell culture plate was centrifuged at 400 × g for 5 min. After an incubation time of 6 h, the medium was replaced with 1 ml of fresh supplemented medium, and the incubation was continued for 48 h before the further analysis. The supernatants were harvested to detect the level of mIL-12 with enzyme-linked immunosorbent assay (ELISA) by using a commercial kit for IL-12 p70 (Endogen, Woburn, Mass.). All samples were assayed in triplicate.
Immunization of mice.
For immunization, 75 nmol of peptide [K]18S12 was mixed with 20 pmol of plasmid pIL-12 or pTCAE in 500 μl of HBS at the peptide/DNA charge ratio of 4.0 to prepare two kinds of mimoviruses, respectively, named mimovirus-12 and mimovirus-T. Mice (five mice per group) were immunized subcutaneously at the tail base with 100 μl of mimovirus solution containing 15 nmol of peptide [K]18S12 and 4 pmol of each plasmid. As controls, mice were immunized with 15 nmol of peptide HBsAg28-39 and 4 pmol of pIL-12 or with only 15 nmol of peptide [K]18S12 in 100 μl HBS.
Cytotoxicity assay.
Spleens were removed from immunized mice 8 to 10 days postimmunization. Single-cell suspensions of splenocytes were prepared using stainless steel mesh screens and red blood cell lysing solution (0.144 M NH4Cl, 0.017 M Tris [pH 7.6]), and cultured in the same medium as P815 cells. Responder cells (about 3× 107) were cocultured with 1 μM synthetic HBsAg28-39 12 mer peptide in 10 ml of medium containing human rIL-2 (20 U/ml; prepared in our laboratory) in upright 25-cm2 culture flasks in 5% CO2 at 37°C. After 5 days, cytotoxic effector populations were harvested. Serial dilutions of effector cells were cultured with 104 51Cr-labeled targets in 200-μl V-bottom wells. Specific cytolytic activity of cells was tested in 51Cr release assays against P815/S or HBsAg28-39 peptide-pulsed P815 targets or naked P815 as control targets. After a 4-h incubation at 37°C, 100 μl of supernatant were collected for gamma radiation counting. The percent specific release was calculated as [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100. Total counts were measured by incubating target cells with 100 μl of HCl (2 M). In our experiments, spontaneous released counts always measured less than 15% of the total counts. Data shown are the means of triplicate cultures. The standard deviation of triplicate data was always less than 20% of the mean.
RESULTS
Peptide design, synthesis, and characterization.
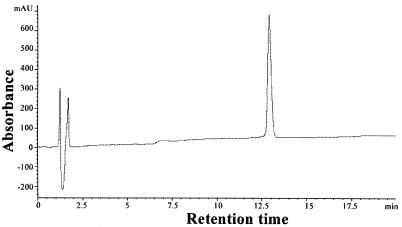
The 30-mer cationic peptide N-[K]18IPQSLDSWWTSL ([K]18S12) was designed to contain a CTL epitope, HBsAg28-39 (H-2Ld restricted) at its carboxyl terminus followed by a DNA-binding moiety of 18 lysines at the amino terminus. To avoid the possibly poor yields due to the inhibitory effect of the C-terminal oligo-l-lysine domain on the following synthesis of the HBsAg28-39 sequence, we positioned the oligo-l-lysine domain at the N terminus, which could allow the ready synthesis of the HBsAg28-39 sequence followed by the addition of the l-lysine chain (11). Moreover, many previous studies indicated that the immunogenicity of CTL epitope could be significantly influenced by the C-terminal residue(s) (4, 10, 32). In our designed peptide [K]18S12, the HBsAg28-39 group was placed at the C terminus with a free C-terminal residue, thus probably keeping well the immunogenicity of the CTL epitope during its presentation in professional APCs in vivo. The identity of this peptide was confirmed by electrospray ionization mass spectrometry (ESI-MS) and amino acid composition analysis (data not shown), and the homogeneity shown by reversed-phase HPLC (Fig. 2).
FIG. 2.
Analytical HPLC profile for peptide [K]18S12, using a low rate of 1 ml/min and a linear, binary gradient (30 ml) between 100% buffer A(0.1% aqueous trifluoroacetic acid) and 60% buffer B(0.1% trifluoroacetic acid-acetonitrile). The peptide(1 mg) was eluted at 38.2% buffer B and was monitored at a wavelength of 214 nm.
Formation of the peptide-DNA complex (mimovirus).
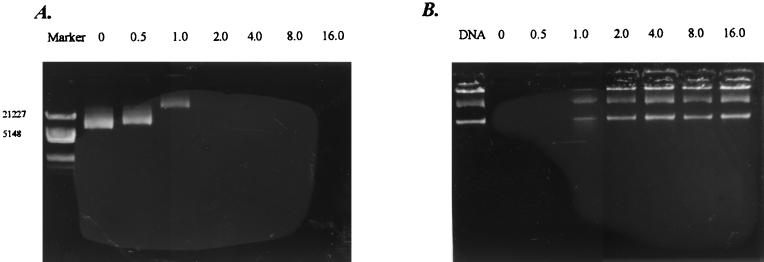
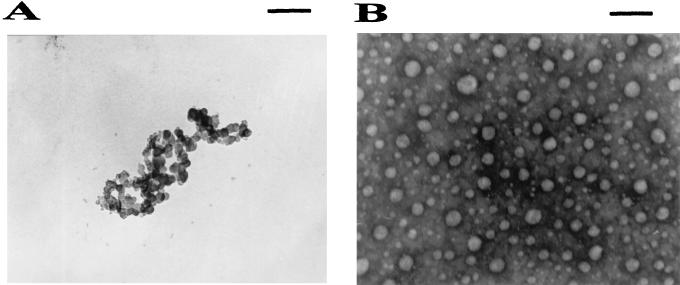
The formation of the peptide-plasmid DNA complexes, namely mimovirus, was examined by their electrophoretic mobility on an agarose gel stained with ethidium bromide at various charge ratios of the peptide to plasmid DNA (Fig. 3A). No migration of the plasmid DNA band was found at the charge ratio of 2.0 for both pIL-12 and pTCAE. This lack of migration indicated neutralization of the nucleic acid by the cationic peptide and/or formation of a large complex between the peptide and the plasmid DNA. With the formation of peptide-DNA complexes, the plasmid DNA is expected to be prevented from digestion by DNase I. We evaluated this ability of each complex by DNase I protection assay (Fig. 3B). The protection of the DNA began at the peptide/DNA ratio of 1.0, at which the DNA was partly retarded in the DNA retardation assay. To assess the structures of the DNA complex with the peptide [K]18S12, we used transmission electron microscopy with negative staining. The relaxed circular plasmid DNA is shown in Fig. 4A. At the peptide/DNA charge ratio of 4.0, the complexes formed a group of heterogeneous, dense particles with diameters ranging from 10 to 100 nm, as shown in Fig. 4B.
FIG. 3.
Agarose gel electrophoresis. (A) DNA retardation assay. The plasmid pIL-12 (1 μg) and several amounts of peptide [K]18S12 were mixed in 20 μl of HBS, followed by electrophoresis on a 0.8% agarose gel stained with ethidium bromide. The charge ratios (peptide/DNA) are indicated above. Marker represents the DNA size marker (λDNA/HindIII+EcoRI; Sangon). (B) DNase I protection assay. The plasmid pIL-12 was preincubated with the peptide [K]18S12 at the charge ratio indicated above, followed by treatment with DNase I as described in Materials and Methods. The integrity of the DNA was compared with that of native DNA.
FIG. 4.
Electron photomicrographs of mimovirus. Mimoviruses were prepared by mixing 1 μg of the plasmid pIL-12, respectively, with the peptide [K]18S12 at various peptide/DNA charge ratios in 50 μl of HBS. The methods used for electron microscopy are described in detail in Materials and Methods. Here we only show the appearance of the naked plasmid DNA (A) and that of mimovirus prepared at a peptide/DNA charge ratio of 4.0 (B). Bar = 200 nm.
Mimovirus-mediated gene transfer in vitro.
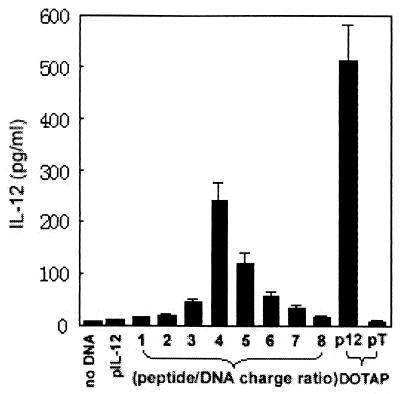
It has been shown that many cationic peptides such as oligolysine could transfer DNA into mammalian cells by condensing plasmid to form particles or aggregates, which could be easily internalized into cells via endocytosis pathway. We therefore reasoned that the mimovirus particle might also have such a gene transfer effect. To test this speculation, we next evaluated the ability of mimovirus to deliver pIL-12 into P815 cells in vitro. The mimoviruses were prepared by mixing 3 μg of plasmid pIL-12, respectively, with peptide [K]18S12 at peptide/DNA charge ratios of 0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, and8.0. The transfection efficiency was evaluated by measuring the IL-12 level in the supernatants of transfected cells with ELISA. As shown in Fig. 5, IL-12 was not detectable above background levels in untransfected control or negative controls (naked pIL-12 or pTCAE complexed with DOTAP). In contrast, while cells were incubated with mimovirus, IL-12 became detectable at the charge ratio of 2.0, at which the DNA was fully retarded as described above (Fig. 3A). As the ratio rose to 4.0, the expression of IL-12 reached the highest level. The level of expression obtained at this ratio was approximately 50% of the level obtained by the liposome DOTAP-based technique. Hereafter the level of expression was significantly reduced as the ratio continued rising to 8.0. As other studies and ours showed, a peptide/DNA ratio greater than 4.0 might turn the mimovirus into even more condensed smaller particles, which probably prevented the transcription of the complexed DNA in the cells so that the level of expression fell markedly. So, we concluded that mimovirus formed at the charge ratio of 4.0 might be the most effective for gene delivery and expression in our present studies.
FIG. 5.
Gene transfer effect of mimovirus in vitro. Mimoviruses were prepared by mixing 3 μg of plasmid pIL-12, respectively, with peptide [K]18S12 at various peptide/DNA charge ratios in 50 μl of HBS. P815 cells were incubated with the mimoviruses or controls for 6 h in a 24-well plate. The control group includes three negative controls—no DNA (naked P815 cell), naked pIL-12, and pTCAE (pT) mixed with DOTAP—and a positive control: pIL-12 mixed with DOTAP. The medium was then replaced by 1 ml of supplemented medium, and the incubation was continued for a total of 48 h before the supernatants were harvested to detect the level of IL-12 by ELISA. The results of one of three similar experiments are shown here.
Mimovirus induced antigen specific CTLs in vivo.
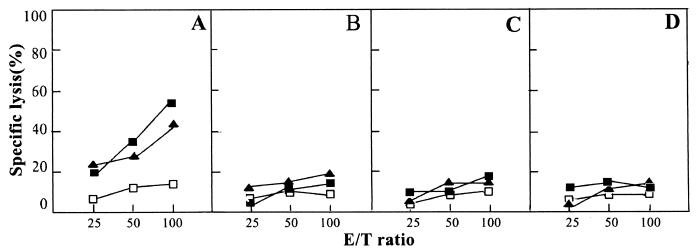
For immunization, we prepared two kinds of mimoviruses, mimovirus-12 and mimovirus-T, by, respectively, mixing peptide [K]18S12 with plasmid pIL-12 or pTCAE at a peptide/DNA charge ratio of 4.0. Mice were immunized subcutaneously at the tail base with 100 μl of mimovirus solution containing 15 nmol of peptide [K]18S12 and 4 pmol of each plasmid. As controls, mice were immunized with 15 nmol of peptide S28-39 and 4 pmol of pIL-12 or with only 15 nmol of peptide [K]18S12 in 100 μl of HBS. The HBsAg28-39-specific CTL responses in the immunized mice were determined by release of 51Cr from target cell P815 pulsed with the synthetic S28-39 peptide or P815/S. The specificity of CTL-mediated lysis was determined by using naked P815 cells as control targets. As shown in the Fig. 6A, an efficient, HBsAg28-39-specific CTL response was observed in the mice immunized with mimovirus-12. By contrast, there was no detectable cytotoxic activity observed in the mice immunized with mimovirus-T, with peptide S28-39 and pIL-12, or with only peptide [K]18S12 (Fig. 6B, C, and D). However, because we did not test other doses of peptide to immunize mice in the present studies, we could not exclude the possibility that Mimovirus-T, or peptide S28-39 and pIL-12, or only peptide [K]18S12 might induce CTL responses at certain higher doses.
FIG. 6.
Inducing specific CTLs in vivo with mimovirus. BALB/c mice were immunized, respectively, with mimovirus-12 which was prepared by mixing peptide [K]18S12 with plasmid pIL-12 at a charge ratio of 4.0 (A), mimovirus-T which was prepared by mixing peptide [K]18S12 with control plasmid pTCAE at a charge ratio of 4.0 (B), HBsAg28-39 peptide and pIL-12 (C), or only peptide [K]18S12 (D). Splenocytes isolated 8 to 10 days after immunization and restimulated with HBsAg28-39 peptide in vitro for 5 days were employed as effector cells. Then, the standard 51Cr-release assays were made at three effector-to-target (E/T) ratios, with P815/S (▪) or peptide-pulsed P815(▴) as the target of cytotoxicity and the naked P815 (□) as the control target. Values of specific lysis are from one representative of three performed experiments and are presented as mean specific lysis of triplicate cultures.
DISCUSSION
The term cross-priming mainly denotes the stimulation of an MHC-I-restricted specific CTL response with exogenous (extracellular) antigens by professional APCs. This mechanism has been recognized for its role in immune responses against viruses, tumor immunity, and vaccine development. Till now, many kinds of particulate antigen delivery systems have been proved efficient to target exogenous antigen into the MHC-I pathway and to elicit CTL, such as immune-stimulating complexes (35), liposomes (2), virus-like particles (18), microspheres (22), and phage-displayed particles (8, 38). It has been suggested that, compared with soluble antigens, particulate antigens are more easily captured by professional APCs such as DCs and macrophages and then become more efficiently transferred into the class I pathways (namely cross-presentation). In our former study, we showed that recombinant filamentous phage particles that displayed the HBsAg28-39 could induce specific CTL responses in vivo (38). Our present studies clearly indicate that mimovirus, as a novel particulate vaccine, could efficiently cross-prime an HBsAg28-39-specific CTL responses in vivo. After subcutaneous injection of mimovirus, this kind of particle might be easily internalized via the endocytosis pathway by the resident cells, especially professional APCs (3, 36). This might be due to not only its particulate nature (2, 8,18, 22, 28, 35, 38), with the size equivalent to a virus, but also its abundant cationic charges which help mimovirus bind electrostatically to the overall negatively charged cell surface (14, 17). Several studies have shown that DCs, macrophages, and even B cells were able to cross-present antigens under specific circumstances at least in vitro (1, 3,9, 12, 15, 16, 23, 27, 29). Only recently, Bevan's group provided the first evidence that CD8+ DCs are responsible for cross-priming in vivo (9, 12). Following Ag uptake, DCs may mature and migrate to the nearest draining lymph node where they select and activate naive Ag-specific T cells (3).
Among a number of nonviral gene delivery systems, many cationic synthetic peptides containing several cationic amino acids have been employed, such as oligolysine (11, 37) and α-helical peptide (20, 21). Many studies have indicated that these peptides could bind to plasmid DNA and form small cationic particles or large aggregates with DNA competent to be internalized into cells via the endocytosis pathway. Furthermore, by conjugating certain receptor-binding ligands (domains) to these cationic peptides, a number of receptor-mediated gene delivery approaches have been developed (11, 24). Here we have designed and synthesized the antigenic cationic peptide [K]18S12, which contains 18 lysines and a CTL epitope, HBsAg28-39. By DNA retardation assay, DNase I protection assay, and transmission electron microscopy, we proved that [K]18S12 could bind to the selected plasmids to form condensed particles, namely, mimovirus, at an appropriate charge ratio of peptide and DNA. Like other cationic peptide delivery systems, mimovirus was shown to be able to transfer pIL-12 gene into mammalian cells such as P815 cells at least in vitro.
IL-12 has not only been suggested to induce production of IFN-γ, then promoting Th1 polarization and CTL responses, but also has been suggested to play a major role in regulating the migration and proper positioning of effector cells (7, 34). In our present study, only mimovirus-12 was proved to be able to induce efficient CTL responses against HBsAg28-39. Considering the gene transfer effect of mimovirus shown in vitro, it is reasonable to speculate that mimovirus-12 might transfer pIL-12 into certain cells, probably including professional APCs, in vivo. Here it is worth noting a similar work reported by Irvine et al. during the preparation of our manuscript (13). They used complexes of plasmid DNA and the cationic peptide CL22, transfecting human or murine DCs much more efficiently than alternative nonviral agents.
Therefore, we concluded that both the particulate entity and the IL-12 gene transfer ability of mimovirus might contribute to its effect of inducing the specific CTL responses in vivo. The design of mimovirus rationally combined the epitope-based peptide vaccine approach with the cationic peptide gene transfer system. As a kind of particular vaccine, mimovirus may represent a novel, efficient delivery system for T-cell-epitope peptides to be targeted into professional APCs to facilitate the induction of potent immune responses. Furthermore, there still exists a great flexibility to further optimize the design of mimovirus by combining (via cationic peptides) any given plasmids encoding either lymphokines or antigens or both, with one or several kinds of functional peptides containing either epitope peptides or peptides targeting DCs or others. Considering its effectiveness, flexibility, and defined composition, mimovirus has the potential to be developed as an efficient vaccine strategy against tumors and intracellular pathogens, including human immunodeficiency virus.
Acknowledgments
Y.-Z. Wu and J.-P. Zhao contributed equally to this study.
This work was supported by National Key Basic Research Program of China (2001CB510001) and National Natural Science Foundation of China (NSFC no. 30170882).
We sincerely thank Yufang Shi of the American Red Cross for his kind help during the preparation of the manuscript.
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Alving, C. R. 1992. Immunologic aspects of liposomes: presentation and processing of liposomal protein and phospholipid antigens. Biochim. Biophys. Acta 1113:307-322. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann, C. C., Q. Yao, C. K. Ho, and S. L. Buckwold. 1996. Flanking residues alter antigenicity and immunogenicity of multi-unit CTL epitopes. J. Immunol. 157:3242-3249. [PubMed] [Google Scholar]
- 5.Bona, C. A., S. Casares, and T. D. Brumeanu. 1998. Towards development of T-cell vaccines. Immunol. Today. 19:126-133. [DOI] [PubMed] [Google Scholar]
- 6.Chow, Y. H., B. L. Chiang, Y. L. Lee, W. K. Chi, W. C. Lin, Y. T. Chen, and M. H. Tao. 1998. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J. Immunol. 160:1320-1329. [PubMed] [Google Scholar]
- 7.Curtsinger, J. M., C. S. Schmidt, A. Mondino, D. C. Lins, R. M. Kedl, M. K. Jenkins, and M. F. Mescher. 1999. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 162:3256-3262. [PubMed] [Google Scholar]
- 8.De Berardinis, P., R. Sartorius, C. Fanutti, R. N. Perham, G. Del Pozzo, and J. Guardiola. 2000. Phage display of peptide epitopes from HIV-1 elicits strong cytolytic responses. Nat. Biotechnol. 18:873-876. [DOI] [PubMed] [Google Scholar]
- 9.Den Haan, J. M., S. M. Lehar, and M. J. Bevan. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gileadi, U., A. Gallimore, P. Van der Bruggen, and V. Cerundolo. 1999. Effect of epitope flanking residues on the presentation of N-terminal cytotoxic T lymphocyte epitopes. Eur. J. Immunol. 29:2213-2222. [DOI] [PubMed] [Google Scholar]
- 11.Harbottle, R. P., R. G. Cooper, S. L. Hart, A. Ladhoff, T. McKay, A. M. Knight, E. Wagner, A. D. Miller, and C. Coutelle. 1998. An RGD-oligolysine peptide: a prototype construct for integrin-mediated gene delivery. Hum. Gene Ther. 9:1037-1047. [DOI] [PubMed] [Google Scholar]
- 12.Heath, W. R., and F. R. Carbone. 2001. Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol. 1:126-134. [DOI] [PubMed] [Google Scholar]
- 13.Irvine, A. S., P. K. E. Trinder, D. L. Laughton, H. Ketteringham, R. H. McDermott, S. C. H. Reid, A. M. R. Haines, A. Amir, R. Husain, R. Doshi, L. S. Young, and A. Mountain. 2000. Efficient nonviral transfection of dendritic cells and their use for in vivo immunization. Nat. Biotechnol. 18:1273-1278. [DOI] [PubMed] [Google Scholar]
- 14.Kabanov, A. V. 1999. Taking polycation gene delivery systems from in vitro to in vivo. Pharm. Sci. Technol. Today 2:365-372. [DOI] [PubMed] [Google Scholar]
- 15.Ke, Y., and J. A. Kapp. 1996. Exogenous antigens gain access to the major histocompatibility complex class I processing pathway in B cells by receptor-mediated uptake. J. Exp. Med. 184:1179-1184. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovacsovics-Bankowski, M., K. Clark, B. Benacerraf, and K. L. Rock. 1993. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc. Natl. Acad. Sci. USA 90:4942-4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laus, R., T. J. Graddis, I. Hakim, and D. Vidovic. 2000. Enhanced major histocompatibility complex class I-dependent presentation of antigens modified with cationic and fusogenic peptides. Nat. Biotechnol. 18:1269-1273. [DOI] [PubMed] [Google Scholar]
- 18.Layton, G. T., S. J. Harris, A. J. Gearing, M. Hill Perkins, J. S. Cole, J. C. Griffiths, N. R. Burns, A. J. Kingsman, and S. E. Adams. 1993. Induction of HIV-specific cytotoxic T lymphocytes in vivo with hybrid HIV-1 V3: Ty-virus-like particles. J. Immunol. 151:1097-1107. [PubMed] [Google Scholar]
- 19.Luo, D., and W. M. Saltzman. 2000. Synthetic DNA delivery systems. Nat. Biotechnol. 18:33-37. [DOI] [PubMed] [Google Scholar]
- 20.Niidome, T., K. Takaji, M. Urakawa, N. Ohmori, A. Wada, T. Hirayama, and H. Aoyagi. 1999. Chain length of cationic alpha-helical peptide sufficient for gene delivery into cells. Bioconjug. Chem. 10:773-780. [DOI] [PubMed] [Google Scholar]
- 21.Niidome, T., M. Urakawa, H. Sato, Y. Takahara, T. Anai, T. Hatakayama, A. Wada, T. Hirayama, and H. Aoyagi. 2000. Gene transfer into hepatoma cells mediated by galactose-modified alpha-helical peptides. Biomaterials 21:1811-1819. [DOI] [PubMed] [Google Scholar]
- 22.Nixon, D. F., C. Hioe, P. D. Chen, Z. Bian, P. Kuebler, M. L. Li, H. Qiu, X. M. Li, M. Singh, J. Richardson, P. McGee, T. Zamb, W. Koff, C. Y. Wang, and D. O'Hagan. 1996. Synthetic peptides entrapped in microparticles can elicit cytotoxic T cell activity. Vaccine 14:1523-1530. [DOI] [PubMed] [Google Scholar]
- 23.Norbury, C. C., B. J. Chambers, A. R. Prescott, H. G. Ljunggren, and C. Watts. 1997. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 27:280-288. [DOI] [PubMed] [Google Scholar]
- 24.Perales, J. C., G. A. Grossmann, M. Molas, G. Liu, T. Ferkol, J. Harpst, H. Oda, and R. W. Hanson. 1997. Biochemical and functional characterization of DNA complexes capable of targeting genes to hepatocytes via the asialoglycoprotein receptor. J. Bio. Chem. 272:7398-7407. [DOI] [PubMed] [Google Scholar]
- 25.Raychaudhuri, S., and K. L. Rock. 1998. Fully mobilizing host defense: building better vaccines. Nat. Biotechnol. 16:1025-1031. [DOI] [PubMed] [Google Scholar]
- 26.Rea, D., M. E. Johnson, M. J. Havenga, C. J. M. Melief, and R. Offringa. 2001. Strategies for improved antigen delivery into dendritic cells. Trends. Mol. Med. 7:91-94. [DOI] [PubMed] [Google Scholar]
- 27.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reimann, J., and R. Schirmbeck. 1999. Alternative pathways for processing exogenous and endogenous antigens that can generate peptides for MHC class I-restricted presentation. Immunol. Rev. 172:131-152. [DOI] [PubMed] [Google Scholar]
- 29.Rock, K. L., S. Gamble, and L. Rothstein. 1990. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science 249:918-921. [DOI] [PubMed] [Google Scholar]
- 30.Schijns, V. E. 2000. Immunological concepts of vaccine adjuvant activity. Curr. Opin. Immunol. 12:456-463. [DOI] [PubMed] [Google Scholar]
- 31.Schirmbeck, R., J. Wild, and J. Reimann. 1998. Similar as well as distinct MHC class I-binding peptides are generated by exogenous and endogenous processing of hepatitis B virus surface antigen. Eur. J. Immunol. 28:4149-4161. [DOI] [PubMed] [Google Scholar]
- 32.Shastri, N., T. Serwold, and F. Gonzalez. 1995. Presentation of endogenous peptide/MHC class I complexes is profoundly influenced by specific C-terminal flanking residues. J. Immunol. 155:4339-4346.7594593 [Google Scholar]
- 33.Singh, M., and D. O'Hagan. 1999. Advances in vaccine adjuvants. Nat. Biotechnol. 17:1075-1081. [DOI] [PubMed] [Google Scholar]
- 34.Sinigaglia, F., D. D. Ambrosio, P. Panina-Bordigon, and L. Rogge. 1999. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immun. Rev. 170:65-72. [DOI] [PubMed] [Google Scholar]
- 35.Sjolander, A., J. C. Cox, and I. G. Barr. 1998. ISCOMs: an adjuvant with multiple functions. J. Leukoc. Biol. 64:713-723. [DOI] [PubMed] [Google Scholar]
- 36.Théry, C., and S. Amigorena. 2001. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 13:45-51. [DOI] [PubMed] [Google Scholar]
- 37.Vaysse, L., and B. Arveiler. 2000. Transfection using synthetic peptides: comparison of three DNA-compacting peptides and effect of centrifugation. Biochim. Biophys. Acta 1474:244-250. [DOI] [PubMed] [Google Scholar]
- 38.Wan, Y., Y. Wu, J. Bian, X. Z. Wang, W. Zhou, Z. C. Jia, Y. Tan, and L. Zhou. 2001. Induction of hepatitis B virus-specific cytotoxic T lymphocytes response in vivo by filamentous phage display vaccine. Vaccine 19:2918-2923. [DOI] [PubMed] [Google Scholar]