Abstract
Herpesviruses are associated with several diseases of marine turtles, including lung-eye-trachea disease (LETD) and fibropapillomatosis. Two approaches were used to identify immunodominant antigens of LETV, the LETD-associated herpesvirus. The first approach targeted glycoprotein B, which is known to be immunogenic and neutralizing in other species. The second strategy identified LETV proteins recognized on Western blots by antibodies in immune green turtle plasma. A 38-kDa protein was resolved by two-dimensional gel electrophoresis, sequenced, and identified as a scaffolding protein encoded by the overlapping open reading frames of UL26 and UL26.5. Glycoprotein B and the scaffolding protein were cloned and expressed in Escherichia coli. The expressed proteins were recognized on Western blots by antibodies in immune green turtle plasma. Phylogenetic studies based on UL26, DNA polymerase, and glycoprotein B revealed that LETV clusters with the alphaherpesviruses.
Herpesviruses are associated with several diseases of marine turtles, including lung-eye-trachea disease (LETD) and fibropapillomatosis (FP). Recent efforts to understand the role of these herpesviruses and their impact on threatened and endangered marine turtle populations have focused on the development of serological assays for seroepidemiological studies. An LETD-associated herpesvirus (LETV)-specific enzyme-linked immunosorbent assay (ELISA) has been developed by using LETV-infected cell lysates as the antigen for detection of antibodies. This assay demonstrated that 21.6% of juvenile green turtles along the east coast of Florida were exposed to LETV or an LETV-like herpesvirus (3). In addition, 12 out of 13 nesting green and loggerhead turtles were shown to be seropositive for antibodies to LETV, suggesting that, as is the case with many human and mammalian herpesvirus infections, individuals are exposed at some point during their lifetimes. Clinical signs consistent with LETD have been observed in juvenile green turtles in the wild (8), and LETV has been found to be associated with LETD lesions in green turtles reared in captivity (11).
LETV is a relatively low-titer and slow-growing virus. Generation of antigen for the LETV ELISA is labor intensive and subject to batch-to-batch variation. Expressed recombinant herpesvirus proteins could provide a source of antigen that would permit more extensive seroepidemiological studies of LETV infections. In this study, two strategies were used to select LETV proteins for expression. One protein was previously identified as a potential immunodominant LETV antigen (2, 3). This 38-kDa protein was recognized by many, but not all, individual wild turtles with antibodies to LETV and was targeted for cloning and expression. A second LETV protein, glycoprotein B (gB), was also selected for cloning and expression. gB has been identified as a major target of the antiherpesvirus immune response in many host species and includes virus-neutralizing activity (6, 12, 19). gB also has been extensively targeted for the development of many mammalian herpesvirus vaccines and for the development of serological assays for detection of herpesvirus infections (1, 5, 7, 13, 14). gB is the most conserved glycoprotein (17), and the gene that encodes it is useful for assigning a herpesvirus to a particular herpesvirus subfamily (12).
LETV gB and the 38-kDa protein were cloned and expressed and shown to react in Western blots with antibodies in immune green turtle plasma. These are the first proteins from a reptilian herpesvirus to be cloned, expressed, and identified as immunogenic in their host species. In addition, phylogenetic studies revealed that LETV clusters with the alphaherpesviruses.
To obtain LETV DNA for the construction of a partial genomic library, terrapene heart cells (1.0 × 107) were infected with LETV clone 221 (2) and then embedded in 0.6% low-temperature agarose. LETV 221 genomic DNA was separated by pulsed-field gel electrophoresis (200 V, 24 h, 50 to 90 s) at 4°C in a 1.0% 0.5× TBE agarose gel. Bands corresponding to the LETV genome (approximately 150 kb) were cut out of the gel. Melted gel slices were digested in β-agarase (5 U/100 mg), and the mixture was concentrated and washed in a Centricon YM-100 (Millipore, Medford, Mass.). LETV DNA was ligated into SmaI-linearized pUC18 cloning vector. The ligated product was electroporated into Escherichia coli host TOP10, and the sequences of the resulting clones were compared to other sequences by BLASTX (basic local alignment search tool in protein database) searching of the National Center for Biotechnology Information database. Clones matching herpesvirus genes were arranged according to the gene order of the model alphaherpesvirus, human herpes simplex virus type 1 (HSV-1). Homologs to 29 HSV-1 genes were detected (data not shown). The LETV partial genomic library contains about 18.7% of the LETV genome.
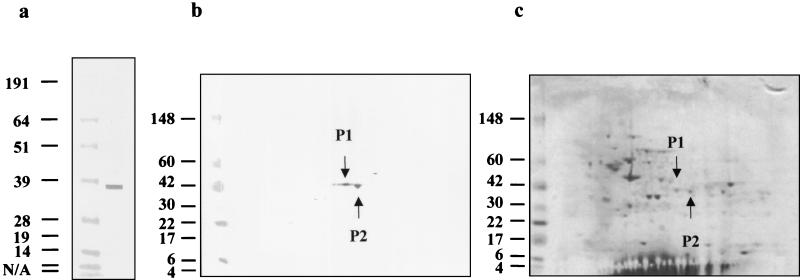
To obtain virus as a source of antigen for two-dimensional (2D) gel electrophoresis analysis, LETV 221 was grown and pelleted by ultracentrifugation as previously described (2). Pellets were washed with 30 ml of cold 10 mM Tris (pH 7.4) and then resuspended in 9 M urea-4% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS)-2 M thiourea-65 mM dithiothreitol-0.1% sodium dodecyl sulfate-trace orange G. The virus sample was prepared and resolved by 2D electrophoresis as follows. A 549-μg sample of total protein plus 2% ampholyte pH 3-10NL (Amersham Pharmacia Biotech, Piscataway, N.J.) was incubated with Immobiline strips pH 3-10NL (Amersham Pharmacia Biotech). The proteins were resolved in the first dimension in an isoelectric focusing apparatus (Amersham Pharmacia Biotech) under the following conditions: 300 V, 100 mA, and 30 W for 1 min; 300 V, 150 mA, and 30 W for 2 h 30 min; 3,500 V 100 mA, and 50 W for 3 h; and 3,500 V, 150 mA, and 100 W for 22 h. The Immobiline strips were equilibrated and then loaded into 4 to 20% Tris-glycine ZOOM gels (Invitrogen, Carlsbad, Calif.) to resolve the second dimension at 125 V. One gel was stained with colloidal blue stain (Pierce, Rockford, Ill.) or Coomassie stain. Another gel was electroblotted onto polyvinylidene difluoride membrane at 30 V for 1.5 h. The membrane was blocked with 5% nonfat dried milk in phosphate-buffered saline-0.2% sodium azide (PBS/A)-0.5% Tween 20/A (FBS/A) overnight at 4°C. After washing, the membrane was incubated with 1-month postimmunization plasma from LETV-immunized turtle 99A-1 (2) diluted 1:100 in 2% fetal bovine serum in PBS for 1 h with rocking to identify the 38-kDa protein. The membrane was washed and then incubated with biotinylated anti-green turtle immunoglobulin G monoclonal antibody HL858 (9) at 1 μg/ml in PBS/A for 1 h at room temperature with rocking and then further developed as previously described (2). The 38-kDa protein was detectable in virus-infected cell lysate preparations but not in uninfected-cell controls. The 38-kDa band visualized in a one-dimensional gel (Fig. 1a) resolved into two protein spots when visualized by 2D gel electrophoresis (Fig. 1b). When the protein spots were cut out of the polyvinylidene difluoride membrane and submitted for direct sequencing, both were N terminally blocked. The corresponding protein spots were identified in the colloidal-blue-stained 2D gels (Fig. 1c), cut out with a sterile scalpel, and submitted for analysis. Protein sequence was obtained from three fractions of each of the protein spots (Fig. 2e). Two of the fraction sequences overlapped and are shown as one shaded bar (Fig. 2e). The sequence information identified these two spots as the same protein, and the two spots are likely the result of differential posttranslation modification such as phosphorylation. As demonstrated in Fig. 2, the 38-kDa sequence fractions matched a portion of the LETV genome containing the overlapping open reading frames corresponding to HSV-1 genes UL26 (minor capsid protein or protease) and UL26.5 (assembly or scaffolding protein). In other herpesviruses, UL26 encodes a polyprotein that is autoproteolytically cleaved to yield protease and another scaffolding protein that is distinct from the assembly or scaffolding protein encoded by UL26.5 (10, 18, 20). The UL26 LETV homolog is diagrammed with these putative cleavage sites (Fig. 2d) and the two hypothetical protein products (Fig. 2d) indicated. The putative product of UL26.5 is a truncated form (approximately 40 amino acids less) of the cleavage product of UL26. The 2D fraction sequences map to the overlapping region of UL26 and UL26.5 encoding the putative LETV assembly or scaffolding proteins (Fig. 2e). This suggests that the 38-kDa protein is likely an assembly or scaffolding protein responsible for proper capsid formation. It is not known whether the 38-kDa protein recognized by green turtle antibodies corresponds to the putative cleavage product of UL26 or the product of UL26.5.
FIG. 1.
Identification of herpesvirus 38-kDa protein by 2D gel electrophoresis and Western blot analysis. The 38-kDa protein was recognized in an LETV-infected cell lysate by plasma from an LETV-immunized green turtle assayed by Western blotting (a). The same plasma sample recognized two protein spots (P1 and P2, indicated by arrows) when the LETV-infected cell lysate was resolved by 2D gel electrophoresis and then assayed by Western blotting (b). The LETV-infected cell lysate was resolved by 2D gel electrophoresis and then Coomassie stained to identify proteins corresponding to the two spots recognized in the Western blot (c). The corresponding proteins, P1 and P2, are indicated by arrows. The values on the left are molecular sizes in kilodaltons. N/A, not available.
FIG. 2.
Schematic illustrating the process of obtaining sequence information of gB and of mapping sequences of P1 and P2 to the overlapping UL26 and UL26.5 portion of the genome. (a) Diagram representing a portion of a typical alphaherpesvirus genome. (b) Clones from the LETV partial genomic library containing the corresponding portion of the genome (indicated by hatched boxes). Primers (shown as arrows with numbers) were designed on the basis of the sequence of these clones to PCR amplify the entire open reading frame of the LETV gB gene, as well as additional sequences for UL26.5 and UL26. (c) Diagram representing the two hypothetical independently transcribed messages of the overlapping LETV UL26 and UL26.5 genes (indicated by arrows). (d) The two expected protein products, protease and scaffolding protein, of the putative polyproteins encoded by LETV UL26 (shown along with the anticipated sizes). The two hypothetical cleavage sites are indicated by arrowheads. The protease portion possesses a histidine tag, and the scaffolding protein has a histidine-rich region, as shown. (e) Protein sequences of the 38-kDa protein resolved by 2D electrophoresis (indicated by hatched boxes) aligned with the scaffolding or assembly portion of the overlapping region of UL26 and UL26.5. aa, amino acids.
Primers were designed on the basis of sequence information obtained from the LETV partial genomic library to PCR amplify the open reading frame of the UL26 or gB gene. Total DNA from LETV-infected cells (100 ng) was added to a 50-μl PCR mixture containing 1.5 mM MgCl2, 0.4 mM deoxynucleoside triphosphate, 0.2 μM each primer, and 2 U of Proof start polymerase with supplied buffer (Qiagen, Valencia, Calif.). The PCR cycles were performed with a Perkin-Elmer Gene Amp 2400 thermal cycler under the following conditions: 2 min at 94°C, followed by 94°C for 10 s, 65°C for 30 s, and 72°C for 4 min for a total of 35 cycles. The resulting PCR products were gel purified (Roche Indianapolis, Ind.) and then T/A cloned into plasmid pTAdv (Clontech, Palo Alto, Calif.). Each clone of the appropriate size (gB gene, 2,598 bp; UL26, 1,743 bp) was sequenced and compared to other herpesvirus homologs in the database. Each clone was subcloned into Nde/Xho-linearized expression vector pET16b (Novagen, Madison, Wis.). The open reading frame of the gB gene was initially cloned in two parts (Fig. 2b) with primers 9 (CCG CCT TTA GAG CTG TAC GAC GAG AGC) and 6 (GCA GCA GCT CGT CCA GGT TAT TGA CG) and primers 5 (TGT TCG ACG GCG TGT ACA TGA TCT ACG) and 7 (GCA GAA CCT ATT CCG CGT CAT GAT GG). The genome organization matched that of an alphaherpesvirus and was consistent with the results of a phylogenetic analysis based on these genes (Fig. 2a). Once the entire sequence of the LETV gB gene was obtained (GenBank accession no. AY124577), primers (TCT CCT CGA ATT CTC ATA TGC GGG AGC GAT GGG CGT CGT GG and GAG AGG AGC GGC CGC TAC TCG AGT CAT ACC GGC GAC GTG TCG GAA AGT TCG) were designed to amplify the entire open reading frame for cloning and expression. Similarly, a region of the genome spanning UL26 was PCR amplified (GenBank accession no. AY124578) and primers (TCT CCT CGG ATC CGC ATA TGG CCG CCG AGA CGT CGT ACC CGG ACG and AGG AAT TCC TAC TCG AGT TAT TTC ATC ATC ATT TGC TTT ACA AAT TCG) were designed for cloning and expression of UL26.
The LETV UL26 and gB genes were expressed successfully in strain HMS174(DE3) cells (Novagen). Pellets from isopropyl-β-d-thiogalactopyranoside (IPTG)-induced and uninduced cell cultures were prepared and boiled, and the same amount of each sample was loaded onto a NuPage 10% Bis-Tris gel (Invitrogen) and run at 175 V in morpholinepropanesulfonic acid (MOPS) buffer. The gel was then incubated in colloidal blue stain or electroblotted onto nitrocellulose membrane at 30 V for 1 h. Nitrocellulose membranes were blocked with 1% alkali-soluble casein (Novagen) overnight at 4°C. The membrane was washed three times in Tris-buffered saline plus Tween 20 and incubated with monoclonal anti-His antibody (Novagen) at 0.2 μg/ml for 1 h with rocking. The membrane was washed and incubated with rabbit anti-mouse immunoglobulin G-alkaline phosphate (Sigma, St. Louis, Mo.) for 1 h at room temperature with rocking. The membrane was further developed as previously described (2). Expression of the LETV UL26 gene clone resulted in two proteins of 26 and 38 kDa (Fig. 3a, lane 1). On the basis of homologs of UL26 in other herpesviruses, this gene encodes a polyprotein that is autoproteolytically cleaved during expression and yields the assembly protein and protease (10, 18, 20). The two expressed LETV proteins likely correspond to these protein products. As diagrammed in Fig. 2d, it is hypothesized that the protease portion of the putative polyprotein maintained the His tag while, serendipitously, the assembly or scaffolding protein contained an internal His-rich region (HPHHHH) such that the monoclonal anti-His antibody recognized both products of the UL26 gene (Fig. 3a, lane 1). A band corresponding to the expected molecular weight of gB was also observed (Fig. 3b, lane 1).
FIG. 3.
Western blot analysis of expressed UL26 (a) and gB (b). In the odd-numbered lanes, lysates of induced E. coli were used as a source of antigen, and in the even-numbered lanes, lysates of the uninduced E. coli control were used as a source of antigen. Molecular weight markers were in lanes MW. Lanes 1 and 2 were incubated with anti-His monoclonal antibody to identify expression of cloned herpesvirus proteins. The LETV scaffolding protein (arrowhead) and protease were recognized by antihistidine antibody. Lanes 3 and 4 were incubated with 1-month post-LETV immunization plasma (a). Lanes 5 and 6 were incubated with plasma from a wild LETV antibody-positive green turtle shown previously to recognize the 38-kDa protein in LETV-infected cell lysates. The expressed LETV glycoprotein (arrowhead) was recognized by antihistidine antibody (b). Lanes 3 and 4 were incubated with 3-month post-LETV immunization plasma. Lanes 5 and 6 were incubated with plasma from an adult LETV antibody-positive green turtle. The values on the left are molecular sizes in kilodaltons.
The immunogenicity of the expressed proteins was evaluated by Western blot analysis. The induced and uninduced bacterial lysates were resolved and transferred to nitrocellulose membrane. Both UL26 and gB were expressed successfully, as detected by anti-His antibody. The antigenicity of the expressed proteins was evaluated by Western blot analysis with plasma from an LETV-immunized green turtle and a wild LETV antibody-positive green turtle. The blocked membrane was incubated with 1-month and 3-month post-LETV immunization plasma samples from green turtle 99A-1 (2) diluted 1:100 in 2% FBS/A or with wild green turtle plasma diluted 1:25 in 5% nonfat dried milk in Tris-buffered saline plus Tween 20 for 1 h with rocking at room temperature. The membrane was further developed as described previously (2). Plasma from the LETV-immunized green turtle was incubated with the UL26 expression products and recognized the 38-kDa protein specifically and not the 26-kDa protein (Fig. 3a, lane 3). This is consistent with the 2D gel electrophoresis study in which the fraction sequences were mapped to the putative assembly protein portion of the polyprotein (Fig. 2e). A wild LETV-positive green turtle plasma sample shown previously to recognize the 38-kDa protein in LETV-infected cell lysates (2) also recognized the E. coli-expressed 38-kDa protein (Fig. 3a, lane 5). This indicates that the expressed 38-kDa protein is a competent antigen for use in immunoassays. Many other proteins in the induced and uninduced preparations were also recognized, demonstrating a high level of background when an E. coli lysate is used as the antigen (Fig. 3a, lane 5 and 6). It was expected that green turtles would have antibodies to E. coli.
Plasma samples from an LETV-immunized green turtle and a wild LETV antibody-positive green turtle were also incubated with expressed LETV gB. The plasma from the LETV-immunized green turtle reacted strongly with a protein corresponding to the appropriate mass (approximately 96 kDa) of unglycosylated gB (Fig. 3b, lane 3). Furthermore, the antibodies in the plasma from the LETV-immunized turtle did not react with the uninduced E. coli lysate that lacked gB (Fig. 3b, lane 4). Interpretation of the reaction of the wild-turtle plasma with gB is more difficult. While a weak reaction with a protein corresponding to the appropriate molecular weight of gB was observed, there was a very high background level (Fig. 3b, lanes 5 and 6).
To determine the relationship of LETV to other herpesviruses, independent analyses were performed with the herpesvirus UL26, DNA polymerase (L. H. Herbst, R. L. Garber, L. Lockwood, and P. A. Klein, Proc. 16th Annu. Symp. Sea Turtle Biol. Conserv., NOAA Tech. Memo NMFS-SEFSC-412, p. 67, 1998) (GenBank accession no. AY124579), and gB genes. The amino acid sequences encoded by the herpesvirus UL26, DNA polymerase, and gB genes were obtained from the National Center for Biotechnology Information database. LETV sequences were obtained from the LETV partial genomic library or from LETV clones described above. Protein sequences were aligned by using the ClustalX analysis program at the website of the European Bioinformatics Institute (http://www2.ebi.ac.uk/clustalw/). Phylogenetic trees were generated by using the programs PROTDIST and FITCH (Fitch-Margoliash method) from the PHYLIP package (Phylogeny Inference Package; University of Washington, Seattle). The phylogenetic trees were constructed by using the cladogram output option and the program TreeView, version 1.6.6 (15). The percentages of amino acid sequence identity between the UL26, DNA polymerase, and gB genes of LETV and those of other herpesviruses were also determined. LETV UL26 was most similar to that of equine herpesvirus 1 (GenBank accession no. NC_001491), at 27.7% identity, and LETV gB was most similar to gallid herpesvirus 1 (GenBank accession no. D13713), at 34.34% identity. LETV DNA polymerase was most similar (approximately 54% identity) to other herpesviruses (GenBank accession no. AF035004, AF035005, and AF049904) associated with FP of several species of marine turtles. LETV consistently clustered with alphaherpesviruses in all of these analyses (data not shown).
Improved diagnostic assays are needed to detect exposure of endangered marine turtles to disease-associated herpesviruses. Recombinant herpesvirus proteins can serve as a valuable source of antigen for the development of immunodiagnostic assays in the absence of cultivated virus and as an alternative to virus-infected cell lysates for large-scale seroepidemiological studies. To our knowledge, these are not only the first proteins from a reptilian herpesvirus to be cloned and expressed, but they represent the first herpesvirus proteins to be identified as immunogenic in a reptilian host species.
Prior to this study, genomic sequence information for marine turtle herpesviruses was limited. The only genomic sequence information for LETV was a 181-bp portion of the herpesvirus DNA polymerase gene (Herbst et al., Proc. 16th Annu. Symp. Sea Turtle Biol. Conserv., NOAA Tech. Memo NMFS-SEFSC-412). To obtain additional sequence information, a partial LETV genomic library was constructed by using DNA obtained by pulsed-field gel electrophoresis. Pulsed-field gel electrophoresis is a useful tool for isolating genomic DNA from herpesviruses that are tightly cell associated and has been utilized for other herpesviruses, such as Marek's disease-associated herpesvirus (21). LETV genomic DNA was identified by pulsed-field gel electrophoresis and determined to be approximately 150 kb in size. This is within the range of the genome sizes of members of the Herpesviridae family (12). While this library only contained 18.7% of the genome, it provided enough sequence information for PCR amplification of genes of interest.
The entire coding sequence of the LETV gB gene was obtained and found to be similar to those of other alphaherpesviruses, with the greatest homology to that of gallid herpesvirus 1, at approximately 34% identity. gB is the most conserved herpesvirus glycoprotein, and the gene that encodes it is the representative gene routinely used for phylogenetic studies (12). The herpesvirus subfamily to which LETV belongs is undecided. However, the organization of the portion of the LETV genome containing the gB gene (Fig. 2a) supports the hypothesis that LETV is an alphaherpesvirus.
Immunogenic proteins of marine turtle herpesviruses have not been previously described. The 38-kDa protein recognized by antibodies of several marine turtles (Fig. 3a) (2, 3) was identified as a scaffolding protein encoded by the overlapping LETV UL26 and UL26.5 genes. Translation of the LETV UL26 message resulted in two products, which are presumed to be the result of autoproteolytic cleavage of the putative polyprotein, such as that which occurs in other herpesviruses (10, 18, 20). On the basis of what is known about other herpesviruses, the proteins are herpesvirus protease and scaffolding protein. The putative protease portion of this hypothetical polyprotein is well conserved, with approximately 25% identity with the human herpesvirus protease amino acid sequence, while the scaffolding protein is more poorly conserved (<19% homology).
Antibodies to histidine identified a protein of the appropriate molecular weight as expressed His-tagged unglycosylated gB (Fig. 3b, lane1). The plasma from the LETV-immunized green turtle reacted strongly with a protein identical in size in E. coli lysates (Fig. 3b, lane 3). This suggests that expressed gB is a competent antigen in this immunoassay format. However, the utility of this protein for use in the screening of herpesvirus-infected wild turtles is unresolved until a larger numbers of turtles can be tested. It is likely that a glycosylated form of this protein would be a better antigen for serologic tests.
Recombinant expressed UL26 and gB have been evaluated previously for development of serological assays in other species. The product of UL26 (minor capsid protein) has been previously used as the antigen in immunodiagnostic assays for the detection of exposure to other disease-associated herpesviruses (4). Specifically, peptides derived from UL26 (or ORF65) was used as the antigen in an ELISA for the detection of antibodies to Kaposi's sarcoma-associated herpesvirus (16). gB has also been used extensively for the development of serological assays (5, 19) and, like UL26, had the most diagnostic strength when used in combination with other herpesvirus proteins (4).
LETV is the only marine turtle herpesvirus to be maintained in culture, and as such, it can serve as a model for marine turtle herpesviruses. Phylogenetic analysis has demonstrated a closer evolutionary relationship between LETV and FP herpesvirus (GenBank accession no. AF035004) (54% amino acid identity) than other herpesviruses in the database. Furthermore, antibodies to FP herpesvirus cross-react with LETV, suggesting a level of conservation of viral proteins between these two viruses (F. C. Origgi, E. R. Jacobson, L. H. Herbst, P. A. Klein, and S. S. Curry, Proc. 20th Annu. Symp. Sea Turtle Biol. Conserv., U.S. Dept. Commerce, NOAA Tech. Memo NMFS-SEFSC, abstr., in press). On the basis of these findings, the assumption is that many of the immunodominant proteins of marine turtle herpesviruses are likely to be conserved. Seroepidemiological studies using these recombinant antigens will help determine the role of these herpesviruses in their respective diseases.
Acknowledgments
We thank Susanna Lamers of Gene Genie Co. (Thibodaux, La.) and Maureen Goodenow of the University of Florida (Gainesville) for assistance with the phylogenetic analysis and construction of the phylogenetic trees.
This research was supported by research grants RWO 194 and RWO 213 from the U.S. Fish and Wildlife Service, Department of the Interior, administered by the Cooperative Fish and Wildlife Unit, University of Florida, Gainesville.
REFERENCES
- 1.Caselli, E., P. G. Balboni, C. Incorvaia, R. Argnani, F. Parmeggiani, E. Cassai, and R. Manservigi. 2001. Local and systemic inoculation of DNA or protein gB1s-based vaccines induce a protective immunity against rabbit ocular HSV-1 infection. Vaccine 19:1225-1231. [DOI] [PubMed] [Google Scholar]
- 2.Coberley, S. S., L. H. Herbst, D. R. Brown, L. M. Ehrhart, D. A. Bagley, S. A. Schaf, R. H. Moretti, E. R. Jacobson, and P. A. Klein. 2001. Detection of antibodies to a disease-associated herpesvirus of the green turtle, Chelonia mydas. J. Clin. Microbiol. 39:3572-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coberley, S. S., L. H. Herbst, L. M. Ehrhart, D. A. Bagley, S. Hirama, E. R. Jacobson, and P. A. Klein. 2001. Survey of Florida green turtles for exposure to a disease-associated herpesvirus. Dis. Aquat. Org. 47(3):157-167. [DOI] [PubMed] [Google Scholar]
- 4.Corchero, J. L., E. C. Mar, T. J. Spira, P. E. Pellet, and N. Inque. 2001. Comparison of serological assays for detection of antibodies against human herpesvirus 8. Clin. Diagn. Lab. Immunol. 8(5):913-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggers, M., K. Radsak, G. Enders, and M. Reschke. 2001. Use of recombinant glycoprotein antigens gB and gH for diagnosis of primary human cytomegalovirus infection during pregnancy. J. Med. Virol. 63:135-142. [PubMed] [Google Scholar]
- 6.Franti, M., J. T. Aubin, G. De Saint-Maur, H. Kosuge, K. Yamanishi, A. Gautheret-Dejean, A. Garbarg-Chenon, J. M. Huraux, and H. Agut. 2002. Immune reactivity of human sera to the glycoprotein B of human herpesvirus 7. J. Clin. Microbiol. 40:44-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao, Y., C. Wang, and G. A. Splitter. 1999. Mapping T and B lymphocyte epitopes of bovine herpesvirus-1 glycoprotein B. J. Gen. Virol. 80(10):2699-2704. [DOI] [PubMed] [Google Scholar]
- 8.Glazebrook, J. S., R. S. Campbell, and A. T. Thomas. 1993. Studies on an ulcerative stomatitis-obstructive rhinitis-pneumonia disease complex in hatchling and juvenile sea turtles, Chelonia mydas and Caretta caretta. Dis. Aquat. Org. 16:133-147. [Google Scholar]
- 9.Herbst, L. H., and P. A. Klein. 1995. Monoclonal antibodies for the measurement of class specific antibody responses in the green turtle, Chelonia mydas. Vet. Immunol. Immunopathol. 46:317-335. [DOI] [PubMed] [Google Scholar]
- 10.Hoog, S. S., W. W. Smith, X. Qiu, C. A. Janson, B. Hellmig, M. S. McQueney, K. O'Donnell, D. O'Shannessy, A. G. DiLella, C. Debouck, and S. S. Abdel-Meguid. 1997. Active site cavity of herpesvirus proteases revealed by the crystal structure of herpes simplex virus protease/inhibitor complex. Biochemistry 36:14023-14029. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson, E. R., J. M. Gaskin, M. Roelke, E. Greiner, and J. Allen. 1986. Conjunctivitis, tracheitis, and pneumonia associated with herpesvirus infection of green sea turtles. J. Am. Vet. Med. Assoc. 189:1020-1023. [PubMed] [Google Scholar]
- 12.Knipe, D. M., and P. M. Howley. 2001. Fields virology, 4th edition. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 13.Kutinova, L., P. Hainz, V. Ludvikova, L. Maresova, and S. Nemeckova. 2001. Immune response to vaccinia virus recombinants expressing glycoproteins gE, gB, gH, and gL of varicella-zoster virus. Virology 280(2):211-220. [DOI] [PubMed] [Google Scholar]
- 14.Loomis-Huff, J. E., R. Eberle, K. M. Lockridge, G. Rhodes, and P. A. Barry. 2001. Immunogenicity of a DNA vaccine against herpes B virus in mice and rhesus macaque. Vaccine 19:4865-4873. [DOI] [PubMed] [Google Scholar]
- 15.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 16.Pau, C. P., L. L. Lam, T. J. Spira, J. B. Black, J. A. Stewart, P. E. Pellett, and R. A. Respess. 1998. Mapping and serodiagnostic application of a dominant epitope within the human herpesvirus 8 ORF 65-encoded protein. J. Clin. Microbiol. 36(6):1574-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira, L. 1994. Function of glycoprotein B homologues of the family Herpesviridae. Infect. Agents Dis. 3(1):9-28. [PubMed] [Google Scholar]
- 18.Sheaffer, A. K., W. W. Newcomb, J. C. Brown, M. Gao, S. K. Weller, amd D. J. Tenney. 2000. Evidence for controlled incorporation of herpes simplex virus type 1 UL26 protease into capsids. J. Virol. 74:6838-6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speckner, A., B. Kropff, S. Knor, and M. Mach. 2000. The antigenic domain 1 of human cytomegalovirus glycoprotein B contains an intramolecular disulphide bond. J. Gen. Virol. 81:2659-2663. [DOI] [PubMed] [Google Scholar]
- 20.Tigue, N. J., P. J. Matharu, N. A. Roberts, J. S. Mills, J. Kay, and R. Jupp. 1996. Cloning, expression, and characterization of the proteinase from human herpesvirus 6. J. Virol. 70:4136-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson, M. R., and P. M. Coussens. 1991. Purification and characterization of infectious Marek's disease virus genomes using pulse field electrophoresis. Virology 185:673-680. [DOI] [PubMed] [Google Scholar]