Abstract
Influenza virus has been described to enter host cells via clathrin-mediated endocytosis. However, it has also been suggested that other endocytic routes may provide additional entry pathways. Here we show that influenza virus may enter and infect HeLa cells that are unable to take up ligands by clathrin-mediated endocytosis. By overexpressing a dominant-negative form of the Eps15 protein to inhibit clathrin-mediated endocytosis, we demonstrate that while transferrin uptake and Semliki Forest virus infection were prevented, influenza virus could enter and infect cells expressing Eps15Δ95/295. This finding is supported by the successful infection of cells with influenza virus in the presence of chemical treatments that block endocytosis, namely, chlorpromazine and potassium depletion. We show also that influenza virus may infect cells incapable of uptake by caveolae. Treatment with the inhibitors nystatin, methyl-β-cyclodextrin, and genistein, as well as transfection of cells with dominant-negative caveolin-1, had no effect on influenza virus infection. By combining inhibitory methods to block both clathrin-mediated endocytosis and uptake by caveolae in the same cell, we demonstrate that influenza virus may infect cells by an additional non-clathrin-dependent, non-caveola-dependent endocytic pathway. We believe this to be the first conclusive analysis of virus entry via such a non-clathrin-dependent pathway, in addition to the traditional clathrin-dependent route.
Clathrin-mediated endocytosis has been described as an internalization pathway for many viruses (for reviews, see references 31, 46, and 52). Along with Semliki Forest virus (SFV) and vesicular stomatitis virus, influenza virus has been observed in clathrin-coated vesicles upon entry into host cells based on analysis by transmission electron microscopy (30, 33, 34). However, influenza virions were also observed in uncoated vesicles in these experiments (33). It has remained unclear whether the presence of these uncoated vesicles was due to shedding of the clathrin coat or to the existence of a non-clathrin-mediated endocytic pathway. It is also unclear if virions within these uncoated vesicles could proceed to cause a productive infection. More recent studies have examined influenza virus entry by using the K44A dynamin mutant in Mv-1 lung cells (45). It was originally thought that dynamin, a small GTPase which mediates the pinching of endocytic vesicles, was specific to clathrin-mediated endocytosis (20, 50). However, it has become clear that dynamin functions in several endocytic vesicle scission events, including the formation of caveolae, budding of Golgi complex-derived vesicles, phagocytosis, and non-clathrin-mediated endocytosis (15, 18, 24, 26, 39). Here we have used more-specific inhibitors of clathrin-mediated endocytosis to determine the specific action of this pathway on influenza virus and to examine if additional, alternative virus entry pathways might exist.
Several endocytic pathways have been implicated previously in pathogen entry (46). In the case of influenza virus, all of these appear to have the capacity to deposit the virus in a low-pH compartment, which is required for hemagglutinin-mediated viral fusion and uncoating (11, 53, 58). Clathrin-mediated endocytosis is the best characterized of these pathways and is considered the primary route of endocytic entry into cells (10). Although implicated as a route of entry for many viruses, clathrin-mediated endocytosis has to date been defined in molecular terms only as the internalization pathway for SFV and Sindbis virus (12, 13). As an alternative to clathrin, caveolae have recently been described as mediating the entry of simian virus 40 (SV40), and in this case the virus enters so-called caveosomes before being transported to the endoplasmic reticulum (41). Other routes of virus internalization include macropinocytosis, which has been suggested for vaccinia virus and Epstein-Barr virus entry. Macropinocytosis is considered a nonspecific mechanism of internalization mediated by the closure of plasma membrane ruffles to form large vesicles known as macropinosomes (29). A fourth route of entry, as yet ill defined, is a non-clathrin-mediated, non-caveola-mediated internalization pathway (8, 38). This pathway has been implicated in the entry of many viruses, including picornaviruses and influenza virus, primarily based on electron microscopic analysis (28, 33).
Assembly of clathrin into a coated pit occurs at the inside face of the plasma membrane in response to a receptor-mediated internalization signal. Clathrin is recruited to the plasma membrane by the AP-2 adapter complex, and while the assembly process is known to require many other proteins, the function of most is not yet clear (32). One of these regulatory proteins is Eps15, which was originally identified as a substrate for the receptor-activated epidermal growth factor tyrosine kinase (14). Eps15 has since been found to be ubiquitously and constitutively associated with AP-2, suggesting a role in clathrin assembly regulation (5, 55). This hypothesis has been supported by the inhibition of transferrin and epidermal growth factor internalization in cells microinjected with antibodies against Eps15 or transiently transfected with dominant-negative forms of the protein (6, 12).
Eps15 consists of three structural domains. The N-terminal domain contains three EH (Eps homology) domains which bind regulatory proteins containing NPF amino acid motifs, including epsin, AP180, and synaptojanin (47). The central domain allows for oligomerization of Eps15 into homodimers (55). The C-terminal domain contains an AP-2 binding site which, together with the EH domains, is required for targeting of Eps15 to clathrin-coated pits (4, 7, 21). It has been reported previously that overexpression of Eps15 lacking the second and third EH domains results in a dominant-negative protein that inhibits clathrin-mediated endocytosis (3). Expression of this construct, Eps15Δ95/295, results in the mislocalization of AP-2 and dynamin, suggesting that Eps15 may play a role in AP-2 recruitment to the plasma membrane.
Here we have used the green fluorescent protein (GFP)-Eps15Δ95/295 construct to determine the role of clathrin-mediated endocytosis in influenza virus entry. We demonstrate that cells expressing this construct, and thus disabled in clathrin-mediated uptake, allow the entry and infection of influenza virus, while SFV infection and transferrin uptake are prevented. These data are supported by experiments using chemical inhibition and potassium depletion to prevent clathrin-mediated endocytosis. Additionally, using a series of chemical inhibitors and dominant-negative caveolin-1 to disrupt caveolae, we show that influenza virus infection occurs in the absence of uptake by caveolae. By combining methods to inhibit both clathrin-mediated endocytosis and uptake by caveolae in the same cell, we demonstrate that influenza virus may enter and infect cells via a non-clathrin-mediated, non-caveola-mediated alternative endocytic pathway, in addition to entry by clathrin-mediated endocytosis.
MATERIALS AND METHODS
Cells, viruses, and infections.
HeLa cells (American Type Culture Collection) were maintained in α-minimal essential medium (α-MEM) containing 10% calf serum, 100 U of penicillin/ml, and 10 μg of streptomycin/ml and were passaged twice-weekly. Influenza virus A/WSN/33 (H1N1) was grown in MDBK cells and plaque titered on MDCK cells. SFV strain M1 was provided by Margaret Kielian, Albert Einstein College of Medicine. Infections were performed essentially as described previously (43). Briefly, viral stocks were diluted in RPMI 1680 medium containing 0.2% bovine serum albumin and buffered to pH 6.8 with HEPES. Influenza virus was adsorbed for 90 min at 4°C, and cells were then maintained in growth medium containing 2% serum at 37°C for either 60 min (at ∼100 to 200 PFU per cell) or 4 h (at ∼1 or <0.1 PFU per cell). SFV was bound as above (at ∼1 PFU per cell) and then incubated at 37°C for 5 h. The chemical inhibitors bafilomycin A (25 nM; Alexis Biochemicals), chlorpromazine (10 μg/ml; Alexis), nystatin (25 μg/ml; Calbiochem), methyl-β-cyclodextrin (MβCD) (10 mM; Sigma), and genistein (100 μg/ml; Alexis) were added during both the virus adsorption and incubation periods.
Transfections.
The dominant-negative, GFP-tagged plasmid construct Eps15Δ95/295 (3) was provided by Jennifer Lippincott-Schwartz, National Institutes of Health (37). Plasmid constructs of wild-type and dominant-negative caveolin-1 were provided by Ari Helenius, ETH-Zurich. Transfections were performed by using the Effectene transfection kit (Qiagen) according to the manufacturer's protocols and routinely exceeded 70% efficiency. For transfection, HeLa cells were grown on coverslips in 24-well plates and transfected with 0.5 μg of DNA. Transfections were typically allowed to proceed for 16 to 18 h before infection or transferrin uptake.
Indirect immunofluorescence microscopy.
Cells were prepared for indirect immunofluorescence microscopy as described previously (59). Influenza virus nucleoprotein (NP) was detected by using monoclonal antibody H10, L16-4R5 (American Type Culture Collection). SFV was detected with monoclonal antibody E1-1 (provided by Margaret Kielian). Eps15 expression was detected by using an anti-Eps15 monoclonal antibody (BD Transduction Laboratories). Secondary antibodies used were Alexa 488-labeled (green), Alexa 568-labeled (red), and Alexa 633-labeled (far red) goat anti-mouse immunoglobulin G (IgG) (Molecular Probes). Cells were viewed by using a Zeiss Axioskop 2 plus microscope with a 63× objective lens. Images were captured with a Zeiss Axiocam using Axiovision 3.0.6.1 software before being transferred into Adobe Photoshop. Confocal microscopy was performed using an Olympus FluoView confocal station. Alexa 488 was excited with the 488-nm line of an argon laser, Alexa 594 was excited with the 568-nm line of a krypton laser, and Alexa 633 was excited with the 635-nm line of a He-Ne laser. Cells were viewed with a 60× objective lens, and images were captured with FluoView software (Olympus) before being transferred into Adobe Photoshop.
Fluorescence-activated cell sorter (FACS) analysis.
For flow cytometry preparation, cells were trypsinized, washed in phosphate-buffered saline (PBS), fixed in 3% paraformaldehyde-PBS, and permeabilized in 0.075% saponin in 10% goat serum-PBS. Cells were incubated with a monoclonal antibody to influenza virus NP or Eps15 for 30 min, followed by Alexa 488-labeled or Alexa 568-labeled goat anti-mouse IgG for 30 min. Cells were analyzed on a FACSCalibur cytometer using CellQuest 3.1f software (Becton Dickinson Immunocytometry Systems). At least 10,000 cells were analyzed for each sample.
Transferrin uptake.
Transferrin uptake assays were performed using Alexa 594-labeled human transferrin (kindly provided by Colin Parrish, Cornell University). HeLa cells were serum starved for 30 min, incubated with 50 μg of Alexa 594-labeled transferrin/ml for 20 min at 4°C for binding, washed, and transferred to 37°C for 15 min. Cells were washed with 0.1 M glycine-0.1 M NaCl, pH 3.0, to remove any uninternalized ligand, unless stated otherwise.
K+ depletion.
Potassium depletion was performed as described previously (27). Briefly, HeLa cells were shocked in a hypotonic medium for 5 min. Virus or transferrin was bound and internalized in the presence of an isotonic K+-free medium (α-MEM with 50 mM Na-HEPES and 100 mM NaCl [pH 7.4]).
RESULTS
Overexpression of GFP-Eps15Δ95/295 prevents transferrin uptake.
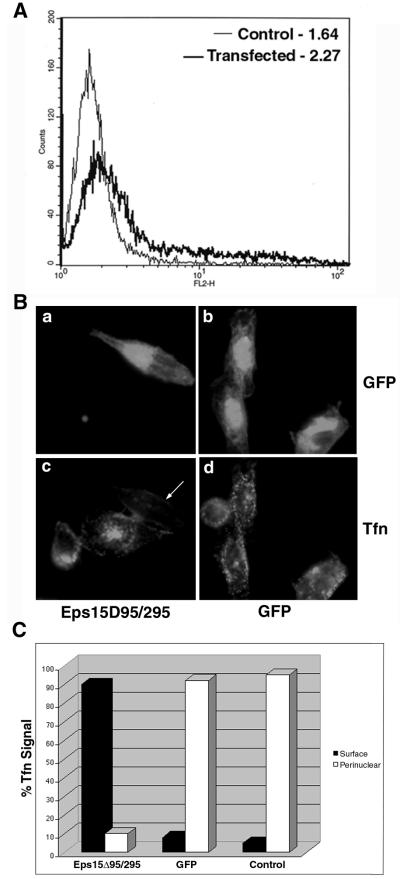
To determine the overexpression efficiency of the Eps15Δ95/295 plasmid in our cell culture and transfection system, we compared Eps15 expression in transfected and untransfected cells by using a monoclonal antibody against Eps15. Analysis by flow cytometry indicated that Eps15 expression in transfected cells increased approximately 1.8 times over that in untransfected cells, based on the median signal expression determined after adjustment for 70% transfection efficiency (Fig. 1A).
FIG. 1.
GFP-Eps15Δ95/295 is overexpressed and prevents transferrin uptake. (A) HeLa cells were either transiently transfected with GFP-Eps15Δ95/295 or left as untransfected controls. Cells were prepared for FACS analysis by using the monoclonal antibody against Eps15 and an Alexa 568-labeled secondary antibody. Shaded curve, untransfected cells; solid curve, transfected cells. Numbers indicate median fluorescence expression as determined by FluoView software. At least 10,000 cells were counted. (B) HeLa cells were transiently transfected with either GFP-Eps15Δ95/295 or GFP alone. Alexa 594-labeled transferrin (Tfn) was bound to serum-starved cells for 20 min at 4°C. Cells were washed, transferred to 37°C for 15 min, washed with low-pH glycine to remove uninternalized ligand, and fixed for viewing. (C) Transferrin uptake quantitated by indirect immunofluorescence. One hundred cells from each category were counted, and residual surface transferrin was distinguished from a strong perinuclear signal.
To further test the efficacy of the plasmid in our system, we transiently transfected HeLa cells, using GFP alone as a positive control, and then performed a transferrin uptake assay. It has been reported previously that expression of Eps15Δ95/295 prevents transferrin internalization (3). Our results agreed with this finding, since cells transfected with GFP allowed normal transferrin uptake (Fig. 1B, panel d), whereas cells expressing GFP-Eps15Δ95/295 were unable to internalize the ligand (panel c). Quantification of transferrin uptake indicated that among GFP-transfected or untransfected control cells, at least 90% displayed strong perinuclear signals, indicative of transferrin in recycling endosomes (Fig. 1C). In contrast, in approximately 90% of cells transfected with Eps15Δ95/295, transferrin was undetectable or remained as a residual signal on the surface after the low-pH wash. These data indicate that the Eps15Δ95/295 construct is well expressed in our transfection system, resulting in inhibition of ligand uptake by clathrin-mediated endocytosis.
Overexpression of GFP-Eps15Δ95/295 prevents SFV infection.
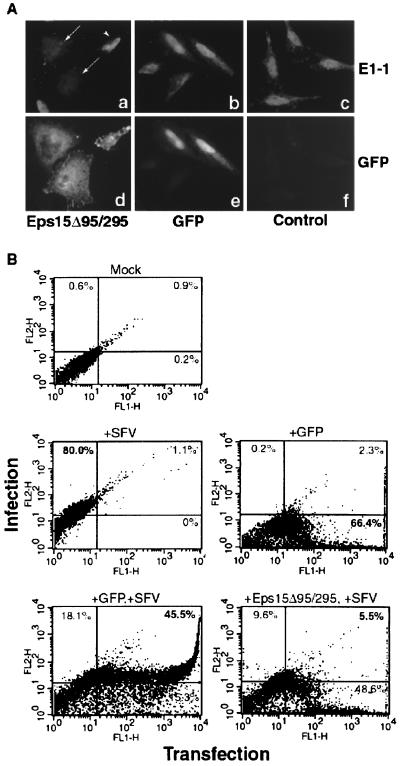
Entry of SFV has been demonstrated to be dependent on clathrin-mediated endocytosis by injection of anti-clathrin antibodies, which prevented infection (12). Therefore, we employed SFV as a clathrin-dependent virus with which to compare our subsequent influenza virus results. After transfection with GFP or GFP-Eps15Δ95/295, cells were infected with SFV for 5 h before examination by indirect immunofluorescence or FACS analysis. As with infection in untransfected control cells, cells transfected with GFP were infected with SFV, as indicated by the strong cytoplasmic antibody signal (Fig. 2A, panel b). However, cells expressing Eps15Δ95/295 were not infected with SFV (Fig. 2A, panel a), due to inhibition of clathrin-mediated entry. Rarely, a cell appeared to be both transfected and infected (Fig. 2A, panel a). We attribute this to the long infection time required and unavoidable residual wild-type Eps15 protein. The percentage of these double-labeled cells viewed under the microcope correlates with the data obtained by FACS analysis. The immunofluorescence data were quantified by flow cytometry, which allows distinctions between infected versus noninfected cells and transfected versus nontransfected cells. When nontransfected cells were infected with SFV, 80% of the cell population expressed an E1-1 signal above background, indicating a large infected-cell population (Fig. 2B). As a monitor of transfection efficiency, transfection with GFP alone resulted in more than 66% of the cells expressing the fluorescent tag. Cells transfected with GFP and infected with SFV showed a major shift (more than 45% of the cell population) to the upper right-hand quadrant, representing cells that were both transfected and infected. Conversely, cells expressing Eps15Δ95/295 did not demonstrate this population shift, as indicated by the fact that only 6% of the cell population was located in the upper right-hand quadrant, confirming that SFV infection is inhibited.
FIG. 2.
SFV infection is prevented in GFP-Eps15Δ95/295-expressing cells. HeLa cells either were transiently transfected with either GFP or GFP-Eps15Δ95/295 or were left as untransfected controls before 5 h of infection. Infection was monitored using monoclonal antibody E1-1. (A) Indirect immunofluorescence monitoring of infection. Arrows indicate uninfected cells; arrowhead indicates a transfected, infected cell. (B) FACS analysis to quantitate immunofluorescence results. Infection is monitored on the y axis, and transfection is monitored on the x axis. The percentage of cells within each quadrant is given.
Influenza virus infection is not inhibited by GFP-Eps15Δ95/295 expression.
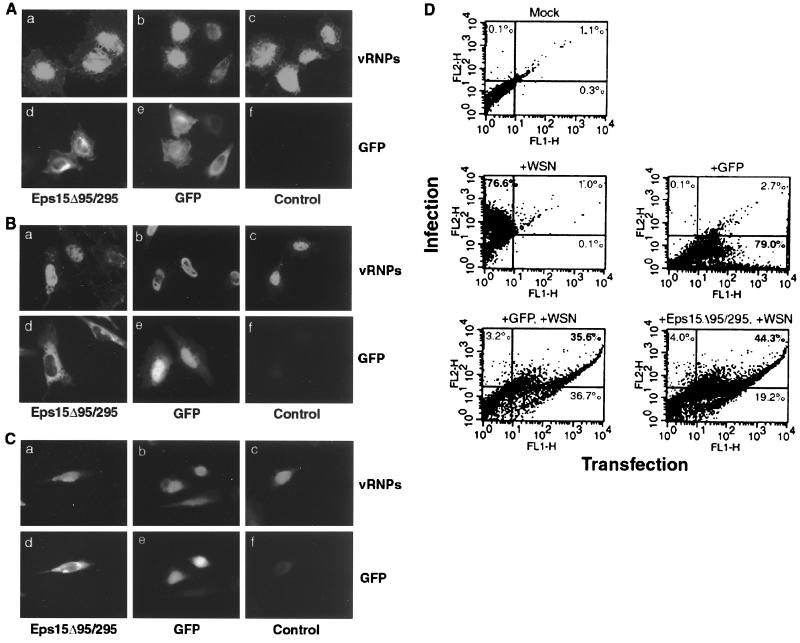
Entry of influenza virus into MDCK, BHK, and Mv-1 lung cells by clathrin-mediated endocytosis has been well described. However, we wished to determine whether additional entry pathways could exist. To examine this, we blocked clathrin-mediated uptake with Eps15Δ95/295 and monitored influenza virus entry and infection. As in transiently transfected cells expressing GFP alone, influenza virus was capable of entering cells expressing GFP-Eps15Δ95/295 and of releasing viral RNPs (vRNPs) into the nucleus, as indicated by the strong nuclear vRNP signal (Fig. 3A). To examine productive infection, cells were allowed to be infected at a low multiplicity of infection (MOI) of approximately 1 PFU/cell for 4 h after transfection. Cells expressing Eps15Δ95/295 were capable of being infected by influenza virus, as indicated by the similar vRNP signals in GFP-expressing and control cells (Fig. 3B). To guard against the possibility that Eps15Δ95/295 was providing only partial inhibition of clathrin-mediated endocytosis, and that extraneous virions were eluding this block, the 4-h infection was repeated at a very low MOI (<0.1 PFU per cell). As with the above assays, Eps15Δ95/295-expressing cells were infected with influenza virus (Fig. 3C). The data for the 4-h low-MOI infection were quantified by flow cytometry and are summarized in Fig. 3D. In GFP-transfected cells, 36% of the cell population was both transfected and infected, and such a cell population represented 44% of the Eps15Δ95/295-transfected cells (Fig. 3D). It is not clear at present why Eps15Δ95/295 would result in this apparent increase in influenza virus infectivity (36% versus 44%). These data show that influenza virus is capable of entering and causing a productive infection in cells lacking a functional clathrin-mediated endocytic pathway.
FIG. 3.
GFP-Eps15Δ95/295 expression does not prevent influenza virus infection. HeLa cells either were transiently transfected with either GFP or GFP-Eps15Δ95/295 or were left as untransfected controls before infection with influenza virus. Infection was monitored using monoclonal antibody H10, L16-4R5 against NP. (A) Entry assay. Cells were infected with ∼100 to 200 PFU per cell, and the virus was allowed to internalize for 60 min at 37°C. (B) Infection. Cells were infected with ∼1 to 5 PFU per cell and then incubated at 37°C for 4 h. (C) Low-MOI infection. Cells were infected with ∼0.1 PFU per cell and incubated at 37°C for 4 h. (D) FACS analysis of infection assay. Infection is monitored on the y axis, and transfection is monitored on the x axis. The percentage of cells within each quadrant is given.
Confocal microscopy of Eps15Δ95/295-transfected cells.
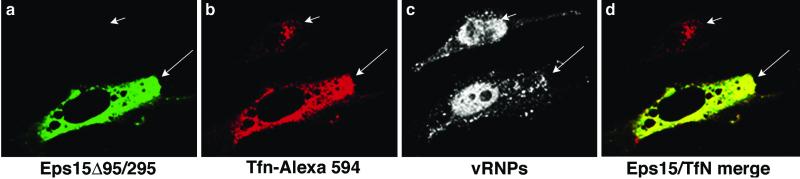
To demonstrate the inhibitory effects of Eps15Δ95/295 expression on transferrin uptake and the ability of influenza virus to overcome this inhibition, we performed confocal microscopy on cells that were transfected, infected, and then exposed to transferrin (Fig. 4). In nontransfected cells, the influenza virus vRNP signal was present primarily in the nucleus (Fig. 4c). Transferrin was internalized and concentrated in the perinuclear region of the cell (Fig. 4b). In transfected cells, vRNPs were again present in the nucleus; however, transferrin remained on the surface of the cell, unable to be internalized (Fig. 4b). As the plane of view is through the nuclear region of the cell, the plasma membrane surface above the nucleus has been eliminated, accounting for the lack of transferrin in this area. It is interesting that much of the uninternalized transferrin appears to colocalize with Eps15Δ95/295 (Fig. 4d), as though it has bound to nonfunctioning sites of clathrin-mediated endocytosis at the plasma membrane.
FIG. 4.
Confocal microscopy of GFP-Eps15Δ95/295-expressing cells with influenza virus and transferrin (Tfn). HeLa cells were transiently transfected with GFP-Eps15Δ95/295 before high-MOI infection with influenza virus. The transferrin uptake assay was then performed; note that the cells were not acid washed after incubation. Influenza virus localization was determined by indirect immunofluorescence with an anti-NP monoclonal antibody. Images were created using Fluoview software and were transferred into Adobe Photoshop. The short arrow in panel b and the long arrow in panel c indicate transferrin and influenza vRNP signal localization, respectively, in untransfected cells.
Influenza virus infects in the presence of chlorpromazine.
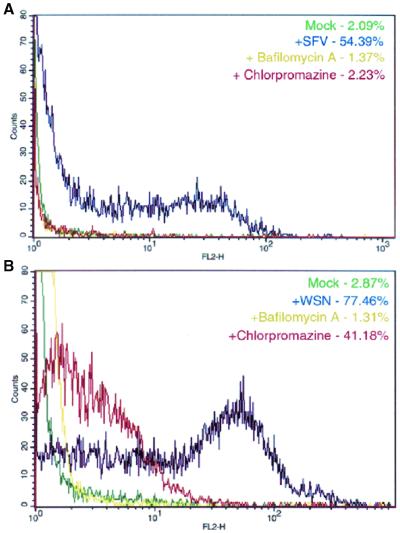
To provide additional support for our Eps15 data, we employed chemical and physiological inhibitors of clathrin-mediated endocytosis. We first inhibited clathrin by treating cells with chlorpromazine, which induces the misassembly of clathrin-coated pits at the plasma membrane (57). Cells were treated with chlorpromazine or bafilomycin A, a vacuolar ATPase inhibitor that blocks endosomal acidification and prevents endocytic trafficking during entry of pH-dependent viruses such as influenza virus or SFV (9, 16), and then were analyzed by flow cytometry. The control SFV infection (without any drug) showed more than 54% of the cells expressing a signal above background (mock) (Fig. 5A). The percent infected cells dropped to approximately 1% with the addition of bafilomycin A and to 2% upon chlorpromazine treatment. The control influenza virus infection resulted in 77% of the cells being infected (Fig. 5B). Treatment with bafilomycin A caused the infection rate to drop to 1%, while chlorpromazine treatment allowed 41% of the cells to be infected with the influenza virus. The discrepancy between the numbers of infected cells in the control and chlorpromazine treatment infections is likely due to the high toxicity of the drug.
FIG. 5.
Chlorpromazine treatment does not inhibit influenza virus infection. (A) HeLa cells were infected with SFV for 5 h, and infection was monitored using monoclonal antibody E1-1. (B) HeLa cells were infected with influenza virus for 4 h, and infection was monitored using an anti-NP monoclonal antibody. Cells were treated with either 25 nM bafilomycin A or 10 μg of chlorpromazine/ml during both the adsorption and incubation periods. Infection was monitored by FACS analysis. Key indicates treatment of cells. Background was determined by residual antibody staining on uninfected cells.
Influenza virus infects despite K+ depletion.
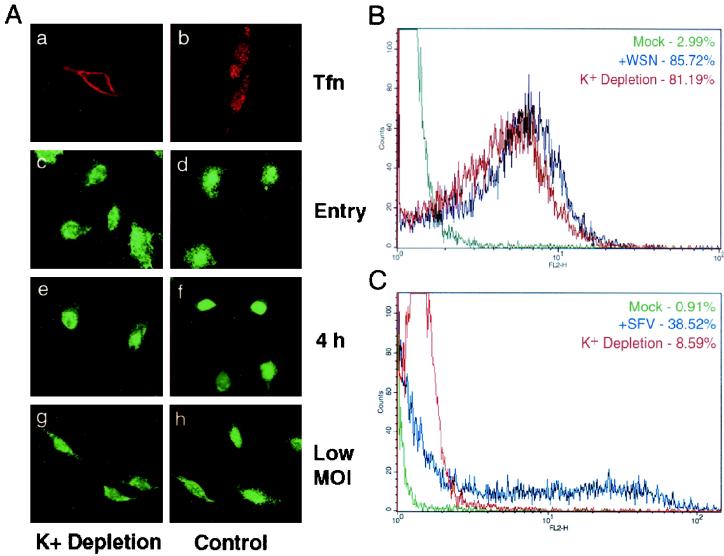
As an alternative to chlorpromazine, potassium depletion combined with hypotonic shock has been used as a possibly more specific and less toxic means of inhibiting clathrin-mediated endocytosis. Such treatment has been well established to reversibly arrest clathrin-coated-pit formation (27). For examination of virus entry, cells underwent a brief hypotonic shock before infection or transferrin uptake in an isotonic K+-free medium. This treatment prevented transferrin uptake (Fig. 6A, panel b) but had no effect on influenza vRNP nuclear entry (panel c) or productive virus infection at either 1 or <0.1 PFU/cell (panels e and g). FACS analysis of potassium-depleted cells confirmed the immunofluorescence data: 81% of K+-depleted cells were infected in an entry assay, compared to 86% of untreated cells (Fig. 6B). A control experiment with SFV demonstrated that potassium depletion prevents the entry of clathrin-dependent viruses, since SFV infection dropped from 39 to 9% upon treatment (Fig. 6C). These data, along with the chlorpromazine results, support our findings from Eps15Δ95/295 transfection experiments that influenza virus may enter and infect cells in the absence of clathrin-mediated endocytosis.
FIG. 6.
K+ depletion does not inhibit influenza virus infection. (A) HeLa cells were shocked in a hyptonic medium for 5 min before either an influenza vRNP entry assay, a 4-h infection (1 PFU/cell), a low-MOI infection (<0.1 PFU/cell), or a transferrin (Tfn) uptake assay in a K+-free medium. Influenza virus infection was detected by indirect immunofluorescence with a monoclonal antibody against NP. Note that cells were not acid washed after transferrin uptake. (B) FACS analysis. HeLa cells were shocked in a hyptonic medium for 5 min before an influenza virus entry assay in a K+-free medium. Influenza virus was detected by a monoclonal antibody against NP. (C) FACS analysis of SFV infection in cells depleted of potassium. HeLa cells were shocked in a hypotonic medium for 5 min before 5 h of infection with SFV in a K+-free medium. Virus infection was detected by monoclonal antibody E1-1.
Influenza virus infection is independent of caveolae.
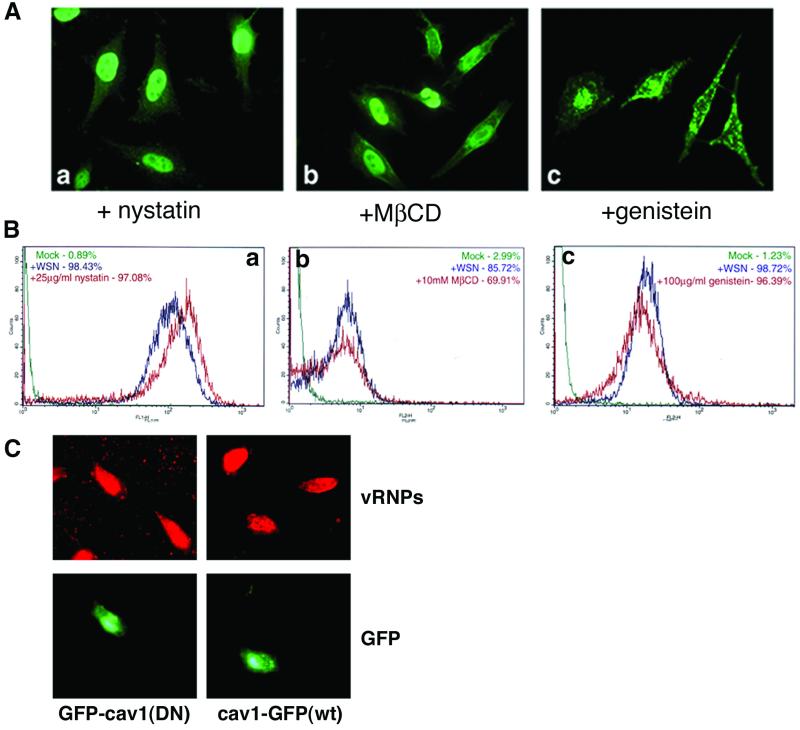
Although caveolae have recently been described as an internalization pathway for several pathogens (51), their role in influenza virus entry has not been addressed. To examine the role of this pathway, cells were treated with caveola-inhibiting drugs during infection. Nystatin and MβCD are sterol-binding drugs that sequester cholesterol and are not believed to affect clathrin-mediated endocytosis (36, 44). We treated cells with concentrations of these drugs known to deplete the cholesterol content of HeLa cell plasma membranes by 60% (54). Genistein is a tyrosine kinase inhibitor which is thought to inhibit the receptor-induced formation of caveolae (40). Drug-treated cells were monitored for infection both by FACS analysis after an entry assay infection and by indirect immunofluorescence after both an entry assay (data not shown) and a 4-h infection. Immunofluorescence analysis shows that drug-treated cells contain a strong nuclear vRNP staining pattern, characteristic of influenza virus-infected cells (Fig. 7A). The vRNP staining pattern in genistein-treated cells is slightly different, containing some additional cytoplasmic staining. Although the cells are clearly infected, we do not yet know if this difference is relevant or is due to toxic effects of the drug. Quantitative FACS analysis shows that the percentage of infected cells is not reduced by drug treatment (Fig. 7B), since at least 96% of cells treated with nystatin or genistein expressed vRNP signals above background and 70% of cells treated with MβCD were infected with influenza virus. This infection rate is comparable to that of untreated control cells and indicates that influenza virus does not require uptake by caveolae for infection. As a more specific way to examine uptake by caveolae, cells were transfected with GFP-tagged wild-type or dominant-negative forms of caveolin-1, the major structural protein of caveolae (kindly provided by Ari Helenius). In both cases, cells displayed normal influenza virus infection when analyzed by indirect immunofluorescence (Fig. 7C) and flow cytometry (data not shown). To confirm the inhibition of caveolar endocytosis, we analyzed HeLa cells for the ability to be infected by SV40, which is known to use caveolae for productive entry. SV40 infection was prevented by transfection with the dominant-negative caveolin-1 construct but was unaffected by overexpression of wild-type caveolin (data not shown), confirming that our protocol for inhibiting caveolar uptake is functioning properly.
FIG. 7.
Treatment with caveola-inhibiting drugs does not prevent influenza virus infection. HeLa cells were treated with either 25 μg of nystatin/ml, 10 mM MβCD, or 100 μg of genistein/ml during both the adsorption and incubation periods of influenza virus infection. Influenza virus infection was detected with a monoclonal antibody against NP. (A) Indirect immunofluorescence analysis of 4-h influenza virus infection in drug-treated cells. (B) FACS analysis of entry assay infection in drug-treated cells. Key indicates treatment of cells. Background was determined by residual antibody staining on uninfected cells. (C) HeLa cells were transiently transfected with wild-type or dominant-negative caveolin-1 constructs before entry assay infection with influenza virus. Transfected cells are indicated by GFP expression; infection was monitored via indirect immunofluorescence using a monoclonal antibody against NP.
Influenza virus infects cells in which both clathrin-mediated endocytosis and uptake by caveolae are inhibited.
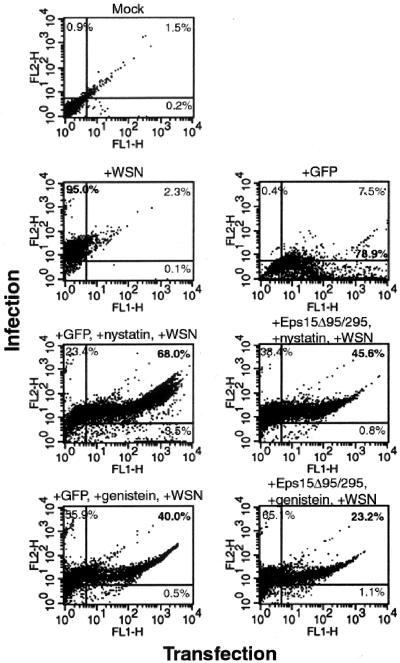
Our data suggested that influenza virus was unaffected by inhibition of either the clathrin or the caveolar route of internalization. However, to determine if influenza virus could truly employ a non-clathrin-mediated, non-caveola-mediated endocytic pathway, both of these routes needed to be inhibited within the same cell. To do this, inhibitory protocols were combined so that cells were first transiently transfected with either GFP or GFP-Eps15Δ95/295 and then treated with either nystatin, MβCD, or genistein. Influenza virus infection was monitored by flow cytometry (Fig. 8). As with each individual inhibition experiment, influenza virus was able to infect cells in which both clathrin-mediated endocytosis and uptake by caveolae were disabled. Transfection with Eps15Δ95/295 followed by treatment with 25 μg of nystatin/ml resulted in 40% of cells expressing both GFP-Eps15Δ95/295 and an influenza virus vRNP signal. Eps15Δ95/295-transfected cells treated with 100 μg of genistein/ml were infected at the rate of 23% of the cell population. These percentages are comparable to that for transfection with GFP alone before drug treatment. Similar results were observed after treatment with MβCD (data not shown). It is also significant that in all cases of transfection and drug-treatment, fewer than 4% of cells remained transfected alone, indicating that almost all available transfected cells were being infected by the influenza virus. Also, the results of the reciprocal experiment, in which cells were transiently transfected with dominant-negative caveolin-1 and treated with chlorpromazine during infection, support the above data (data not shown). These results indicate that influenza virus is capable of using a non-clathrin-mediated, non-caveola-mediated endocytic pathway for entry and infection of host cells, in addition to the traditional clathrin-mediated endocytic route.
FIG. 8.
Inhibition of both clathrin-mediated endocytosis and uptake by caveolae does not prevent influenza virus infection. HeLa cells were transiently transfected with either GFP or GFP-Eps15Δ95/295. Cells were treated with either 25 μg of nystatin/ml or 100 μg of genistein/ml during a high-MOI infection with influenza virus. Infection was monitored by FACS analysis using a monoclonal antibody against NP. Infection is monitored on the y axis, and transfection is monitored on the x axis. The percentage of cells within each quadrant is given.
DISCUSSION
We demonstrate here that in addition to clathrin-mediated endocytosis, influenza virus may enter and infect HeLa cells by a non-clathrin-mediated, non-caveola-mediated internalization pathway. Through transient transfection with a dominant-negative acting Eps15 construct, we demonstrate that while clathrin-dependent transferrin and SFV are prohibited from entering cells, internalization of influenza virus is unaffected. This finding is supported by treatment of cells with chlorpromazine and by exposure to potassium depletion. Potassium depletion has been used to investigate the role of clathrin-mediated endocytosis in rhinovirus infection (2, 28), and chlorpromazine has been used previously to demonstrate clathrin-mediated uptake for the polyomavirus JC virus, parechovirus 1, and Epstein-Barr virus (23, 35, 42). Although chlorpromazine has been shown to reduce influenza virus yields by 2 to 3 log PFU in chicken embryo fibroblasts (25), this may be due to the severe toxic effects of the drug itself or disruptions in viral replication rather than a direct effect on entry. Also, by employing dominant-negative acting caveolin-1 and a tyrosine kinase inhibitor, we demonstrate that influenza virus does not require caveolae for entry, and by use of selective sterol-binding agents, we demonstrate that influenza virus does not seem to depend on specific cholesterol-rich lipid microdomains. By combining inhibitory protocols, we show that influenza virus may infect cells deficient in both clathrin-mediated endocytosis and uptake by caveolae. Again, we believe that these data demonstrate an alternative entry pathway for influenza virus in the form of a non-clathrin-dependent route, in addition to clathrin-mediated endocytosis, as described previously (33).
Such a non-clathrin-dependent pathway has been implicated in virus entry before, but its nature is still ill defined (8, 38). As yet, no specific means to inhibit this pathway exists, and therefore it must remain as a default route when all other endocytic routes have been disabled. However, several recent reports have described entry by this non-clathrin-dependent, non-caveola-dependent alternative pathway. Expression of Eps15Δ95/295 in caveola-deficient T lymphocytes has been shown not to prevent uptake and delivery to lysosomes of interleukin-2 (IL-2) (26). The IL-2 entry pathway has been characterized as requiring dynamin, like clathrin-mediated endocytosis and caveolae, and occurring on detergent-resistant membranes (DRMs). Other studies have examined the uptake of glycosylphosphatidylinositol (GPI)-anchored proteins via a pathway that may bypass the endosomal system to deliver contents to the Golgi apparatus (37). The GPI anchor has also been examined in the context of diphtheria toxin. This GPI-linked toxin can enter epithelial cells deficient in both clathrin-mediated and caveolar uptake (54). In contrast to IL-2, entry of diphtheria toxin occurs independently of dynamin, and since sterol-binding drugs do not affect diphtheria toxin entry, it appears to be independent of DRMs.
That influenza virus infects in the presence of the sterol-binding drugs nystatin and MβCD suggests that virus entry is independent of DRMs, and we are currently investigating this further. This possibility is supported by recent data demonstrating that the influenza virus hemagglutinin fusion peptide does not associate with DRMs (1). However, it remains possible that a novel and to date uncharacterized type of DRM, which is insensitive to the drug treatments used here, is involved in uptake. In addition, it is clear that use of pharmocological treatments alone makes it difficult to draw conclusions on microdomain function at the plasma membrane (22). The use of dominant-negative caveolin allows a firm conclusion to be drawn about the role of caveolin in influenza virus entry, but the role of other lipid microdomains remains an open question at present. The transmembrane domain of hemagglutinin does, however, appear to have a known affinity for DRMs with regard to virus budding from the host cell plasma membrane (48, 49), and such DRMs within the virus envelope are likely to affect the fusion properties of the virus.
We have demonstrated previously, by using the K44A dynamin mutant in Mv-1 lung cells, that influenza virus entry is dependent on dynamin. Although dynamin activity was previously thought to be specific to clathrin-mediated endocytosis, it is now clear that this small GTPase functions in a variety of endocytic vesicle scission events (15, 18, 24, 39), and it has been described as a essential component of non-clathrin-mediated endocytosis (26). Although we have not yet differentiated the roles of dynamin in influenza virus entry via clathrin- versus non-clathrin-mediated endocytosis, it is quite likely that dynamin plays a crucial role in both endocytic pathways. Although we have yet to examine the role of dynamin in influenza virus entry in a HeLa cell system, we would expect that entry is similarly inhibited by dominant-negative dynamin. A non-clathrin- and non-caveola-dependent, dynamin-dependent pathway characterized for influenza virus may represent a novel alternative internalization pathway. Together with data for other ligands, this leads to the suggestion that there may be multiple non-clathrin-mediated endocytic routes, which may vary in requirements for entry and trafficking within the cell. Additionally, there may be cell type differences in the relative action or abundance of the endocytic pathways discussed here, and such differences may have effects on the preferred route of entry for influenza virus. The next step will be to determine which endocytic vesicles the influenza virus is trafficked through when it enters cells by clathrin-mediated endocytosis and by its alternative route of entry. It can be hypothesized that the trafficking events from both entry pathways will be similar to that already proposed for influenza virus—passage through early or sorting endosomes followed by fusion from late endosomes in a pH-dependent manner.
As yet, it is not clear why and how influenza virus has the capacity to enter cells through multiple pathways. One obvious possibility relates to the nonspecific influenza virus receptor sialic acid, which can take the form of either a glycoprotein or a glycolipid (19). Other viruses that have been described as entering cells solely by clathrin-mediated endocytosis have also been determined to have specific receptors for binding. For instance, SFV is known to bind to major histocompatibility complex class I molecules (17), while Sindbis virus binds to laminin receptor (56). Binding to these specific receptors may target the virus to a particular pathway of entry, in this case clathrin-mediated endocytosis. Influenza virus, due to its nonspecific binding, may lack this targeting, and this may, in turn, lead to multiple pathways of entry. In this regard, influenza virus may share common properties with Shiga toxin, which binds the glycosphingolipid receptor globotriaosyl ceramide and has been shown to enter cells by both clathrin-dependent and -independent pathways (for a review, see reference 22).
Taken together, our data would indicate that influenza virus may enter cells by a non-clathrin-mediated, non-caveola-mediated endocytic pathway, in addition to entry by clathrin-mediated endocytosis. It is therefore possible that other viruses may have multiple entry pathways that could be dependent on receptor binding or cell type.
Acknowledgments
We thank Benjamin Briggs for preliminary work on caveolin constructs and SV40 control experiments. We thank Ari Helenius for helpful discussions and for providing reagents. We also thank Jennifer Lippincott-Schwartz, Colin Parrish, and Margaret Kielian for their kind contributions of reagents.
S.B.S. was supported by a Training Grant from the National Institutes of Health (T32AI07618). This work was supported by a Scientist Development Grant from the American Heart Association and by grant R01AI48678 from the National Institutes of Health (to G.R.W.).
REFERENCES
- 1.Ahn, A., D. L. Gibbons, and M. Kielian. 2002. The fusion peptide of Semliki Forest virus associates with sterol-rich membrane domains. J. Virol. 76:3267-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 276:3952-3962. [DOI] [PubMed] [Google Scholar]
- 3.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 4.Benmerah, A., B. Begue, A. Dautry-Varsat, and N. Cerf-Bensussan. 1996. The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps 15 protein. J. Biol. Chem. 271:12111-12116. [DOI] [PubMed] [Google Scholar]
- 5.Benmerah, A., J. Gagnon, B. Begue, B. Megarbane, A. Dautry-Varsat, and N. Cerf-Bensussan. 1995. The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J. Cell Biol. 131:1831-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benmerah, A., V. Poupon, N. Cerf-Bensussan, and A. Dautry-Varsat. 2000. Mapping of Eps15 domains involved in its targeting to clathrin-coated pits. J. Biol. Chem. 275:3288-3295. [DOI] [PubMed] [Google Scholar]
- 8.Bishop, N. E. 1997. An update on non-clathrin coated endocytosis. Rev. Med. Virol. 7:199-207. [DOI] [PubMed] [Google Scholar]
- 9.Bowman, E. J., A. Siebers, and K. Altendorf. 1988. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 85:7972-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodsky, F. M., C. Y. Chen, C. Knuehl, M. C. Towler, and D. E. Wakeham. 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17:517-568. [DOI] [PubMed] [Google Scholar]
- 11.Bui, M., G. Whittaker, and A. Helenius. 1996. Effect of M1 protein and low pH on nuclear transport of influenza virus ribonucleoproteins. J. Virol. 70:8391-8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone, R., S. Fre, G. Iannolo, F. Belleudi, P. Mancini, P. G. Pelicci, M. R. Torrisi, and P. P. Di Fiore. 1997. eps15 and eps15R are essential components of the endocytic pathway. Cancer Res. 57:5498-5504. [PubMed] [Google Scholar]
- 13.Doxsey, S. J., F. M. Brodsky, G. S. Blank, and A. Helenius. 1987. Inhibition of endocytosis by anti-clathrin antibodies. Cell 50:453-463. [DOI] [PubMed] [Google Scholar]
- 14.Fazioli, F., L. Minichiello, B. Matoskova, W. T. Wong, and P. P. Di Fiore. 1993. eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol. Cell. Biol. 13:5814-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold, E. S., D. M. Underhill, N. S. Morrissette, J. Guo, M. A. McNiven, and A. Aderem. 1999. Dynamin 2 is required for phagocytosis in macrophages. J. Exp. Med. 190:1849-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guinea, R., and L. Carrasco. 1995. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J. Virol. 69:2306-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helenius, A., B. Morein, E. Fries, K. Simons, P. Robinson, V. Schirrmacher, C. Terhorst, and J. L. Strominger. 1978. Human (HLA-A and HLA-B) and murine (H-2K and H-2D) histocompatibility antigens are cell surface receptors for Semliki Forest virus. Proc. Natl. Acad. Sci. USA 75:3846-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henley, J. R., E. W. A. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 20.Hinshaw, J. E. 2000. Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol. 16:483-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannolo, G., A. E. Salcini, I. Gaidarov, O. B. Goodman, Jr., J. Baulida, G. Carpenter, P. G. Pelicci, P. P. Di Fiore, and J. H. Keen. 1997. Mapping of the molecular determinants involved in the interaction between eps15 and AP-2. Cancer Res. 57:240-245. [PubMed] [Google Scholar]
- 22.Johannes, L., and C. Lamaze. 2002. Clathrin-dependent or not: is it still the question? Traffic 3:443-451. [DOI] [PubMed] [Google Scholar]
- 23.Joki-Korpela, P., V. Marjomaki, C. Krogerus, J. Heino, and T. Hyypia. 2001. Entry of human parechovirus 1. J. Virol. 75:1958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, S. M., K. E. Howell, J. R. Henley, H. Cao, and M. A. McNiven. 1998. Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science 279:573-577. [DOI] [PubMed] [Google Scholar]
- 25.Krizanová, O., F. Ciampor, and P. Verber. 1982. Influence of chlorpromazine on the replication of influenza virus in chick embryo fibroblasts. Acta Virol. 26:209-216. [PubMed] [Google Scholar]
- 26.Lamaze, C., A. Dujeancourt, T. Baba, C. G. Lo, A. Benmerah, and A. Dautry-Varsat. 2001. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell 7:661-671. [DOI] [PubMed] [Google Scholar]
- 27.Larkin, J. M., M. S. Brown, J. L. Goldstein, and R. G. Anderson. 1983. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell 33:273-285. [DOI] [PubMed] [Google Scholar]
- 28.Madshus, I. H., K. Sandvig, S. Olsnes, and B. van Deurs. 1987. Effect of reduced endocytosis induced by hypotonic shock and potassium depletion on the infection of Hep 2 cells by picornaviruses. J. Cell. Physiol. 131:14-22. [DOI] [PubMed] [Google Scholar]
- 29.Maniak, M. 2001. Macropinocytosis, p. 78-93. In M. Marsh (ed.), Endocytosis. Oxford University Press, Oxford, United Kingdom.
- 30.Marsh, M., and A. Helenius. 1980. Adsorptive endocytosis of Semliki Forest virus. J. Mol. Biol. 142:439-454. [DOI] [PubMed] [Google Scholar]
- 31.Marsh, M., and A. Helenius. 1989. Virus entry into animal cells. Adv. Virus Res. 36:107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsh, M., and H. T. McMahon. 1999. The structural era of endocytosis. Science 285:215-220. [DOI] [PubMed] [Google Scholar]
- 33.Matlin, K. S., H. Reggio, A. Helenius, and K. Simons. 1981. Infectious entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol. 91:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matlin, K. S., H. Reggio, A. Helenius, and K. Simons. 1982. Pathway of vesicular stomatitis virus leading to infection. J. Mol. Biol. 156:609-631. [DOI] [PubMed] [Google Scholar]
- 35.Miller, N., and L. M. Hutt-Fletcher. 1992. Epstein-Barr virus enters B cells and epithelial cells by different routes. J. Virol. 66:3409-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neufeld, E. B., A. M. Cooney, J. Pitha, E. A. Dawidowicz, N. K. Dwyer, P. G. Pentchev, and E. J. Blanchette-Mackie. 1996. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J. Biol. Chem. 271:21604-21613. [DOI] [PubMed] [Google Scholar]
- 37.Nichols, B. J., A. K. Kenworthy, R. S. Polishchuk, R. Lodge, T. H. Roberts, K. Hirschberg, R. D. Phair, and J. Lippincott-Schwartz. 2001. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153:529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols, B. J., and J. Lippincott-Schwartz. 2001. Endocytosis without clathrin coats. Trends Cell Biol. 11:406-412. [DOI] [PubMed] [Google Scholar]
- 39.Nicoziani, P., F. Vilhardt, A. Llorente, L. Hilout, P. J. Courtoy, K. Sandvig, and B. van Deurs. 2000. Role for dynamin in late endosome dynamics and trafficking of the cation-independent mannose 6-phosphate receptor. Mol. Biol. Cell 11:481-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parton, R. G., B. Joggerst, and K. Simons. 1994. Regulated internalization of caveolae. J. Cell Biol. 127:1199-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelkmans, L., K. J., and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 42.Pho, M. T., A. Ashok, and W. J. Atwood. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 74:2288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Root, C. R., E. G. Wills, L. L. McNair, and G. R. Whittaker. 2000. Entry of influenza viruses into cells is inhibited by a highly specific protein kinase C inhibitor. J. Gen. Virol. 81:2697-2705. [DOI] [PubMed] [Google Scholar]
- 44.Rothberg, K. G., J. E. Heuser, W. C. Donzell, Y. S. Ying, J. R. Glenney, and R. G. Anderson. 1992. Caveolin, a protein component of caveolae membrane coats. Cell 68:673-682. [DOI] [PubMed] [Google Scholar]
- 45.Roy, A.-M. M., J. S. Parker, C. R. Parrish, and G. R. Whittaker. 2000. Early stages of influenza virus entry into Mv-1 lung cells: involvement of dynamin. Virology 267:17-28. [DOI] [PubMed] [Google Scholar]
- 46.Russell, D. G., and M. Marsh. 2001. Endocytosis in pathogen entry and replication, p. 247-280. In M. Marsh (ed.), Endocytosis. Oxford University Press, Oxford, United Kingdom.
- 47.Salcini, A. E., S. Confalonieri, M. Doria, E. Santolini, E. Tassi, O. Minenkova, G. Cesareni, P. G. Pelicci, and P. P. Di Fiore. 1997. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 11:2239-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 49.Scheiffele, P., M. G. Roth, and K. Simons. 1997. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 16:5501-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmid, S. L., M. A. McNiven, and P. De Camilli. 1998. Dynamin and its partners: a progress report. Curr. Opin. Cell Biol. 10:504-512. [DOI] [PubMed] [Google Scholar]
- 51.Shin, J. S., and S. N. Abraham. 2001. Caveolae as portals of entry for microbes. Microbes Infect. 3:755-761. [DOI] [PubMed] [Google Scholar]
- 52.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. (First published 28 March 2002; 10.1099/vir.0.18346-0.) [DOI] [PubMed] [Google Scholar]
- 53.Skehel, J. J., T. Bizebard, P. A. Bullough, F. M. Hughson, M. Knossow, D. A. Steinhauer, S. A. Wharton, and D. C. Wiley. 1995. Membrane fusion by influenza virus. Cold Spring Harbor Symp. Quant. Biol. 55:573-580. [DOI] [PubMed] [Google Scholar]
- 54.Skretting, G., M. L. Torgersen, B. van Deurs, and K. Sandvig. 1999. Endocytic mechanisms responsible for uptake of GPI-linked diphtheria toxin receptor. J. Cell Sci. 112:3899-3909. [DOI] [PubMed] [Google Scholar]
- 55.Tebar, F., T. Sorkina, A. Sorkin, M. Ericsson, and T. Kirchhausen. 1996. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 271:28727-28730. [DOI] [PubMed] [Google Scholar]
- 56.Wang, K. S., R. J. Kuhn, E. G. Strauss, S. Ou, and J. H. Strauss. 1992. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol. 66:4992-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White, J., J. Kartenbeck, and A. Helenius. 1982. Membrane fusion activity of influenza virus. EMBO J. 1:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whittaker, G., I. Kemler, and A. Helenius. 1995. Hyperphosphorylation of mutant influenza virus matrix (M1) protein causes its retention in the nucleus. J. Virol. 69:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]