Abstract
CD8 T-cell (TCD8+) responses elicited by viral infection demonstrate the phenomenon of immunodominance: the numbers of TCD8+ responding to different viral peptides vary over a wide range in a reproducible manner for individuals with the same major histocompatibility complex class I alleles. To better understand immunodominance, we examined TCD8+ responses to multiple defined viral peptides following infection of mice with influenza virus. The immunodominance hierarchy of influenza virus-specific TCD8+ was not greatly perturbed by the absence of either perforin or T-helper cells or by interference with B7 (CD80)-mediated signaling. These findings indicate that costimulation by antigen-presenting cells (APCs) or killing of APCs by TCD8+ plays only a minor role in establishing the immunodominance hierarchy of antiviral TCD8+ in this system. This points to intrinsic features of the TCD8+ repertoire as major contributors to immunodominance.
Immunodominance is a central feature of CD8+ T-cell (TCD8+) responses to viruses, bacteria, tumors, and minor H antigens. Of the many thousands of peptides present in such complex antigens, relatively few are recognized by responding TCD8+, and responses to these few peptides can be ordered based on the numbers of responding TCD8+ into a relatively stable hierarchy. Despite its importance to understanding immune responses and designing vaccines, immunodominance is poorly understood at the mechanistic level. It is clear that immunodominance is not simply explained by the numbers of peptide complexes generated by antigen-presenting cells (APCs), the affinities of peptides for class I molecules, or the affinities of T-cell receptors for peptide-class I complexes, though each of these parameters contributes to the phenomenon (45).
Recent technical advances in quantitating TCD8+ responses have facilitated detailed mechanistic dissection of immunodominance. It is now possible to accurately enumerate TCD8+ responses to individual peptide determinants of complex antigens ex vivo using intracellular cytokine staining (ICS), enzyme-linked immunospot assay, or major histocompatibility complex (MHC)-peptide tetramer-based techniques (27). These methods enable the definition of immunodominance hierarchies in response to complex antigens, which provides a background for exploration of underlying mechanisms. Determinants eliciting the most vigorous responses are termed immunodominant determinants (IDDs), with other determinants referred to as subdominant determinants (SDDs) (35).
In many respects, the best-characterized system for studying immunodominance in TCD8+ responses is the infection of BALB/c or C57/BL6 mice with influenza virus (IV). Previous findings in this system have demonstrated that multiple factors contribute to immunodominance hierarchies (10, 14). A major factor contributing to the ascendance of IDDs over SDDs is the suppression of SDD-specific TCD8+ by IDD-specific TCD8+, a phenomenon termed immunodomination. Based on findings using mice immunized with multiple synthetic peptide determinants, Sandberg et al. suggested that TCD8+ compete at the level of APCs for activation (33), an idea is supported by the recent findings of Kedl et al. (22). One potential mechanism of competition is that the initial responding (immunodominant) TCD8+ lyse APCs, preventing activation of later-arriving (subdominant) clones. Indeed, Loyer et al. found that TCD8+ specific for minor H antigens can destroy adoptively transferred APCs by a perforin-dependent process (25), and destruction of dendritic cells by tumor- or virus-specific TCD8+ has been reported (31).
An additional possible contributing factor for immunodominance hierarchies is the requirement for assistance provided by TCD4+. TCD4+ aid TCD8+ responses in several ways, including local secretion of cytokines and modification of APCs to enhance their TCD8+-activating capacity (5, 15, 30, 34, 44). Such modifications may include enhanced expression of B7, whose interaction with naïve TCD8+ strongly favors activation (8, 26, 32). An important issue is the role costimulation plays in establishing immunodominance hierarchies. Does it assist, hinder, or not greatly affect the immunodominance hierarchy?
Another factor that can influence immunodominance hierarchies is the presence of responses to new determinants restricted by other class I molecules. In humans, for example, responses to determinants can be rather unpredictable among individuals (7). Given that each individual has a unique history of exposure to foreign antigens, it is difficult to sort out the contributions of nature (i.e., genotype) versus nurture (i.e., prior antigenic experience). Obviously, this question is much more easily addressed using inbred mice maintained under controlled conditions.
To define the importance of these potential factors in establishing immunodominance hierarchies, we studied influenza virus specific-TCD8+ responses in mice deficient in perforin or TCD4+ or following interference with B7 (CD80)-mediated signaling. Our findings support the idea that none of these factors plays an essential role in establishing the immunodominance hierarchy in TCD8+ responses.
MATERIALS AND METHODS
Mice, virus, TCD8+ priming in vivo, antibody blocking, and ICS assay.
C57BL/J6 (B6) (H-2b), B6CD4−/−, B6 I-Ab β-chain−/−, B6 perforin−/−, BALB/c (H-2d), and BALB/c × C57/BL6 F1 (CB6 F1, H-2b × H-2b) mice were purchased from Taconic (Germantown, N.Y.). BALB/c perforin−/− mice were provided by John Harty (43). Eight- to 10-week-old female mice were primed by intraperitoneal (i.p.) injection with 600 hemagglutinating units of influenza virus A/Puerto Rico/8/34 (PR8). For blocking experiments, antibodies and recombinant CTLA-4.Fc at 150 to ∼200 μg/mouse were injected i.p. 1 day before, 1 day after, and on the day of PR8 priming. Primed splenic cells and peritoneal cells were prepared at various days after priming. TCD8+ responses were quantitated by ICS for gamma interferon (IFN-γ) accumulation following peptide stimulation (19) as described previously (10). Briefly, splenocytes or peritoneal exudate cells were stained first with Cychrome-labeled anti-CD8α (BD-Pharmingen, San Diego, Calif.). Cells were then washed and fixed with 1% paraformaldehyde and further stained in the presence of 0.2% saponin with fluorescein isothiocyanate-labeled anti-IFN-γ (BD-Pharmingen). Cells were analyzed by flow cytometry.
Peptides, monoclonal antibodies, and other reagents.
All peptides were synthesized, purified by high-performance liquid chromatography, and analyzed by mass spectrometry by or under the supervision of the Biologic Resource Branch, National Institute of Allergy and Infectious Diseases (Rockville, Md.). All peptides were dissolved in dimethyl sulfoxide at a 1 mM concentration as stock solutions and kept at −30°C. Anti-CTLA-4 (UC10-4F10-11 (42), anti-CD4 (GK1.4) monoclonal antibody (MAb) ascites and anti-CD8α (53.6) MAb were purified from ascites produced in SCID mice and purchased from Charles River Laboratories (Wilmington, Mass.). The production of recombinant CTLA-4.Fc and its mutant form MutCTLA-4.Fc, which lacks the binding site for B7 molecules, has been described (21).
RESULTS
Immunodominance hierarchies in normal mice.
Table 1 lists a number of defined IV peptides that are recognized by virus-specific TCD8+ in B6 and BALB/c mice (2, 3, 4, 10, 17, 40). In addition, we described here a novel Dd-restricted peptide from PB2, PB2289-297, that we identified using the peptide motif prediction algorithm of Rammensee and colleagues (29). The synthetic version of this peptide sensitizes target cells for lysis by PB2-specific TCD8+ in the picomolar range and coelutes with the naturally processed peptide by high-performance liquid chromatography (W. Chen, unpublished observations).
TABLE 1.
Determinants used in this study
| Designation | Sequence | Ranka | Restriction element | Reference |
|---|---|---|---|---|
| B6 | ||||
| PA224-233 | SSLENFRAYV | 1 | Db | 3 |
| NP366-374 | ASNENMETM | 2 | Db | 16 |
| DAMP62-70 | LSLRNPILV | 3 | Db | 11 |
| PB1703-711 | SSYRRPVGI | 4 | Kb | 4 |
| PB2198-206 | ISPLMVAYM | 5 | Kb | 4 |
| NS2114-121 | RTFSFQLI | 6 | Kb | 40 |
| MI128-135 | MGLIYNRM | 7 | Kb | 40 |
| BALB/c | ||||
| NP147-155 | TYQRTRALV | 2 | Kd | 36 |
| PB2289-297 | IGGIRMVDI | 3 | Dd | Chen et al., unpublished |
| HA518-526 | IYSTVASSL | 4 | Kd | 37 |
| NP39-47 | FYIQMCTEL | 5 | Kd | 14 |
| NP218-226 | AYERMCNIL | 6 | Kd | 10 |
| HA462-470 | LYEKVKSQL | 7 | Kd | 10 |
Determinants are ranked according to the magnitude of TCD8+ responses elicited by i.p. infection with PR8.
We have previously described the primary responses to these determinants in B6 and BALB/c mice following i.p. infection with PR8 (10, 12). In Fig. 1 and 2 we replot the data sets originally described in these references as absolute numbers of responding cells. These data define a baseline for the experimental manipulations that follow. Importantly, the variation in responses between individual mice in a given experiment is within the variation that we observe between individuals in different experiments. This enabled us to limit experiments with knockout mice to a manageable size by not always including wild-type mice as controls. Another finding supports the validity of this approach: for any given mouse the relative positions of determinants within the immunodominance hierarchy are independent of the absolute magnitude of the response.
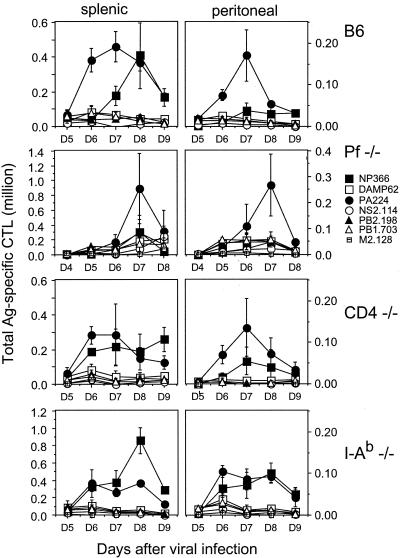
FIG. 1.
Immunodominance hierarchy following i.p. infection with PR8 in B6 and B6 knockout mice. Splenic and peritoneal cells were prepared at the indicated times after PR8 infection, and responses to individual determinants were assessed by ICS using a panel of H-2b-restricted peptides. All responses were normalized by subtracting the background obtained with APCs not exposed to peptides. These percentages were used to calculate the numbers of antigen-specific cells among all TCD8+ recovered from spleens (left panels) or peritoneal exudates (right panels). We used different scales for splenic and peritoneal responses due to differences in total cell numbers. CD4−/− animals and I-Ab−/− animals were assayed on the same day. Each point represents the average value for three individual animals. In a separate experiment, perforin−/− (pf−/−) mice exhibited a day 7 response similar to that shown. Data from wild-type mice are replotted from reference 12; we observed similar responses on day 7 in 10 individual experiments. Ag, antigen.
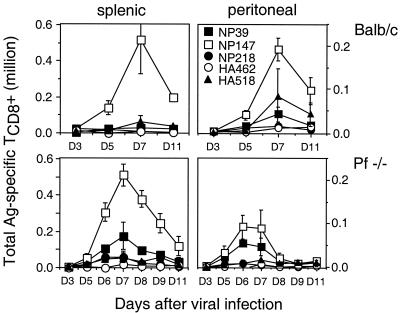
FIG. 2.
Immunodominance hierarchy in response to influenza virus PR8 in BALB/c and BALB/c perforin−/− mice. Splenic and peritoneal cells were prepared at various times after PR8 priming, and determinant-specific responses were assessed by ICS using a panel of H-2d-restricted peptides as described in the legend to Fig. 1. In a separate experiment, perforin−/− (pf−/−) mice exhibited a day 7 response similar to that shown. Data from wild-type mice are replotted from reference 10; we observed similar responses on day 7 in six individual experiments. Ag, antigen.
To recapitulate our previous observations, we collected TCD8+ from spleen and peritoneum 3 to 5 days postinfection and measured the numbers of responding peptide-specific TCD8+ by IFN-γ-ICS. For B6 mice, PA224-233 and NP366-374 represent IDDs (Fig. 1). PA224-233-specific TCD8+ are more prevalent than NP366-374 TCD8+. PA224-233-specific TCD8+ respond more rapidly and decline sooner. Note the compartment-dependent discrepancy in the responses, which peaks on day 7 when the ratio of peritoneal to splenic PA224-233-specific TCD8+ is 1:3 versus 1:4 for NP366-374-specific TCD8+. This is consistent with either differential presentation of the determinants in the spleen versus the peritoneum or differential regulation of the responding TCD8+, e.g., due to increased immunodomination in the peritoneum by PA224-233-specific TCD8+. By contrast, for BALB/c mice (Fig. 2), the IDD NP147-155 is more dominant in splenic TCD8+ than in peritoneal TCD8+. Again, all specificities in both sites peaked on or around day 7.
Perforin has a minor role in establishing immunodominance hierarchies.
Immunodomination is an extensively documented feature of immunodominance in which dominant TCD8+ suppress the response of subdominant TCD8+ (13, 39, 46). A possible mechanism of immunodomination is perforin-mediated lysis of APCs by dominant TCD8+. To test this possibility, we determined the immunodominance hierarchy in perforin−/− B6 (20) and BALB/c mice (1).
As seen in Fig. 1 and 2, TCD8+ responses for perforin−/− B6 mice differed only slightly from those for wild-type mice. In perforin−/− mice on day 7 postinfection, there was an approximate doubling in responses to all determinants except NP366-374. This may be due to increased antigenic presentation as a result of increased viral load or reduced lysis of APCs. The failure of NP366-374-specific TCD8+ to increase may reflect a decrease in its ability to immunodominate other determinants due to a decreased capacity to kill APCs.
By contrast, the number of responding TCD8+ did not demonstrate a general increase in BALB/c perforin−/− mice. The only significant alteration was a relative increase in the frequency of NP39-47-specific TCD8+, which ascended the dominance hierarchy at the expense of HA518-526. Since presentation of NP39-47 is limiting in vivo (10), this may reflect increased antigen presentation due to decreased lysis of APCs.
Immunodominance hierarchy is intact in TCD4+-deficient mice.
It has been documented at the population level that naïve TCD8+ demonstrate variable requirements for TCD4+-mediated help. TCD4+ seem to be particularly important in activation of naïve TCD8+ that are activated by cross-priming, i.e., presentation of exogenous antigens by professional APCs (6). We examined the influence of TCD4+ on the immunodominance hierarchy in B6 mice by using mice with targeted deletions of CD4 (23) or I-Ab β chain (18).
As seen in Fig. 1, the absence of CD4 resulted in a decrease in the number of responding splenic TCD8+ (except NP366-374-specific TCD8+, which responded better) but had little effect on the immunodominance hierarchy. In I-Ab−/− animals (Fig. 1), responses to NP366-374 were enhanced for both the spleen and the peritoneum while responses to PA224-233 were concomitantly reduced. Responses to other subdominant determinants were not significantly altered.
Interference with CD28-B7 interactions has little impact on the immunodominance hierarchy.
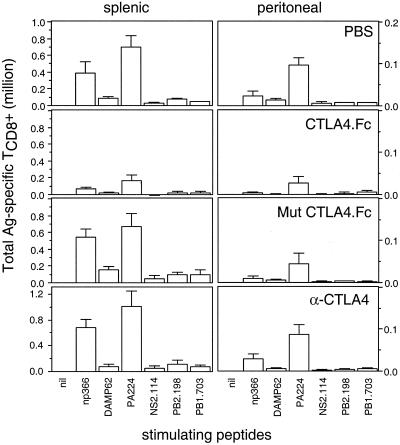
The interaction of APC B7 with TCD8+ CD28 or CD152 (CTLA-4) positively and negatively regulates TCD8+ activation, respectively. To examine the role of B7-mediated immune modulation, we treated B6 mice with soluble CD152, which binds to B7 in vivo and interferes with its costimulatory properties (21). As a control, mice were injected with a mutated version of CD152 which no longer binds B7 (21). Animals were injected with recombinant proteins for three consecutive days beginning the day prior to infection with IV, and TCD8+ responses were measured on day 7. As seen in Fig. 3, injection with soluble CD152 (but not mutant CD152) greatly diminished TCD8+ responses, as previously demonstrated for other viral infections (24). Notably, the immunodominance hierarchy was not significantly affected among the TCD8+ activated under these conditions. To examine the possible influence of B7-CD152 negative regulation, we treated mice with the anti-CD152 MAb UC10-4F10-11 using the same injection schedule. This had no significant effect on the vigor or specificity of TCD8+ responses.
FIG. 3.
Effects of interfering with CD28-B7 interaction immunodominance hierarchy in B6 mice. B6 mice were injected once a day for 3 days with 150 to ∼200 μg of α-CTLA-4, recombinant CTLA-4.Fc, mutated recombinant CTLA-4.Fc, or phosphate-buffered saline (PBS). Mice were infected with PR8 on the second day of the regimen. As described in Fig. 1, splenic and peritoneal cells were prepared 7 days following PR8 priming, and their determinant-specific responses were enumerated by ICS using a panel of known H-2b-restricted peptides. All animals were assessed on the same day. Each point represents the average value from three individual animals. Ag, antigen.
These findings indicate that the B7-CD28 interaction greatly enhances anti-IV TCD8+ responses but does not favor immunodominant TCD8+. Additionally, negative signaling via CD152 appears to exert little impact on the vigor or nature of the peak TCD8+ response.
F1 animals maintain immunodominance hierarchies.
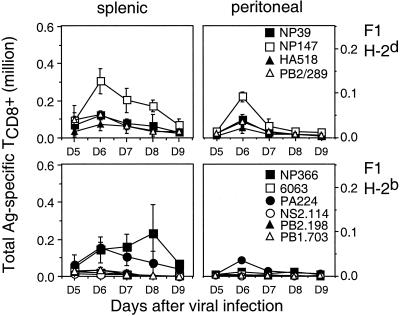
In most mammalian species, individuals express a distinct MHC haplotype obtained from each parent. The presence of additional MHC class I molecules can potentially influence repertoire selection and antigen presentation. This influence can be exerted by both qualitative (novel molecules) and quantitative (50% reduction in expression of each allomorph) mechanisms. It was therefore of interest to determine whether the immunodominance hierarchies of H-2b and H-2d mice are maintained in F1 animals (Fig. 4). Responses to most determinants peaked 1 day earlier than in parental mice. The overall number of responding TCD8+ was similar in F1 and parental mice, indicating that increasing the diversity of the response does not result in a net increase in responsiveness. Moreover, the reduction in responses was spread fairly evenly among determinants, such that the hierarchies were more or less melded with each other. This finding, like those above, point to the stability of immunodominance hierarchies.
FIG. 4.
Immunodominance hierarchy in response to influenza virus PR8 in H-2d×b F1 mice. Splenic and peritoneal cells were prepared at various times after PR8 priming, and determinant-specific responses were assessed by ICS using a panel of H-2b- and/or H-2d-restricted peptides as described in the legend to Fig. 1. H-2b- and H-2d-restricted responses for CB6 F1 animals, although displayed in different panels, were assessed on the same day. Each point represents the average value from three individual animals. Ag, antigen.
This is not to say that dominance hierarchies are always this predictable: indeed there are examples both old (reviewed in reference 46) and new (2) of scrambling of hierarchies associated with mixing of allomorphs. Rather, the present data provide an example that such reordering of immunodominance hierarchies is not inevitable upon introduction of new restriction elements.
DISCUSSION
As strategies for identifying potential TCD8+ gain in sophistication and methods for identifying antigen-specific TCD8+ gain in sensitivity, we nudge closer to appreciating immune responses in all their splendid complexity. While not so long ago it was sufficient to characterize TCD8+ responses to potential antigens in an all or none manner, it is now clear that responses are composed of swarms of TCD8+ clones responding to multiple determinants in predictable hierarchies.
Clearly, two of the important factors in establishing dominance hierarchies in response to different determinants are the efficiency of generating peptide class I complexes and the existence of TCD8+ clones capable of responding to the complex. These alone, however, do not fully account for the positions of determinants within the hierarchy. We therefore explored a number of potential contributing factors. Our results obtained with perforin−/− mice indicate that perforin-mediated elimination of APCs has a limited role in establishing the anti-IV hierarchy (Fig. 1 and 2). Since perforin-dependent lysis is the primary mechanism used by TCD8+ (20, 41), killing of APCs by immunodominant clones probably does not play a major role in immunodominance in this system, echoing prior findings in the response of mice to Listeria monocytogenes (1).
A previous study found that the polyclonal pulmonary TCD8+ response to intranasal IV infection was reduced in I-Ab−/− animals (38). By contrast, we failed to observe a significant difference in the numbers of responding TCD8+ associated with deletion of class II molecules or CD4. This may be related to differences in the virus strains used, the route or dose of infection, or methodologies. We found that the immunodominance hierarchy was largely unaffected by the absence of TCD4+. There was a relatively minor shuffling in the immunodominance hierarchy in I-Ab−/− mice, where NP366-374-specific TCD8+ replace PA224-233 TCD8+ at the top of the hierarchy. Curiously, this was not observed in CD4−/− mice. Rahemtulla have shown that CD4−/− mice maintain functional class II-restricted helper cells in the form of CD8-CD- TCRαβ+ T cells (28). Such help is not available in I-Ab−/− mice, suggesting that the immunodominance of PA224-233 requires help normally provided by TCD4+. Notably, even in I-Ab−/− mice, PA224-233 occupies the β-position in the immunodominance hierarchy, indicating that the requirement for help is relative rather than absolute.
We confirmed previous findings that CTLA-4-Ig strongly interferes with activation of virus-specific TCD8+ in general (24) and IV-specific TCD8+ in particular (26). Notably, PA224-233 and NP366-374 retained their respective positions in the immunodominance hierarchy. This indicates that B7-dependent costimulation does not grossly influence immunodominance, rather exerting relatively equal stimulation among all responding clones. We failed to detect a significant effect of the anti-CD152 MAb UC10-4F10-11 on either the overall α-IV TCD8+ response or the immunodominance hierarchy. Administration of this antibody under similar conditions has been shown to interfere with the CTLA-4-mediated negative regulation of antitumor and antiself responses (32). It has been reported that CD152 has a greater influence on deactivating memory TCD8+ than naïve TCD8+ (9). In future studies it will be of interest to examine the influence of CD152 on the immunodominance hierarchy in memory TCD8+.
Altogether, our findings indicate that while costimulation positively influences the activation of naïve virus-specific TCD8+, it does so in a global manner and does not make a significant contribution to the establishment of immunodominance hierarchies. Rather, hierarchies appear to result from intrinsic properties of the TCD8+ repertoire. Using recombinant vaccinia viruses, numerous studies have shown that increasing the number of peptide class I complexes generated from an inserted gene can boost the response to a nominal determinant relative to the response to vaccinia virus gene products (45). In other words, modifying the relative amounts of peptide class I complexes presented by a given APC can alter the immunodominance hierarchy. The present findings predict that altering the nature of the APC would influence the immunodominance hierarchy only inasmuch as this modifies the relative quantities of the determinants presented by the APC, and not by altering costimulatory signals.
Acknowledgments
Bethany Buschling provided outstanding technical assistance. John Harty (University of Iowa) generously provided perforin knockout BALB/c mice. Thanks to Chris Norbury for insightful suggestions on the manuscript. We are grateful to the NIAID peptide synthesis facility for providing high-quality material.
REFERENCES
- 1.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 290:1354-1358. [DOI] [PubMed] [Google Scholar]
- 2.Belz, G. T., P. G. Stevenson, and P. C. Doherty. 2000. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J. Immunol. 165:2404-2409. [DOI] [PubMed] [Google Scholar]
- 3.Belz, G. T., W. Xie, J. D. Altman, and P. C. Doherty. 2000. A previously unrecognized H-2Db-restricted peptide prominent in the primary influenza A virus-specific CD8+ T-cell response is much less apparent following secondary challenge. J. Virol. 74:3486-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belz, G. T., W. Xie, and P. C. Doherty. 2001. Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific cd8(+) t cell responses. J. Immunol. 166:4627-4633. [DOI] [PubMed] [Google Scholar]
- 5.Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. [DOI] [PubMed] [Google Scholar]
- 6.Bennett, S. R. M., F. R. Carbone, F. Karamalis, J. F. A. P. Miller, and W. R. Heath. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 1:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betts, M. R., J. P. Casazza, B. A. Patterson, S. Waldrop, W. Trigona, T. M. Fu, F. Kern, L. J. Picker, and R. A. Koup. 2000. Putative immunodominant human immunodeficiency virus-specific CD8(+) T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 74:9144-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers, C. A., and J. P. Allison. 1999. Costimulatory regulation of T cell function. Curr. Opin. Cell Biol. 11:203-210. [DOI] [PubMed] [Google Scholar]
- 9.Chambers, C. A., T. J. Sullivan, T. Truong, and J. P. Allison. 1998. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur. J. Immunol. 28:3137-3143. [DOI] [PubMed] [Google Scholar]
- 10.Chen, W., L. C. Anton, J. R. Bennink, and J. W. Yewdell. 2000. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 12:83-93. [DOI] [PubMed] [Google Scholar]
- 11.Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7:1306-1312. [DOI] [PubMed] [Google Scholar]
- 12.Chen, W., C. C. Norbury, Y. Cho, J. W. Yewdell, and J. R. Bennink. 2001. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8(+) T cells at the levels of T cell repertoire and presentation of viral antigens. J. Exp. Med. 193:1319-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole, G. A., T. L. Hogg, M. A. Coppola, and D. L. Woodland. 1997. Efficient priming of CD8+ memory T cells specific for a subdominant epitope following Sendai virus infection. J. Immunol 158:4301-4309. [PubMed] [Google Scholar]
- 14.Deng, Y., J. W. Yewdell, L. C. Eisenlohr, and J. R. Bennink. 1997. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J. Immunol. 158:1507-1515. [PubMed] [Google Scholar]
- 15.Doherty, P. C., D. J. Topham, R. A. Tripp, R. D. Cardin, J. W. Brooks, and P. G. Stevenson. 1997. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159:105-117. [DOI] [PubMed] [Google Scholar]
- 16.Falk, K., O. Rötzschke, K. Deres, J. Metzger, G. Jung, and H.-G. Rammensee. 1991. Identification of naturally processed viral nonapeptides allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. J. Exp. Med. 174:425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn, K. J., G. T. Belz, J. D. Altman, R. Ahmed, D. L. Woodland, and P. C. Doherty. 1998. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8:683-691. [DOI] [PubMed] [Google Scholar]
- 18.Grusby, M. J., R. S. Johnson, V. E. Papaioannou, and L. H. Glimcher. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II- deficient mice. Science 253:1417-1420. [DOI] [PubMed] [Google Scholar]
- 19.Jung, T., U. Schauer, C. Heusser, C. Neumann, and C. Rieger. 1993. Detection of intracellular cytokines by flow cytometry. J. Immunol. Methods 159:197-207. [DOI] [PubMed] [Google Scholar]
- 20.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 21.Kearney, E. R., T. L. Walunas, R. W. Karr, P. A. Morton, D. Y. Loh, J. A. Bluestone, and M. K. Jenkins. 1995. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J. Immunol. 155:1032-1036. [PubMed] [Google Scholar]
- 22.Kedl, R. M., W. A. Rees, D. A. Hildeman, B. Schaefer, T. Mitchell, J. Kappler, and P. Marrack. 2000. T cells compete for access to antigen-bearing antigen-presenting cells. J. Exp. Med. 192:1105-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Killeen, N., S. Sawada, and D. R. Littman. 1993. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO J. 12:1547-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, S. K., D. S. Reed, S. Olson, M. J. Schnell, J. K. Rose, P. A. Morton, and L. Lefrancois. 1998. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl. Acad. Science USA 95:10814-10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loyer, V., P. Fontaine, S. Pion, F. Hetu, D. C. Roy, and C. Perreault. 1999. The in vivo fate of APCs displaying minor H antigen and/or MHC differences is regulated by CTLs specific for immunodominant class I-associated epitopes. J. Immunol. 163:6462-6467. [PubMed] [Google Scholar]
- 26.Lumsden, J. M., J. M. Roberts, N. L. Harris, R. J. Peach, and F. Ronchese. 2000. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J. Immunol. 164:79-85. [DOI] [PubMed] [Google Scholar]
- 27.McMichael, A. J., and C. A. O'Callaghan. 1998. A new look at T cells. J. Exp. Med. 187:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahemtulla, A., T. M. Kundig, A. Narendran, M. F. Bachmann, M. Julius, C. J. Paige, P. S. Ohashi, R. M. Zinkernagel, and T. W. Mak. 1994. Class II major histocompatibility complex-restricted T cell function in CD4-deficient mice. Eur. J. Immunol. 24:2213-2218. [DOI] [PubMed] [Google Scholar]
- 29.Rammensee, H.-G., J. Bachmann, and S. Stevanovic. 1997. MHC ligands and peptide motifs. Landes Bioscience, Austin, Tex.
- 30.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 31.Ritchie, D. S., I. F. Hermans, J. M. Lumsden, C. B. Scanga, J. M. Roberts, J. Yang, R. A. Kemp, and F. Ronchese. 2000. Dendritic cell elimination as an assay of cytotoxic T lymphocyte activity in vivo. J. Immunol. Methods 246:109-117. [DOI] [PubMed] [Google Scholar]
- 32.Salomon, B., and J. A. Bluestone. 2001. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19:225-252. [DOI] [PubMed] [Google Scholar]
- 33.Sandberg, J. K., P. Grufman, E. Z. Wolpert, L. Franksson, B. J. Chambers, and K. Karre. 1998. Superdominance among immunodominant H-2Kb-restricted epitopes and reversal by dendritic cell-mediated antigen delivery. J. Immunol. 160:3163-3169. [PubMed] [Google Scholar]
- 34.Schoenberger, S. P., R. E. Toes, E. I. van der Voort, R. Offringa, and C. J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 35.Sercarz, E. E., P. V. Lehmann, A. Ametani, G. Benichou, A. Miller, and K. Moudgil. 1993. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 11:729-766. [DOI] [PubMed] [Google Scholar]
- 36.Sherman, L. A., T. A. Burke, and J. A. Biggs. 1992. Extracellular processing of antigens that bind class I major histocompatibility molecules. J. Exp. Med. 175:1221-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweetser, M. T., V. L. Braciale, and T. J. Braciale. 1989. Class I major histocompatibility complex-restricted T lymphocyte recognition of the influenza hemagglutinin: Overlap between class I cytotoxic T lymphocytes and antibody sites. J. Exp. Med. 170:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tripp, R. A., S. R. Sarawar, and P. C. Doherty. 1995. Characteristics of the influenza virus-specific CD8+ T cell response in mice homozygous for disruption of the H-2lAb gene. J. Immunol. 155:2955-2959. [PubMed] [Google Scholar]
- 39.van der Most, R. G., R. J. Concepcion, C. Oseroff, J. Alexander, S. Southwood, J. Sidney, R. W. Chesnut, R. Ahmed, and A. Sette. 1997. Uncovering subdominant cytotoxic T-lymphocyte responses in lymphocytic choriomeningitis virus-infected BALB/c mice. J. Virol. 71:5110-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitiello, A., L. Yuan, R. W. Chesnut, J. Sidney, S. Southwood, P. Farness, M. R. Jackson, P. A. Peterson, and A. Sette. 1996. Immunodominance analysis of CTL responses to influenza PR8 virus reveals two new dominant and subdominant Kb-restricted epitopes. J. Immunol. 157:5555-5562. [PubMed] [Google Scholar]
- 41.Walsh, C. M., M. Matloubian, C. C. Liu, R. Ueda, C. G. Kurahara, J. L. Christensen, M. T. Huang, J. D. Young, R. Ahmed, and W. R. Clark. 1994. Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Science USA 91:10854-10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walunas, T. L., D. J. Lenschow, C. Y. Bakker, P. S. Linsley, G. J. Freeman, J. M. Green, C. B. Thompson, and J. A. Bluestone. 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1:405-413. [DOI] [PubMed] [Google Scholar]
- 43.White, D. W., A. MacNeil, D. H. Busch, I. M. Pilip, E. G. Pamer, and J. T. Harty. 1999. Perforin-deficient CD8+ T cells: in vivo priming and antigen-specific immunity against Listeria monocytogenes. J. Immunol. 162:980-988. [PubMed] [Google Scholar]
- 44.Whitmire, J. K., and R. Ahmed. 2000. Costimulation in antiviral immunity: differential requirements for CD4(+) and CD8(+) T cell responses. Curr. Opin. Immunol. 12:448-455. [DOI] [PubMed] [Google Scholar]
- 45.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51-88. [DOI] [PubMed] [Google Scholar]
- 46.Zinkernagel, R. M., and P. C. Doherty. 1979. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv. Immunol. 27:52-180. [DOI] [PubMed] [Google Scholar]