Abstract
The human papillomavirus (HPV) type 16 E7 oncoprotein must inactivate the retinoblastoma tumor suppressor (Rb) pathway to bypass G1 arrest. However, E7 C-terminal mutants that were able to inactivate Rb were unable to bypass DNA damage-induced G1 arrest and keratinocyte senescence, suggesting that the E7 C terminus may target additional G1 regulators. The E7 C-terminal mutant proteins E7 CVQ68-70AAA and E7 Δ79-83 (deletion of positions 79 through 83) were further tested in several models of cell cycle arrest associated with elevated levels of p21. C-terminal mutations rendered E7 unable to induce S phase and endoreduplication in differentiated keratinocytes and rendered it less efficient in delaying senescence of human mammary epithelial cells. Interestingly, when cell cycle arrest was induced with a peptide form of p21, the E7 C-terminal mutants were deficient in overcoming arrest, whereas a mutant defective in Rb binding was competent in inhibiting G1 arrest. These results suggest that the inactivation of both p21 and Rb by E7 contributes to subversion of cell cycle control in normal human epithelia but that neither p21 nor Rb inactivation alone is sufficient.
Human papillomavirus (HPV) replication requires factors provided by differentiating epithelial cells but also requires the host cell's DNA synthesis machinery; thus, HPV must induce S phase in cells that would otherwise exit the cell cycle (57). The E7 oncoprotein of HPV type 16 induces S phase in differentiated cells and in other situations of cell cycle arrest, dependent in part upon E7-mediated inactivation of the retinoblastoma tumor suppressor (Rb). To inactivate Rb fully, E7 prevents Rb from binding to the E2F family of transcription factors (10, 55) and targets Rb for degradation (5, 25, 28, 32, 33). Important for Rb destabilization are conserved region 1 (CR1) and an LXCXE Rb-binding motif in conserved region 2 (CR2), both located in the N terminus of E7 (5, 25, 28, 32, 33).
Both Rb and the cyclin-dependent kinase (CDK) inhibitor p21 prevent S phase in differentiated cells (2, 16, 30, 31, 38, 39). Rb binds to the E2F family of transcription factors in G1 and represses transcription of E2F-controlled S-phase genes (19), while p21 inhibits the activity of G1 and S-phase cyclin/CDK complexes important for cell cycle progression (50). Motifs in the N- and C-terminal halves of p21 can each inhibit CDK activity and arrest cells in G1 (1, 3, 7, 11). While substoichiometric amounts of p21 do not inhibit cyclin/CDK complexes, increased levels of p21 associated, for instance, with differentiation (29), senescence (42), or DNA damage (20) are inhibitory (27, 56). The C terminus of p21 also binds the DNA polymerase δ processivity factor PCNA and inhibits PCNA-dependent DNA synthesis (53); however, the CDK inhibitory function is responsible for inducing G1 arrest (11, 43). E7 restores p21-inhibited CDK activity (23, 31), and the C terminus of E7 is sufficient for p21 inactivation in vitro (23). Furthermore, E7 and p21 interact in cells (23, 31), and it has been proposed that E7 may induce S phase in differentiated cells by inactivating p21 (31).
Propagation of human mammary epithelial cells (HMECs) in culture provides a model of epithelial senescence where the role of Rb can be temporally separated from other pathways of cell cycle arrest (21, 46). The first block, termed M0 (mortality stage 0) or selection, occurs after several passages in culture and is characterized by the presence of large, flat cells (21, 51). A role for Rb in imposing M0 lies in the observations that E7-expressing HMECs bypass M0 (21) depending upon the integrity of CR1 and the LXCXE motif (22) and that HMECs can spontaneously escape M0 by repressing expression of p16INK4A (9, 22), a CDK inhibitor that blocks phosphorylation of Rb (49). The subsequent proliferation arrest, known as M1 or senescence (48, 51), is associated with telomere attrition (37, 48), which triggers a p53-dependent DNA damage response (14). Moreover, increased levels of p53 and p21 have been observed in HMECs approaching M1 (22) and HMECs expressing a dominant negative p53 fail to arrest at M1 (24). Expression of either the HPV 16 E6 oncoprotein (4, 48) or the catalytic subunit of telomerase, hTERT (37), allows HMECs to bypass M1. E7 can extend the life span of HMECs but not beyond M1 (4, 48). Instead, the aging E7-expressing cells begin to grow in tight clumps and eventually cease proliferating (22). Whether the C terminus of E7 contributes to HMEC life span extension has not been explored.
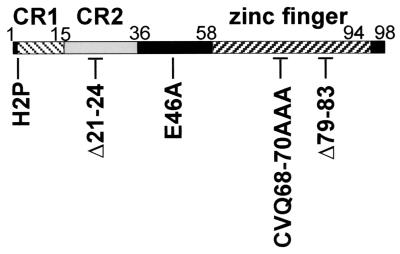
Mutations within the C-terminal zinc finger of E7 separate Rb destabilization from the bypass of DNA damage checkpoints and keratinocyte senescence (28). The objectives of the present study were to further characterize the importance of the E7 C terminus in subverting epithelial cell cycle control and to test the hypothesis that C-terminal mutations prevent E7 from inactivating p21. The E7 mutant proteins tested in these studies included those that target Rb (E7 E46A, E7 CVQ68-70AAA, and E7 Δ79-83 [with a deletion of positions 79 through 83]) (28) and those that do not target Rb (E7 H2P and E7 Δ21-24) (16, 25, 28, 32). The locations of the E7 mutations are indicated in Fig. 1.
FIG. 1.
E7 schematic showing the locations of substitution mutations (e.g., H2P, histidine at position 2 replaced with proline) and deletion mutations (e.g., Δ21-24).
C-terminal mutations prevent E7 from inducing S phase in differentiated keratinocytes.
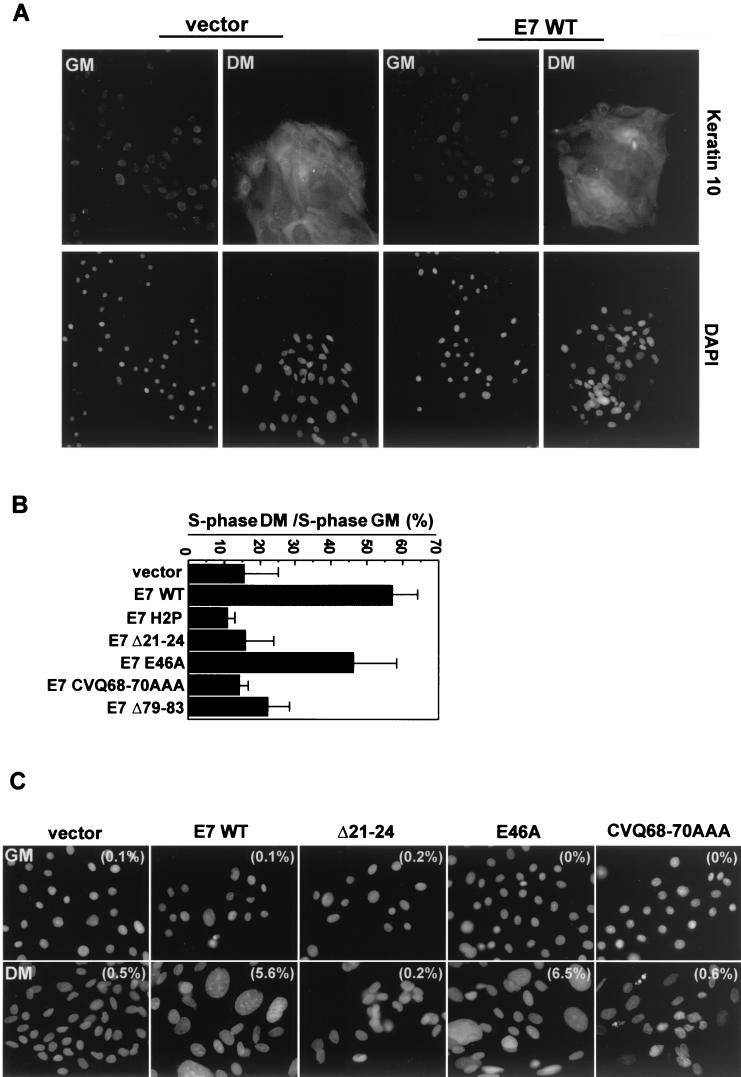
Both Rb and p21 have been implicated in differentiation-induced cell cycle arrest (2, 16, 31, 38, 39); thus, we wished to determine whether E7 activities in addition to Rb inactivation were required for E7 to induce S phase in differentiated human foreskin keratinocytes (HFKs). HFKs expressing wild-type or mutated E7 proteins were generated by retroviral transduction as in earlier studies (16, 26, 28). Cells were induced to differentiate by growth in medium containing 10% calf serum and 2 mM calcium (44, 47). Differentiation was confirmed by immunofluorescence-based detection of keratin 10, an early marker of HFK differentiation. HFKs grown in differentiation medium had elevated keratin 10 levels, regardless of E7 expression (Fig. 2A and data not shown), in accordance with previous reports that E7 alone does not prevent differentiation (6, 12, 16).
FIG.2.
Uncoupling of cell cycle arrest and differentiation by E7. (A) Keratin 10 expression in differentiated HFKs. The upper panels show keratin 10 labeling with an anti-keratin 10 antibody (DE-K10; Neomarkers), and the lower panels show nuclei counterstained with DAPI (Sigma). Images were collected with a 20× objective on a Nikon Eclipse TE300 microscope equipped with a charge-coupled-device camera (Roper Scientific) and Metamorph software (Universal Imaging Co.). GM, growth medium; DM, differentiation medium. (B) S-phase induction in differentiated HFKs. The S-phase populations are graphed normalized to populations of undifferentiated cells. Shown is a summary of the results of three experiments. Error bars represent standard deviations. (Raw data are presented in Table 1). (C) Giant nuclei in E7-expressing, differentiated HFKs. Images of DAPI-stained nuclei were collected at the same magnification with the equipment described for panel A. Examples are shown, with the percentage of cells with giant nuclei (of 300 cells counted) indicated in parentheses on each image. Nuclei were considered giant if their area was at least four times greater than the average size of nuclei in vector-expressing cells.
Growth for 5 days in differentiation medium resulted in an 80 to 90% reduction in the S-phase population of HFKs expressing empty vector, E7 H2P, or E7 Δ21-24 (Table 1 and Fig. 2B), consistent with the behavior of these mutants in organotypic cultures of HFKs (16). In contrast, the S-phase fraction of differentiated HFKs expressing wild type E7 (E7 WT) or E7 E46A was approximately threefold greater than that of vector-expressing cells (Table 1 and Fig. 2B). Neither E7 CVQ68-70AAA nor E7 Δ79-83 was significantly better than empty vector at inducing S phase in differentiated HFKs (Table 1 and Fig. 2B), despite the fact that these mutant E7s inactivate Rb (28). Cells expressing E7 CVQ68-70AAA had a more pronounced G1 arrest than cells expressing E7 Δ79-83, which accumulated in both G1 and G2 (Table 1), suggesting that the two E7 C-terminal mutations may have subtle differences. Both E7 CVQ68-70AAA and E7 Δ79-83 were expressed at somewhat reduced levels compared to that of E7 WT in a previous study (28); however, increasing their expression levels by using the pBABE retroviral vector did not restore the ability to induce S phase in differentiated HFKs (data not shown).
TABLE 1.
Cell cycle analysis of differentiated keratinocytes
| Vector or protein transduced | % of cells ina:
|
|||||
|---|---|---|---|---|---|---|
| G1
|
S
|
G2/M
|
||||
| GM | DM | GM | DM | GM | DM | |
| Vector | 48.6 ± 7.8 | 71.5 ± 11.4 | 25.4 ± 4.0 | 3.9 ± 2.1 | 26.0 ± 3.5 | 22.6 ± 10.9 |
| E7 WT | 50.6 ± 4.4 | 54.4 ± 5.0 | 21.4 ± 5.1 | 12.6 ± 3.5 | 27.0 ± 2.0 | 31.2 ± 7.7 |
| E7 H2P | 48.1 ± 2.2 | 69.9 ± 2.2 | 26.7 ± 1.5 | 2.9 ± 0.2 | 22.2 ± 0.4 | 23.8 ± 0.9 |
| E7 Δ21-24 | 44.3 ± 4.2 | 65.7 ± 9.3 | 25.2 ± 6.9 | 4.0 ± 1.2 | 29.0 ± 4.0 | 28.5 ± 8.9 |
| E7 E46A | 47.9 ± 2.7 | 44.3 ± 10.4 | 23.0 ± 3.4 | 10.6 ± 1.4 | 28.1 ± 3.5 | 46.5 ± 12.4 |
| E7 CVQ68-70AAA | 45.3 ± 0.0 | 65.7 ± 9.5 | 26.3 ± 5.1 | 3.5 ± 0.9 | 26.0 ± 3.9 | 28.0 ± 10.3 |
| E7 Δ79-83 | 52.0 ± 6.5 | 51.3 ± 1.0 | 25.4 ± 6.6 | 5.8 ± 3.0 | 20.8 ± 0.3 | 39.6 ± 0.2 |
Numbers are percentages of cells expressing vector or protein in the indicated phase of the cell cycle and medium determined as described in Materials and Methods. The percentages are averages of values from three experiments ± standard deviations. GM, keratinocyte growth medium; DM, keratinocyte differentiation medium.
Interestingly, Rb and p21 have each been implicated in the control of endoreduplication (30, 35, 52, 54), and E7 WT induces endoreduplication in differentiated keratinocytes (13). While analysis of keratin 10 was being carried out, grossly enlarged, DAPI (4′,6′-diamidino-2-phenylindole)-stained nuclei were noticed in differentiated HFKs expressing E7 WT or E7 E46A (Fig. 2C). Approximately 6% of the cells expressing E7 WT or E7 E46A had giant nuclei, while the frequency was less than 1% in differentiated populations expressing vector, E7 H2P, E7 Δ21-24, or E7 CVQ68-70AAA, and in HFKs maintained in growth medium (Fig. 2C). Together, these data suggest that the ability of E7 to inactivate Rb is not sufficient for induction of S phase and endoreduplication in differentiated HFKs and are consistent with the hypothesis that the C terminus of E7 must target additional G1 regulators.
E7 does not require an intact C terminus to bypass M0 in HMECs.
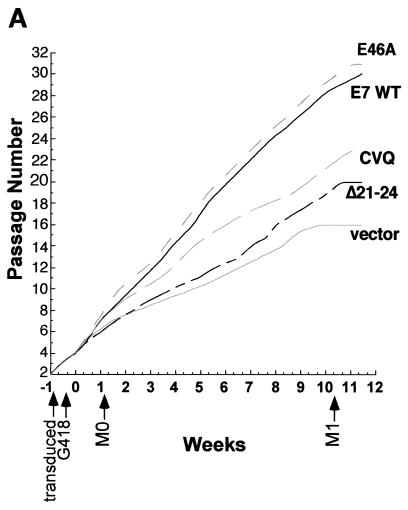
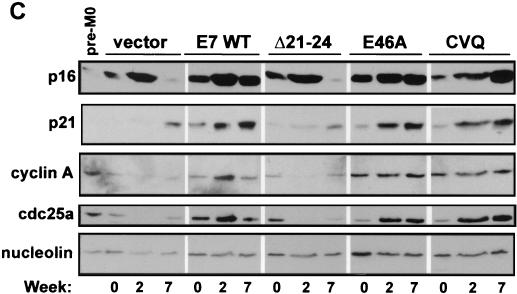
While E7 mutants that do not target Rb do not bypass M0 (22), the importance of the E7 C terminus in bypassing M0 has not been tested. To address whether the E7 C terminus has a role, pre-M0 cells were transduced with retroviral vectors expressing wild-type or mutated E7 proteins (21, 22). Cells were passaged at a 1:3 ratio until the vector control cells began to senesce at the M1 stage (around 11 weeks after G418 selection). Cell lysates and micrographs were collected periodically to evaluate the ability of E7 mutants to bypass M0 and prolong the HMEC life span. Entry into M0 was defined by cell morphology, a reduction in proliferation rate, and by the expression levels of p16 and cyclin A (9, 21, 22, 51). HMECs expressing vector or E7 Δ21-24 entered M0 within the first week after G418 selection, exhibiting reduced proliferation relative to that of HMECs expressing E7 WT (Fig. 3A), characteristic flattened cell morphology (Fig. 3B), and decreased cyclin A and increased p16 levels compared to levels in untransduced pre-M0 cells (Fig. 3C). As previously shown (21, 22), p21 levels were low or undetectable in M0 cells (Fig. 3C).
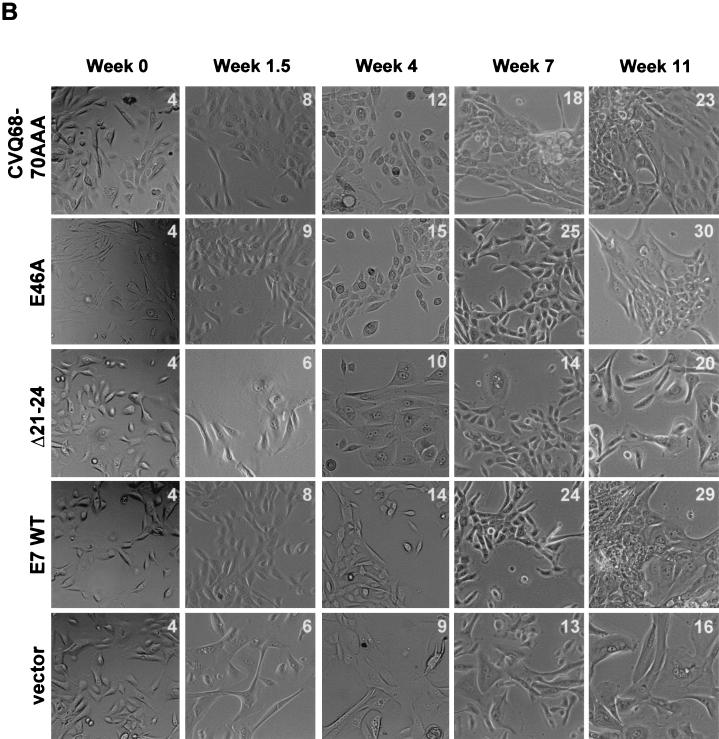
FIG. 3.
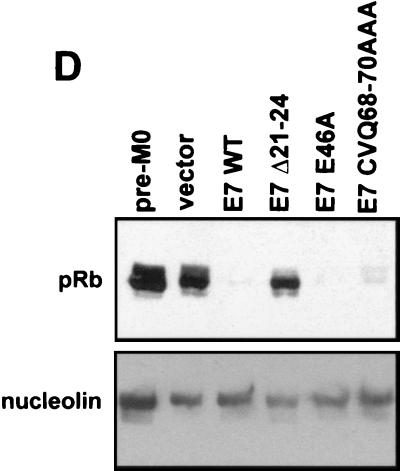
Induction of HMEC proliferation by E7. (A) HMEC passage number relative to time in culture. Times of transduction and G418 selection are indicated. The first passage after drug selection (passage 4) was designated week 0. (B) Micrographs showing HMEC morphologies at different time points. The passage number is indicated on each image. (C) Western blots probed with anti-p16 (Pharmingen), anti-p21 (Ab-2; Calbiochem), anti-cyclin A (BF-683; Pharmingen), anti-cdc25a (F-6; Santa Cruz Biotechnology), and antinucleolin (as a loading control, C23; Santa Cruz Biotechnology). (D) Western blot probed with an anti-Rb antibody (G3-245; Pharmingen) comparing Rb levels in E7-expressing HMECs with that of untransduced, pre-M0 HMECs (first lane).
In contrast, HMECs expressing E7 WT, E7 E46A, or E7 CVQ68-70AAA did not enter M0. The morphology of cells expressing either E7 CVQ68-70AAA or E7 E46A was similar to that of cells expressing E7 WT (Fig. 3B, weeks 0 to 4), and all three populations expressed cyclin A and cdc25a (Fig. 3C). Cyclin A and cdc25a are E2F-responsive genes (15, 34), and thus the increased levels may be due to a combination of cell proliferation and Rb inactivation. Cells expressing E7 WT, E7 E46A, or E7 CVQ68-70AAA had reduced levels of pRb (Fig. 3D) and elevated levels of p16 (Fig. 3C) compared to untransduced pre-M0 cells. p16 levels remained elevated throughout the experiment (Fig. 3D and data not shown), consistent with observations that p16 expression is not selected against in cells lacking Rb function (36). As expected, p21 levels were also increased in response to E7 expression (31, 45) in a manner dependent upon CR1 and CR2 (33). These data show that the activity disrupted in E7 CVQ68-70AAA is not required for bypass of M0 and that, as previously shown (22), the ability of E7 to bypass M0 requires targeting of Rb.
The E7 C terminus contributes to the ability of E7 to delay M1 in HMECs.
After several weeks, the proliferation of HMECs expressing E7 CVQ68-70AAA noticeably slowed relative to that of HMECs expressing E7 WT (Fig. 3A), though cell morphology (Fig. 3B) and cyclin A levels were similar to those of E7 WT-expressing cells (Fig. 3C and data not shown). In contrast, E7 E46A was as efficient as E7 WT in driving HMEC proliferation throughout the experiment (Fig. 3A). Decreasing levels of cdc25a have been associated with the approach to M1 (46); however, the reduced proliferation of E7 CVQ68-70AAA-expressing HMECs did not correlate with reduced levels of cdc25a (Fig. 3C and data not shown). HMECs expressing E7 were previously shown to grow in tightly packed groups as they neared M1 (22). HMECs expressing E7 WT, E7 E46A, or E7 CVQ68-70AAA eventually grew in tightly packed groups as they aged (Fig. 3B). However, this occurred approximately 11 passages and 3 weeks earlier in HMECs expressing E7 CVQ68-70AAA than in E7 WT-expressing cells (around passage 15 for E7 CVQ68-70AAA and passage 26 for E7 WT) (Fig. 3B and data not shown). Large, flat cells became apparent in all three populations as they approached M1. This also occurred at an earlier passage for HMECs expressing E7 CVQ68-70AAA (around passage 18 versus passage 29 for E7 WT) (Fig. 3B and data not shown). At the end of the experiment, HMECs expressing E7 WT, E7 E46A, and E7 CVQ68-70AAA were at passages 30, 31, and 23, respectively (Fig. 3A). Thus, E7 CVQ68-70AAA can destabilize Rb and bypass M0 in HMECs but is less efficient than E7 WT in postponing M1.
HMECs expressing vector or E7 Δ21-24 escaped M0 by downregulating p16 expression (Fig. 3C, week 7, and data not shown) and proliferated for a number of passages before both populations again were dominated by large, flat cells (Fig. 3B). Both post-M0 cultures had increased levels of p21 compared to those of their M0 predecessors and the untransduced, pre-M0 cells (Fig. 3C and data not shown). At the end of the experiment, HMECs expressing vector and E7 Δ21-24 were at passages 16 and 20, respectively (Fig. 3A). Together, these data suggest that both Rb-related and Rb-independent activities of E7, separated temporally in HMECs, contribute to the extension of the HMEC life span.
E7 bypasses G1 arrest of HFKs imposed by a p21-derived peptide.
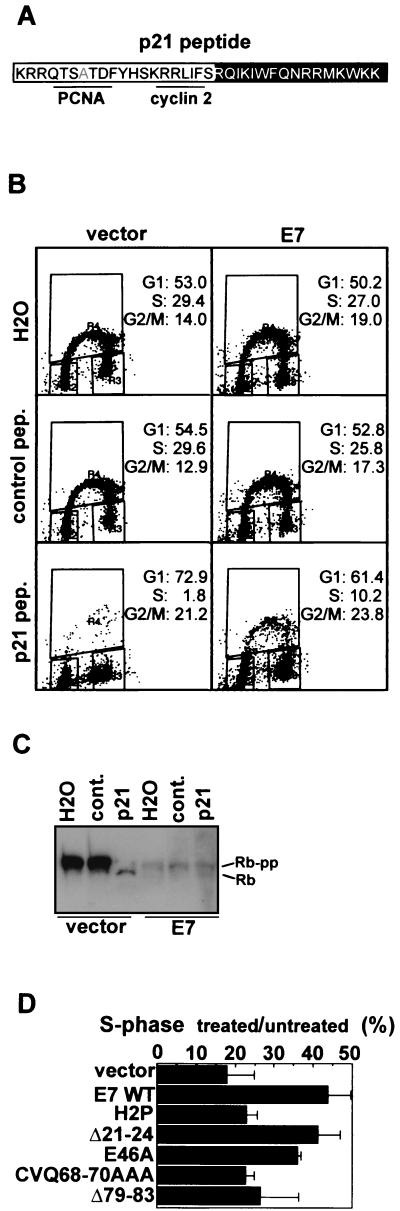
E7 inactivates p21 in vitro (23, 31) depending on an interaction with the C terminus of p21 (23). To determine more directly whether mutations in the E7 C terminus affect the ability of E7 to bypass p21-mediated cell cycle arrest, we employed a previously characterized p21-derived peptide that contains the C-terminal 20 amino acids of p21 fused to an internalization peptide derived from the Antennapedia protein of Drosophila melanogaster (Fig. 4A) (3, 7, 17). This region of p21, which contains the second cyclin binding site and the PCNA binding site of p21, is sufficient to inhibit G1/S-phase CDK activity and arrest cells in G1 (1, 3, 11). The PCNA binding site was mutated to focus on the CDK-inhibitory function of p21, which is responsible for blocking S-phase entry (50). This assay was employed because it allows the control of p21 levels to be independent of other effects induced by either DNA damage or differentiation, because it allows efficient uptake into primary cells (3, 7, 17), and because the region of p21 contained in the peptide was shown by mutagenesis studies to be sufficient for E7 binding (reference 23 and data not shown).
FIG. 4.
Bypass of p21 peptide-induced G1 arrest. (A) Schematic of the p21 peptide. The p21 portion is boxed in white, and the internalization domain is boxed in black. The PCNA site is mutated (indicated by the gray A) such that only the cyclin/CDK-inhibitory site (labeled cyclin 2) is active. (B) Cell cycle profiles of HFKs treated with p21 peptide, with scrambled control peptide, or with water. pep., peptide. (C) Western blot showing Rb phosphorylation state in p21 peptide- or control-treated HFKs. cont., control peptide. (D) S-phase induction inp21 peptide-treated HFKs expressing E7 mutants. The S-phase fraction of peptide-treated HFKs expressing vector, E7 WT, or mutated E7 proteins was measured and graphed normalized to those of water-treated controls. Shown is a summary of the results of three experiments (raw data are presented in Table 2). Error bars represent standard deviations.
HFKs expressing vector or E7 WT were incubated for 24 h either with 10 μg of p21 peptide per ml, with a control peptide containing scrambled p21 sequence fused to the internalization peptide, or with no peptide. Cell cycle profiles were obtained as previously described (28). The phosphorylation state of Rb, a G1 CDK substrate, was assessed via Western blotting of lysates prepared from peptide-treated HFKs. The control peptide did not affect cell cycle progression (Fig. 4B) or Rb phosphorylation state (Fig. 4C) in vector- or E7-expressing HFKs. Treatment with p21 peptide resulted in a pronounced G1 arrest in vector-expressing cells, and the S-phase fraction was reduced from 29 to approximately 2% (Fig. 4B). In contrast, E7 was more efficient than vector at inducing S phase following peptide treatment, with S-phase fractions of 26 and 10% in the control- and p21-treated HFKs, respectively. Rb was hypophosphorylated in the vector-expressing cells following p21 peptide treatment (Fig. 4C). In contrast, both hyperphosphorylated and hypophosphorylated Rb were detectable in the E7-expressing cells (Fig. 4C). The finding that E7 inhibits p21 peptide-imposed G1 arrest in HFKs extends previous reports that E7 restores p21-inhibited CDK activity (23, 31). E7 did not completely reverse the p21 peptide-imposed G1 arrest. However, p21 may have other cellular roles, for instance during differentiation, that are beyond the imposition of cell cycle arrest (18, 40). Therefore, as HPV replication requires host cell differentiation, it may be detrimental if E7 totally eliminates p21 function.
The integrity of CR1 and the C terminus of E7 are required for overriding p21 peptide-mediated arrest.
The C-terminal half of E7 was sufficient for p21 inactivation in vitro (23), but there was evidence that multiple regions of E7 may contribute to the inactivation (23, 31). To determine whether the failure of E7 CVQ68-70AAA and E7 Δ79-83 to bypass cell cycle arrest (28; present study) is associated with a failure to inactivate p21, HFKs expressing these E7 mutants were treated with p21 peptide. CR1 and LXCXE mutants were tested as well. E7 WT and E7 E46A reproducibly induced S phase following peptide treatment (Fig. 4D and Table 2). In contrast, E7 CVQ68-70AAA and, unexpectedly, E7 H2P were not significantly better than vector at inducing S phase (Fig. 4D and Table 2). Results with E7 Δ79-83 were variable, suggesting again that E7 CVQ68-70AAA and E7 Δ79-83 may not be functionally equivalent. Interestingly, E7 Δ21-24 induced S phase following peptide treatment (Fig. 4D and Table 2), suggesting that the targeting of Rb and p21 by E7 are genetically separable activities. Thus, CR1 and the LXCXE motif are important for efficient Rb inactivation (25, 28) while CR1 and the C terminus are important for targeting p21.
TABLE 2.
Cell cycle analysis of keratinocytes treated with p21 peptides
| Vector or protein transduced | % of cells with indicated peptide concn (μg/ml) ina:
|
|||||
|---|---|---|---|---|---|---|
| G1
|
S
|
G2/M
|
||||
| 0 | 10 | 0 | 10 | 0 | 10 | |
| Empty vector | 54.1 ± 2.1 | 70.9 ± 1.2 | 22.9 ± 3.6 | 3.9 ± 1.3 | 20.8 ± 4.0 | 21.7 ± 2.5 |
| E7 WT | 51.1 ± 4.4 | 64.0 ± 2.1 | 24.4 ± 2.7 | 10.1 ± 1.3 | 22.0 ± 3.0 | 21.3 ± 3.5 |
| E7 H2P | 53.8 ± 4.1 | 76.6 ± 0.2 | 23.0 ± 0.7 | 5.2 ± 0.5 | 20.9 ± 1.5 | 16.7 ± 2.3 |
| E7 Δ21-24 | 53.0 ± 2.9 | 64.3 ± 3.6 | 21.3 ± 3.2 | 8.8 ± 2.2 | 23.1 ± 1.3 | 21.4 ± 2.1 |
| E7 E46A | 46.1 ± 2.5 | 67.9 ± 4.6 | 26.7 ± 1.9 | 9.6 ± 0.4 | 24.6 ± 0.9 | 20.3 ± 2.0 |
| E7 CVQ68-70AAA | 48.3 ± 2.5 | 70.3 ± 5.9 | 25.1 ± 2.6 | 5.6 ± 0.8 | 24.2 ± 2.5 | 22.0 ± 5.1 |
| E7 Δ79-83 | 54.1 ± 3.6 | 76.3 ± 1.0 | 24.3 ± 1.3 | 6.4 ± 2.7 | 19.5 ± 1.9 | 16.0 ± 0.3 |
Numbers are percentages of cells expressing vector or protein in the indicated phase of the cell cycle and with the indicated peptide concentration determined as described in Materials and Methods. The percentages are averages of values from three experiments ± standard deviations.
E7 mutants that do not bypass p21 peptide-imposed arrest are not deficient in p21 binding.
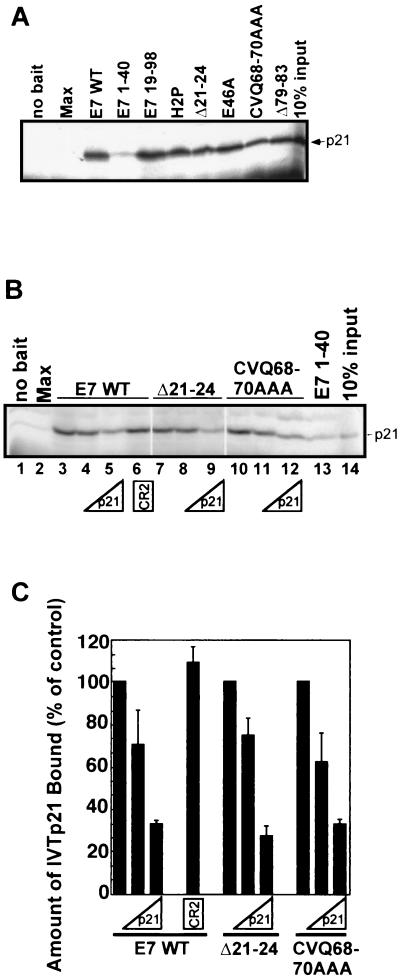
In vitro binding assays were carried out with recombinant E7 and control proteins and 35S-labeled, in vitro-translated p21 (IVT-p21) to determine whether the failure of E7 mutants to override p21 peptide-imposed arrest correlated with a lack of p21 binding. All full-length E7 proteins tested could bind IVT-p21 (Fig. 5A). A truncated form of E7 lacking CR1 (E7 19-98) bound as well as E7 WT, while an N-terminal fragment of E7 (E7 1-40) was severely deficient in binding (Fig. 5A). A control protein, Max, did not bind p21 (Fig. 5A). We carried out competitive binding assays in which p21 peptide or a peptide containing the CR2 portion of E7 was added to the mix of recombinant bait proteins and IVT-p21. The addition of p21 peptide reduced the binding of all three E7 proteins tested (E7 WT, E7 Δ21-24, and E7 CVQ68-70AAA), while the CR2 peptide had no effect (Fig. 5B and C), confirming previous results indicating that binding of E7 to p21 is mediated primarily by the E7 C terminus (23). That E7 mutants unable to bypass p21 peptide-imposed arrest could still bind p21 suggests, however, that the mechanism of p21 inactivation may be more complicated than was previously thought (23, 31). In addition, it is possible that CR1 or C-terminal mutations alter the nature of the p21-E7 interaction such that p21 is not inhibited.
FIG. 5.
Interaction between E7 and p21. (A) Autoradiograph showing binding of 35S-IVT-p21 to recombinant His-tagged E7 proteins. (B and C) Competitive binding experiments showing that E7 WT, E7 Δ21-24, and E7 CVQ68-70AAA bind p21 with similar avidities. (B) Reactions were carried out as described for panel A but with 0 μM (lanes 1 to 3, 7, 10, and 13), 0.1 μM (lanes 4, 8, and 11), or 1 μM (lanes 5, 9, and 12) p21 peptide or 1 μM E7 CR2 peptide (lane 6) included in the reaction mixtures. Shown are the results of a representative experiment (B) and a summary of two experiments (C), where the amount of p21 remaining bound to the E7 proteins was measured via phosphorimager analysis and graphed relative to the amount of p21 bound in the absence of competitor. Error bars represent standard deviations.
The E7 C terminus contributes to the ability of E7 to maintain CDK2 activity in differentiated HFKs.
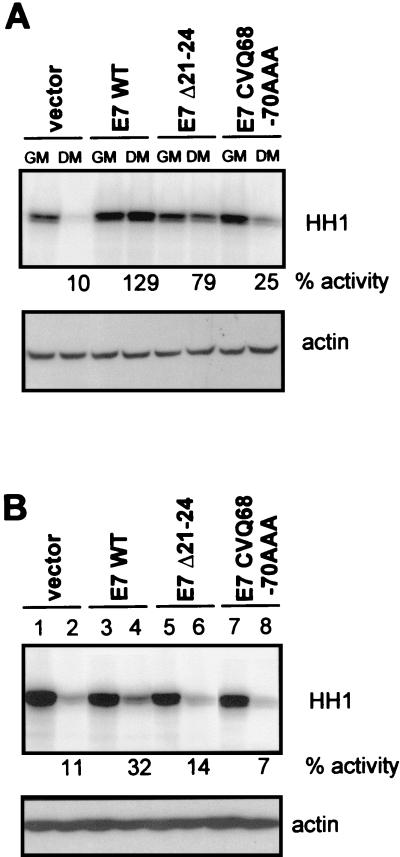
E7 was previously shown to maintain CDK2 activity in HFKs 24 and 72 h after the cells were induced to differentiate, despite the presence of elevated levels of p21 (31). To further examine the importance of the E7 C terminus in antagonizing p21 function, HFKs expressing vector, E7 WT, or mutated E7 proteins were induced to differentiate with serum and calcium. CDK2 was immunoprecipitated from cell lysates with an anti-CDK2 antibody (M2; Santa Cruz) after 1 or 5 days, and kinase assays were carried out as previously described (23). CDK2 activity was reduced in vector- and E7 CVQ68-70AAA-expressing HFKs at both early (Fig. 6A) and late (Fig. 6B) times after differentiation. In contrast, CDK2 activity was retained in E7 WT-expressing HFKs at the earlier time point (Fig. 6A), and after 5 days in differentiation medium, these cells retained approximately 30% of the CDK2 activity found in matched, undifferentiated control cells (Fig. 6B). These data are in accordance with a role of the E7 C terminus in inactivating p21.
FIG. 6.
CDK2 activity in E7-expressing, differentiated HFKs. CDK2 activity of HFKs was assessed after 1 (A) or 5 (B) days of growth in differentiation medium (DM). Histone H1 (HH1) was used as the substrate. Percentages of kinase activity (% activity) relative to the levels retained by matched control cells maintained in growth medium (GM) are indicated. The lower blots in each panel are lysate input controls probed with an anti-actin antibody (I19; Santa Cruz).
Interestingly, differentiated HFKs expressing E7 Δ21-24 maintained a significant level of CDK2 activity at the earlier time point (Fig. 6A), consistent with the ability of E7 Δ21-24 to inactivate p21 in the peptide assay (Fig. 4D and Table 2). However, CDK2 activity was diminished after 5 days (Fig. 6B). Significantly, p21 is not the only cyclin-dependent kinase inhibitor induced during HFK differentiation. p27kip1 and, later, p15ink4b, are also induced (2). While p27 directly inhibits CDK2 activity, p15 was proposed to indirectly block CDK2 activity in differentiated HFKs by inducing the redistribution of p21 and/or p27 to cyclin/CDK2 complexes (2). Possibly, E7 Δ21-24 may not able to counteract all of the CDK inhibitors present at later times during HFK differentiation.
Taken together, the results of the experiments presented here demonstrate that the ability of E7 to deregulate epithelial cell cycle control coincides with the ability to target both Rb and p21. This has an interesting and previously uncharacterized parallel with adenovirus E1A, which induces DNA synthesis in differentiated cells in a manner dependent on inactivation of both Rb and p21 (38). These results also support the idea that the E7 C terminus, and perhaps CR1, have Rb-independent activities required for bypassing cell cycle arrest in normal human epithelial cells. E7 targets at least two regulators of the G1/S transition, Rb and p21, but while the ability of E7 to inactivate either Rb or p21 appears not to be sufficient for E7 to bypass cell cycle arrest associated with DNA damage (16, 28), differentiation (16; present study), or HFK senescence (28), by targeting multiple regulators of the G1/S transition, E7 may ensure S-phase entry of HPV-infected epithelial cells.
Finally, the experiments reported here do not exclude a role for other E7 C-terminal activities in the bypass of cell cycle arrest. While this work was in progress, the E7 C terminus was reported to function in conjunction with the Rb-binding motif to induce cdc25a (41). A C-terminal mutation, L67R, prevented induction of cdc25a (41). Although this mutation is adjacent to CVQ68-70AAA, the mutations had differing effects on the ability of E7 to overcome Rb-mediated arrest of SAOS2 cells (8, 28), suggesting that they are not functionally equivalent. Induction of cdc25a likely did not contribute to the inhibition of p21 peptide-induced arrest, since E7 Δ21-24 does not have the functional Rb-binding motif required for cdc25a induction. Nevertheless, the present study, together with other studies (31, 23, 41), has begun to determine which of the many biochemical and functional interactions mapped to the E7 C terminus contribute to the ability of E7 to subvert cell cycle control in normal human epithelial cells.
Acknowledgments
We thank members of the Galloway laboratory for helpful discussions and critical reviews of the manuscript. We thank Kristin Robinson and Greg Wipf for excellent technical assistance.
This work was supported by grant CA 64795 from the National Cancer Institute to D.A.G. J. O. Funk was supported by the Deutsche Groshungsgemeinschaft.
REFERENCES
- 1.Adams, P. D., W. R. Sellers, S. K. Sharma, A. D. Wu, C. M. Nalin, and W. G. Kaelin, Jr. 1996. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol. Cell. Biol. 16:6623-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alani, R. M., J. Hasskarl, and K. Munger. 1998. Alterations in cyclin-dependent kinase 2 function during differentiation of primary human keratinocytes. Mol. Carcinog. 23:226-233. [DOI] [PubMed] [Google Scholar]
- 3.Ball, K. L., S. Lain, R. Fahraeus, C. Smythe, and D. P. Lane. 1997. Cell-cycle arrest and inhibition of Cdk4 activity by small peptides based on the carboxy-terminal domain of p21WAF1. Curr. Biol. 7:71-80. [DOI] [PubMed] [Google Scholar]
- 4.Band, V., J. A. De Caprio, L. Delmolino, V. Kulesa, and R. Sager. 1991. Loss of p53 protein in human papillomavirus type 16 E6-immortalized human mammary epithelial cells. J. Virol. 65:6671-6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berezutskaya, E., B. Yu, A. Morozov, P. Raychaudhuri, and S. Bagchi. 1997. Differential regulation of the pocket domains of the retinoblastoma family proteins by the HPV16 E7 oncoprotein. Cell Growth Differ. 8:1277-1286. [PubMed] [Google Scholar]
- 6.Blanton, R. A., M. D. Coltrera, A. M. Gown, C. L. Halbert, and J. K. McDougall. 1992. Expression of the HPV16 E7 gene generates proliferation in stratified squamous cell cultures which is independent of endogenous p53 levels. Cell Growth Differ. 3:791-802. [PubMed] [Google Scholar]
- 7.Bonfanti, M., S. Taverna, M. Salmona, M. D'Incalci, and M. Broggini. 1997. p21WAF1-derived peptides linked to an internalization peptide inhibit human cancer cell growth. Cancer Res. 57:1442-1446. [PubMed] [Google Scholar]
- 8.Brehm, A., S. J. Nielsen, E. A. Miska, D. J. McCance, J. L. Reid, A. J. Bannister, and T. Kouzarides. 1999. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J. 18:2449-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner, A. J., M. R. Stampfer, and C. M. Aldaz. 1998. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene 17:199-205. [DOI] [PubMed] [Google Scholar]
- 10.Chellappan, S., V. B. Kraus, B. Kroger, K. Munger, P. M. Howley, W. C. Phelps, and J. R. Nevins. 1992. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc. Natl. Acad. Sci. USA 89:4549-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, J., P. Saha, S. Kornbluth, B. D. Dynlacht, and A. Dutta. 1996. Cyclin-binding motifs are essential for the function of p21CIP1. Mol. Cell. Biol. 16:4673-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng, S., D. C. Schmidt-Grimminger, T. Murant, T. R. Broker, and L. T. Chow. 1995. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 9:2335-2349. [DOI] [PubMed] [Google Scholar]
- 13.Chien, W.-M., F. Noya, H. M. Benedict-Hamilton, T. R. Broker, and L. T. Chow.2002. Alternative fates of keratinocytes transduced by human papillomavirus type 18 E7 during squamous differentiation. J. Virol. 76:2964-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin, L., S. E. Artandi, Q. Shen, A. Tam, S. L. Lee, G. J. Gottlieb, C. W. Greider, and R. A. DePinho. 1999. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97:527-538. [DOI] [PubMed] [Google Scholar]
- 15.DeGregori, J., T. Kowalik, and J. R. Nevins. 1995. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol. Cell. Biol. 15:4215-4224. (Erratum, 15:5846-5847.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demers, G. W., E. Espling, J. B. Harry, B. G. Etscheid, and D. A. Galloway. 1996. Abrogation of growth arrest signals by human papillomavirus type 16 E7 is mediated by sequences required for transformation. J. Virol. 70:6862-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derossi, D., G. Chassaing, and A. Prochiantz. 1998. Trojan peptides: the penetration system for intracellular delivery. Trends Cell Biol. 8:84-87. [PubMed] [Google Scholar]
- 18.Dotto, G. P. 2000. p21WAF1/Cip1: more than a break to the cell cycle? Biochim. Biophys. Acta 1471:M43-M56. [DOI] [PubMed] [Google Scholar]
- 19.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 20.el-Deiry, W. S., J. W. Harper, P. M. O'Connor, V. E. Velculescu, C. E. Canman, J. Jackman, J. A. Pietenpol, M. Burrell, D. E. Hill, Y. Wang, et al. 1994. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 54:1169-1174. [PubMed] [Google Scholar]
- 21.Foster, S. A., and D. A. Galloway. 1996. Human papillomavirus type 16 E7 alleviates a proliferation block in early passage human mammary epithelial cells. Oncogene 12:1773-1779. [PubMed] [Google Scholar]
- 22.Foster, S. A., D. J. Wong, M. T. Barrett, and D. A. Galloway. 1998. Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol. Cell. Biol. 18:1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao, Q., S. H. Hauser, X. L. Liu, D. E. Wazer, H. Madoc-Jones, and V. Band. 1996. Mutant p53-induced immortalization of primary human mammary epithelial cells. Cancer Res. 56:3129-3133. [PubMed] [Google Scholar]
- 25.Gonzalez, S. L., M. Stremlau, X. He, J. R. Basile, and K. Münger. 2001. Degradation of the retinoblastoma tumor suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J. Virol. 75:7583-7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1991. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J. Virol. 65:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper, J. W., S. J. Elledge, K. Keyomarsi, B. Dynlacht, L. H. Tsai, P. Zhang, S. Dobrowolski, C. Bai, L. Connell-Crowley, E. Swindell, et al. 1995. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 6:387-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helt, A.-M., and D. A. Galloway. 2001. Destabilization of the retinoblastoma tumor suppressor by human papillomavirus type 16 E7 is not sufficient to overcome cell cycle arrest in human keratinocytes. J. Virol. 75:6737-6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang, H., J. Lin, Z. Z. Su, F. R. Collart, E. Huberman, and P. B. Fisher. 1994. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21, WAF1/CIP1, expression in the absence of p53. Oncogene 9:3397-3406. [PubMed] [Google Scholar]
- 30.Jiang, Z., P. Liang, R. Leng, Z. Guo, Y. Liu, X. Liu, S. Bubnic, A. Keating, D. Murray, P. Goss, and E. Zacksenhaus. 2000. E2F1 and p53 are dispensable, whereas p21Waf1/Cip1 cooperates with Rb to restrict endoreduplication and apoptosis during skeletal myogenesis. Dev. Biol. 227:8-41. [DOI] [PubMed] [Google Scholar]
- 31.Jones, D. L., R. M. Alani, and K. Munger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones, D. L., and K. Münger. 1997. Analysis of the p53-mediated G1 growth arrest pathway in cells expressing the human papillomavirus type 16 E7 oncoprotein. J. Virol. 71:2905-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, D. L., D. A. Thompson, and K. Munger. 1997. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology 239:97-107. [DOI] [PubMed] [Google Scholar]
- 34.Katich, S. C., K. Zerfass-Thome, and I. Hoffmann. 2001. Regulation of the Cdc25A gene by the human papillomavirus type 16 E7 oncogene. Oncogene 20:543-550. [DOI] [PubMed] [Google Scholar]
- 35.Khan, S. H., and G. M. Wahl. 1998. p53 and pRb prevent rereplication in response to microtubule inhibitors by mediating a reversible G1 arrest. Cancer Res. 58:396-401. [PubMed] [Google Scholar]
- 36.Khleif, S. N., J. DeGregori, C. L. Yee, G. A. Otterson, F. J. Kaye, J. R. Nevins, and P. M. Howley. 1996. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc. Natl. Acad. Sci. USA 93:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 38.Mal, A., D. Chattopadhyay, M. K. Ghosh, R. Y. Poon, T. Hunter, and M. L. Harter. 2000. p21 and retinoblastoma protein control the absence of DNA replication in terminally differentiated muscle cells. J. Cell Biol. 149:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Missero, C., E. Calautti, R. Eckner, J. Chin, L. H. Tsai, D. M. Livingston, and G. P. Dotto. 1995. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc. Natl. Acad. Sci. USA 92:5451-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Missero, C., F. Di Cunto, H. Kiyokawa, A. Koff, and G. P. Dotto. 1996. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 10:3065-3075. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen, D. X., T. F. Westbrook, and D. J. McCance. 2002. Human papillomavirus type 16 E7 maintains elevated levels of the cdc25A tyrosine phosphatase during deregulation of cell cycle arrest. J. Virol. 76:619-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noda, A., Y. Ning, S. F. Venable, O. M. Pereira-Smith, and J. R. Smith. 1994. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res. 211:90-98. [DOI] [PubMed] [Google Scholar]
- 43.Ogryzko, V. V., P. Wong, and B. H. Howard. 1997. WAF1 retards S-phase progression primarily by inhibition of cyclin-dependent kinases. Mol. Cell. Biol. 17:4877-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittelkow, M. R., J. J. Wille, Jr., and R. E. Scott. 1986. Two functionally distinct classes of growth arrest states in human prokeratinocytes that regulate clonogenic potential. J. Investig. Dermatol. 86:410-417. [DOI] [PubMed] [Google Scholar]
- 45.Ruesch, M. N., and L. A. Laimins. 1997. Initiation of DNA synthesis by human papillomavirus E7 oncoproteins is resistant to p21-mediated inhibition of cyclin E-cdk2 activity. J. Virol. 71:5570-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandhu, C., J. Donovan, N. Bhattacharya, M. Stampfer, P. Worland, and J. Slingerland. 2000. Reduction of Cdc25A contributes to cyclin E1-Cdk2 inhibition at senescence in human mammary epithelial cells. Oncogene 19:5314-5323. [DOI] [PubMed] [Google Scholar]
- 47.Schlegel, R., W. C. Phelps, Y. L. Zhang, and M. Barbosa. 1988. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 7:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shay, J. W., W. E. Wright, D. Brasiskyte, and B. A. Van der Haegen. 1993. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene 8:1407-1413. [PubMed] [Google Scholar]
- 49.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 50.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 51.Stampfer, M. R. 1985. Isolation and growth of human mammary epithelial cells. J. Tissue Cult. Methods 9:107-115. [Google Scholar]
- 52.Stewart, Z. A., S. D. Leach, and J. A. Pietenpol. 1999. p21Waf1/Cip1 inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol. Cell. Biol. 19:205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waga, S., G. J. Hannon, D. Beach, and B. Stillman. 1994. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369:574-578. [DOI] [PubMed] [Google Scholar]
- 54.Waldman, T., C. Lengauer, K. W. Kinzler, and B. Vogelstein. 1996. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature 381:713-716. [DOI] [PubMed] [Google Scholar]
- 55.Wu, E. W., K. E. Clemens, D. V. Heck, and K. Münger. 1993. The human papillomavirus E7 oncoprotein and the cellular transcription factor E2F bind to separate sites on the retinoblastoma tumor suppressor protein. J. Virol. 67:2402-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, H., G. J. Hannon, and D. Beach. 1994. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 8:1750-1758. [DOI] [PubMed] [Google Scholar]
- 57.zur Hausen, H. 1996. Papillomavirus infections—a major cause of human cancers. Biochim. Biophys. Acta 1288:F55-F78. [DOI] [PubMed] [Google Scholar]