Abstract
1. The frequency of miniature end-plate potentials (m.e.p.p.s) was recorded from neuromuscular junctions in rat diaphragm phrenic nerve preparations in vitro after preparations had soaked in solutions containing Ca in concentrations between 10-10 and 10-2 M and a similar range of [Mg].
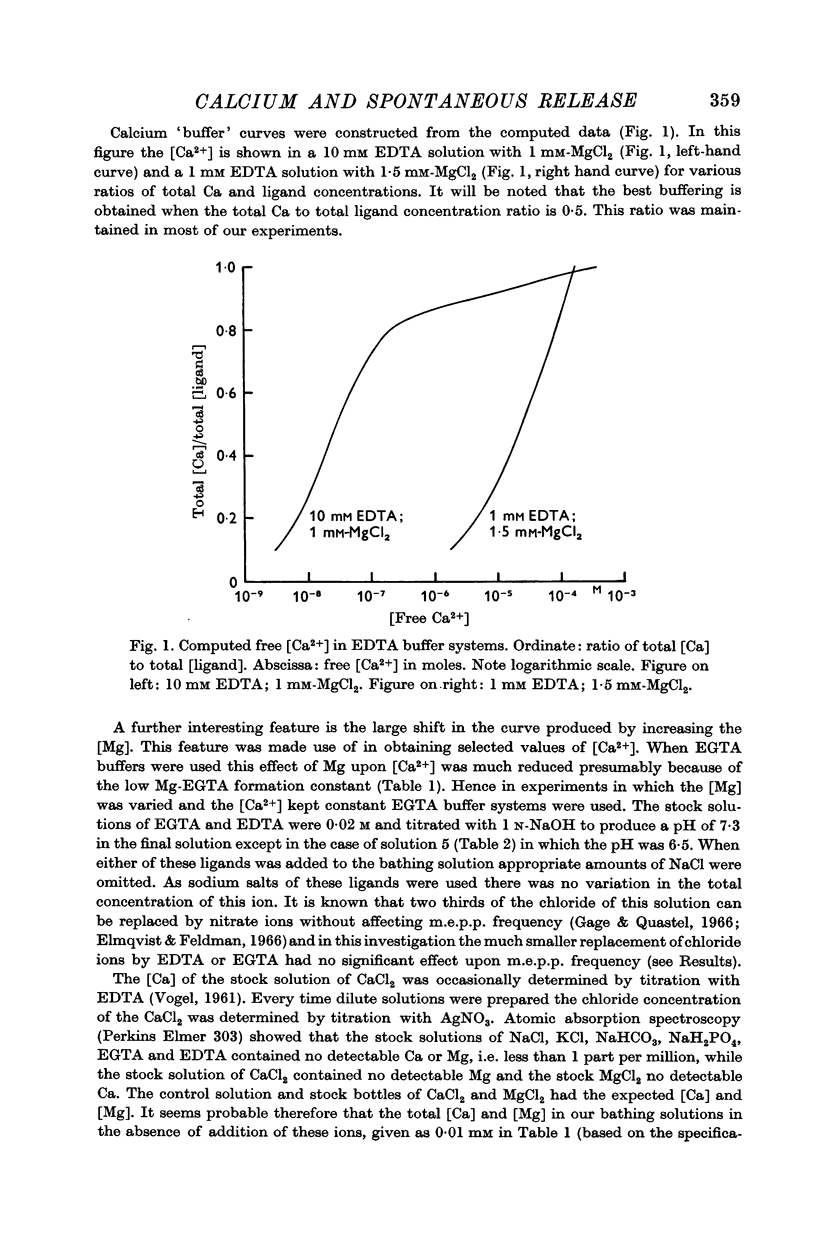
2. Ethylenediamine tetra-acetate (EDTA) and ethyleneglycol bis (β-aminoethyl ether) tetra-acetate (EGTA) buffers were added to prepare solutions with [Ca] and [Mg] below 10-4 M. A computer program was used to estimate the free [Ca2+] in these solutions, and it was shown that the effects of Ca could be attributed to the free [Ca2+] in the bathing solution.
3. M.e.p.p.s could still be detected without difficulty after soaking preparations for 6-8 hr in solutions containing EDTA or EGTA buffers and no added Ca. The basal frequency was unchanged upon exhibition of Ca in concentrations up to 10-5 M and/or Mg in concentrations up to 10-3 M.
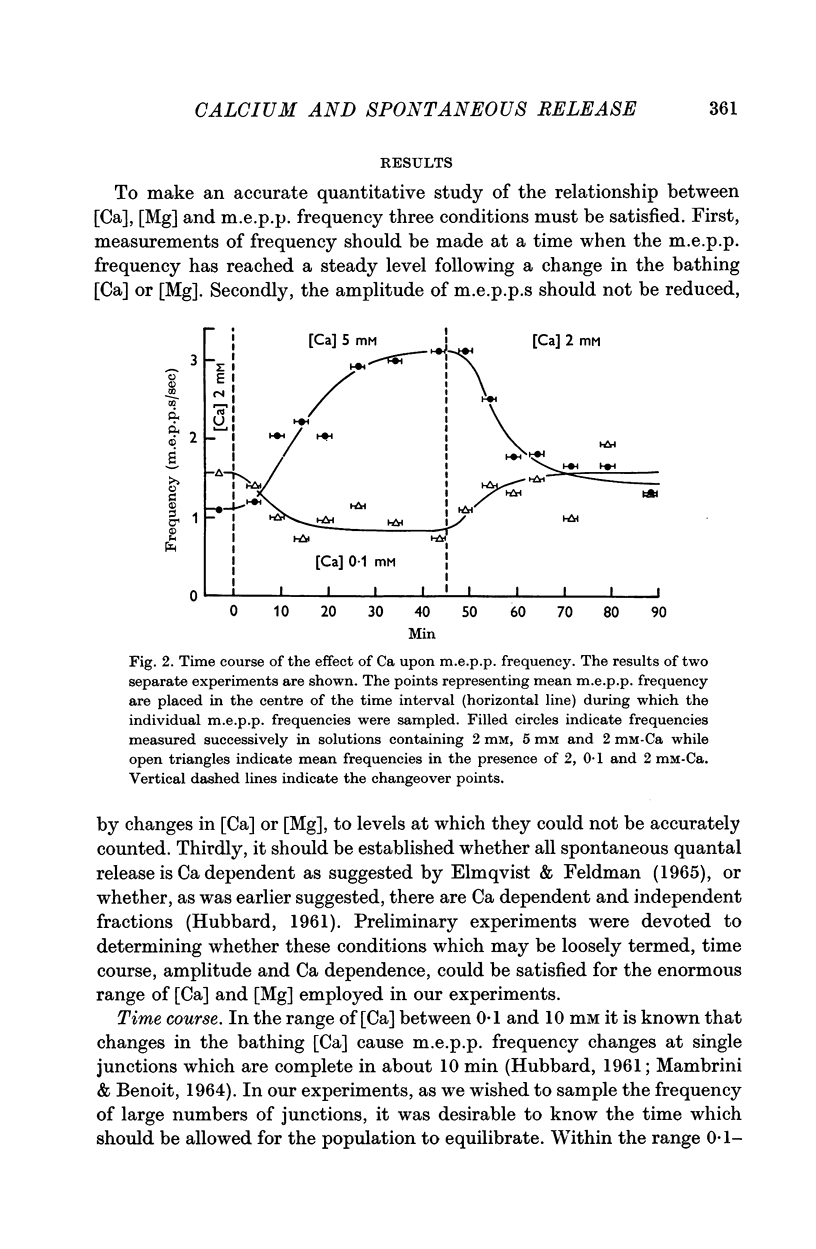
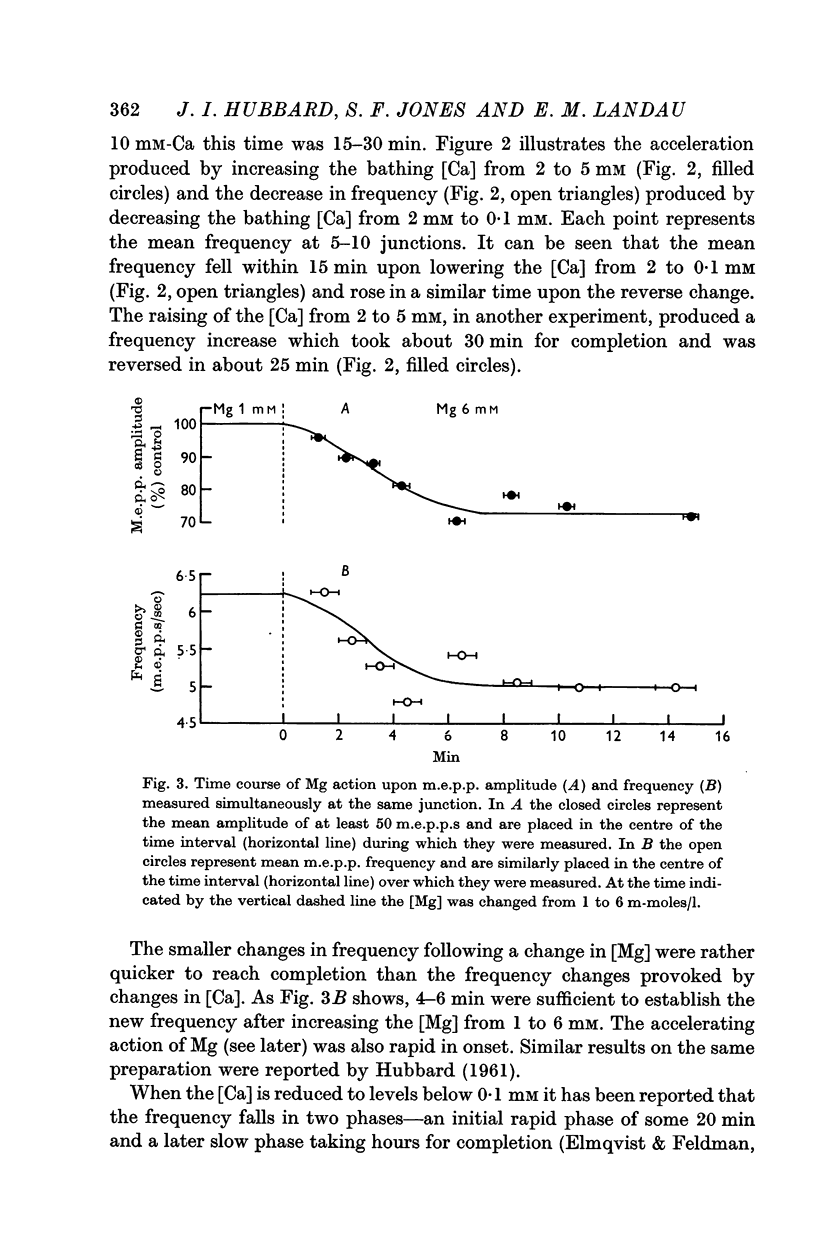
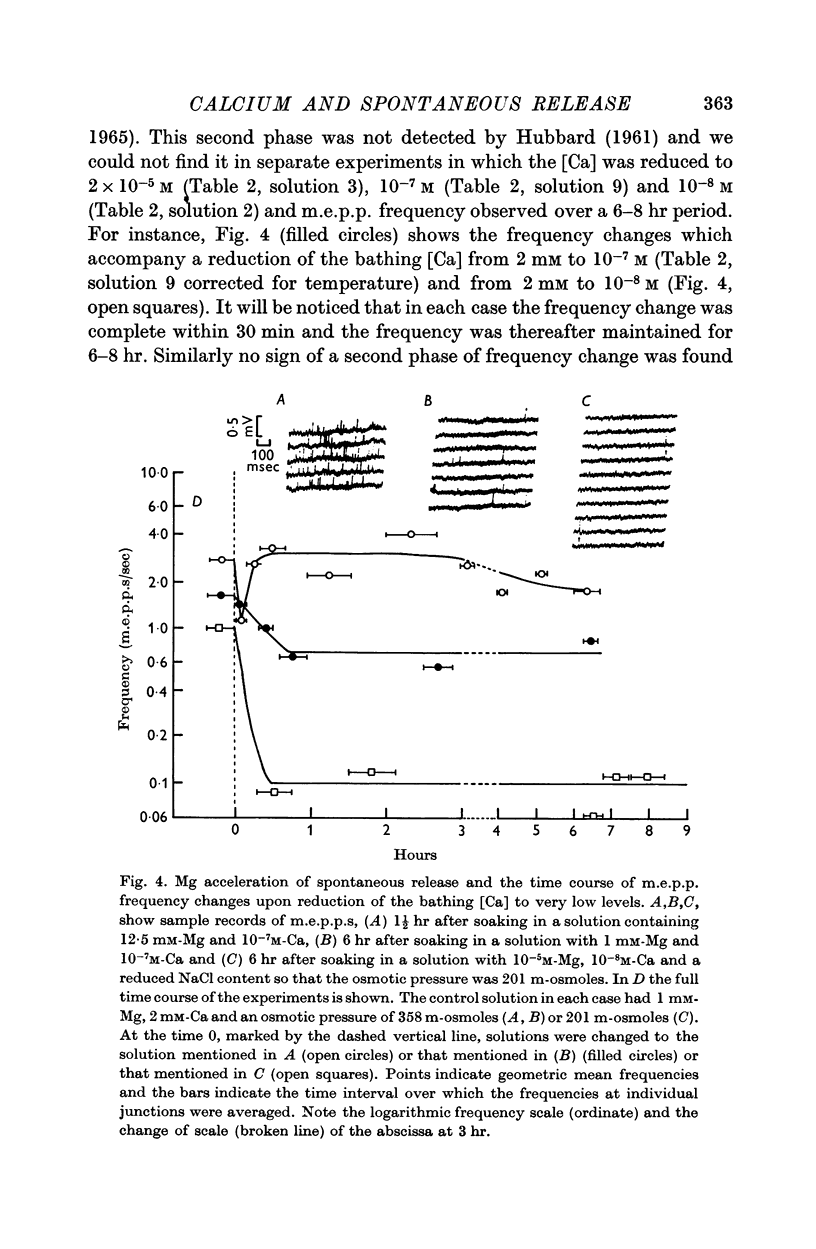
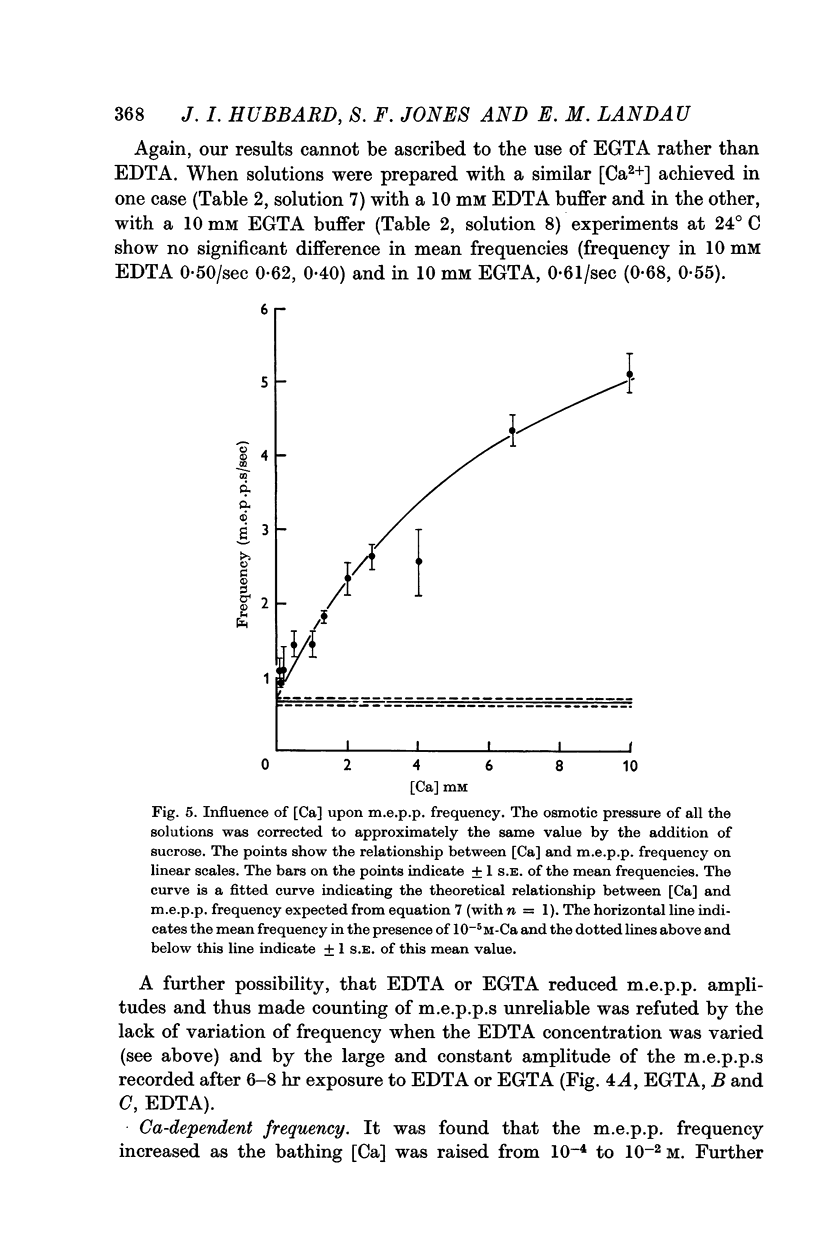
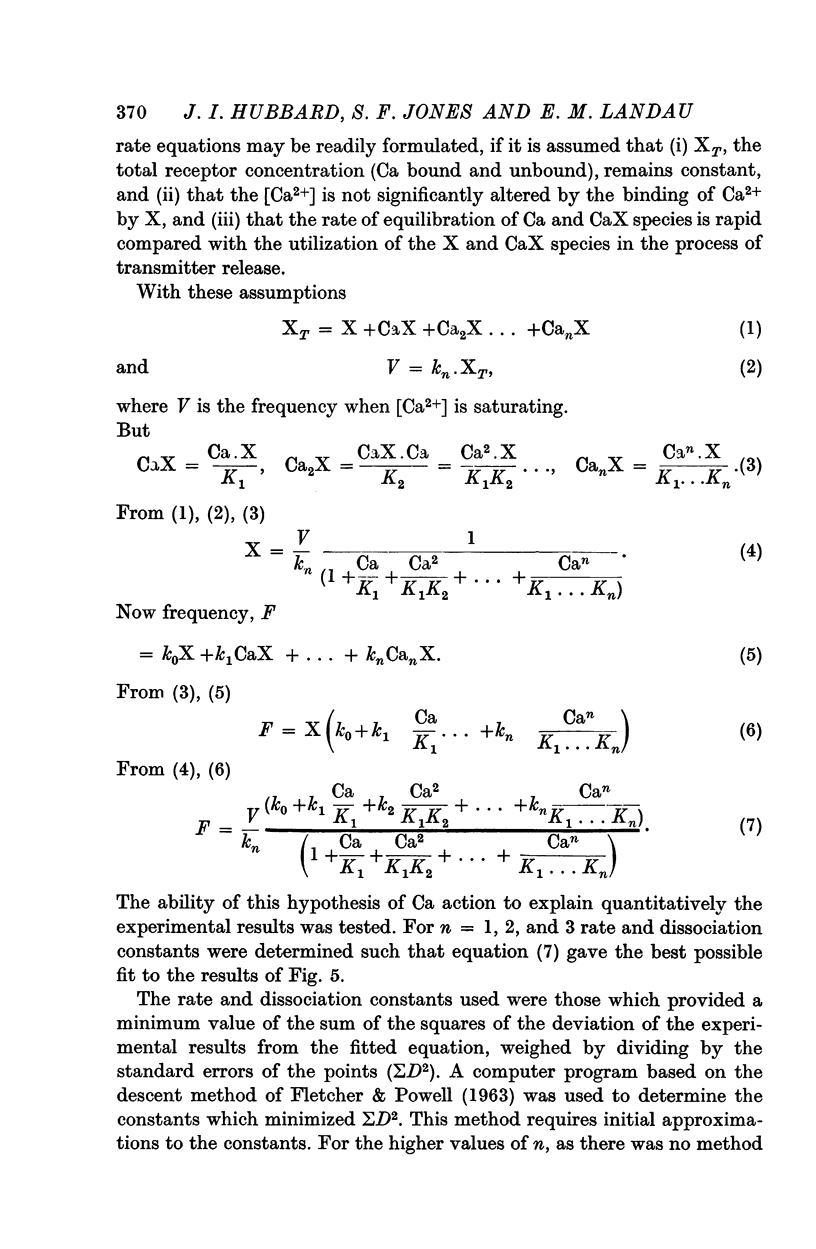
4. Ca in concentrations of and above 10-4 M accelerated m.e.p.p. frequency from the basal level. This effect reached a maximum in [Ca] of 10 mM and raising the [Ca] above this level did not further change frequency. These effects were explained by the combination of Ca molecules with a nerve terminal receptor site. It was postulated that this combination allosterically activated the spontaneous release mechanism.
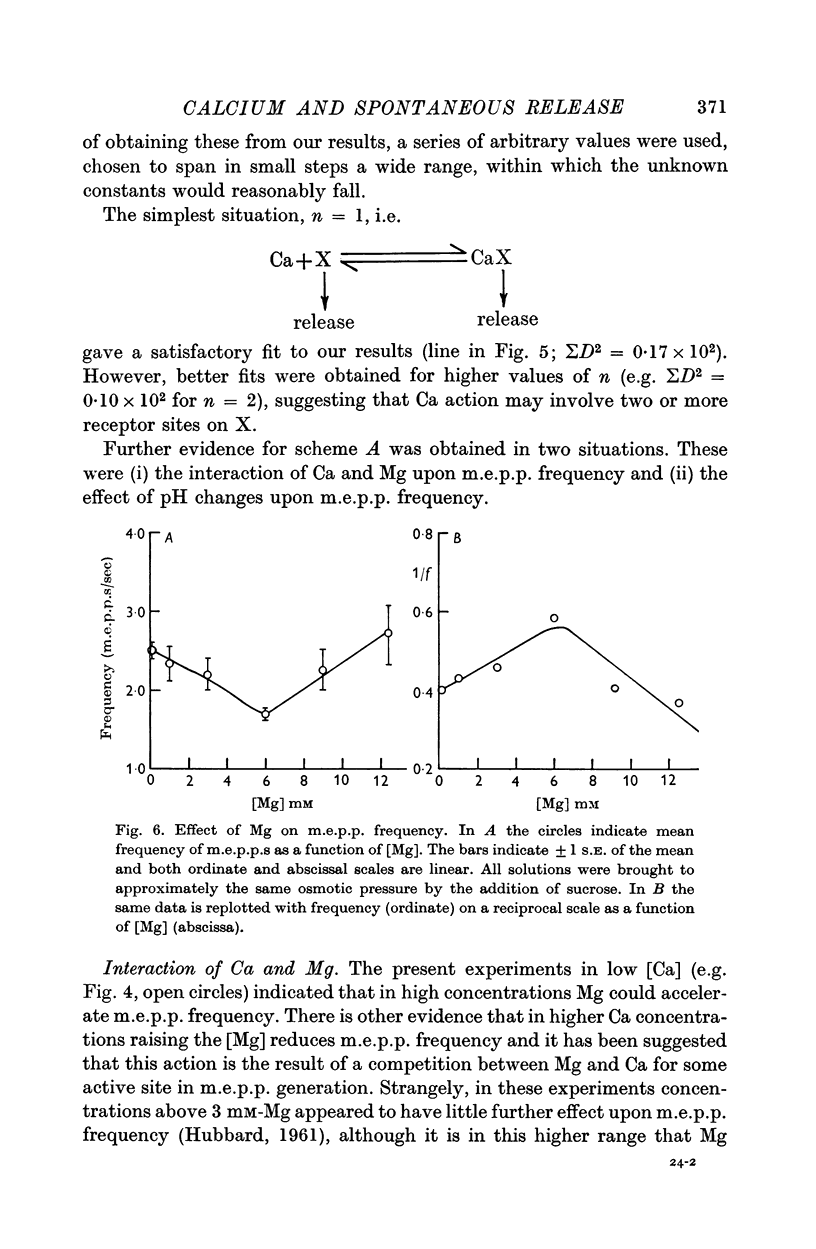
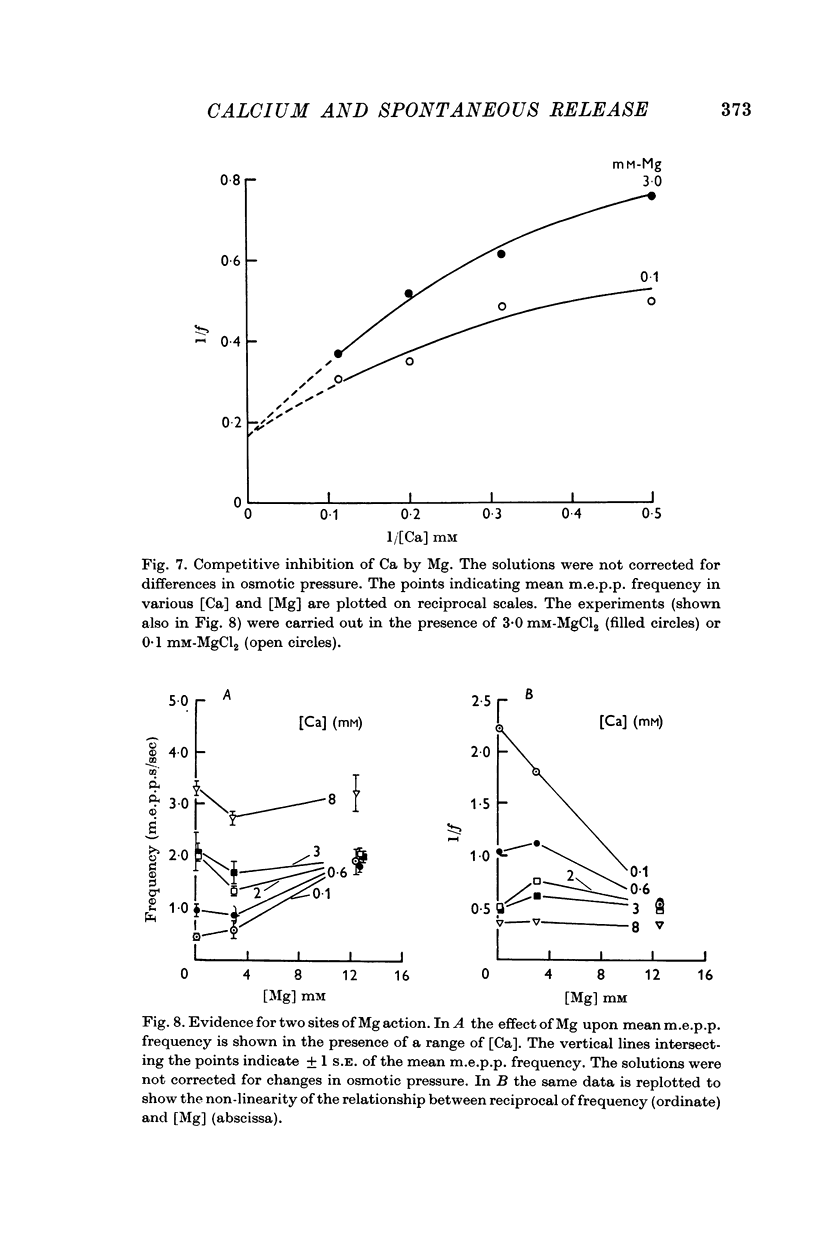
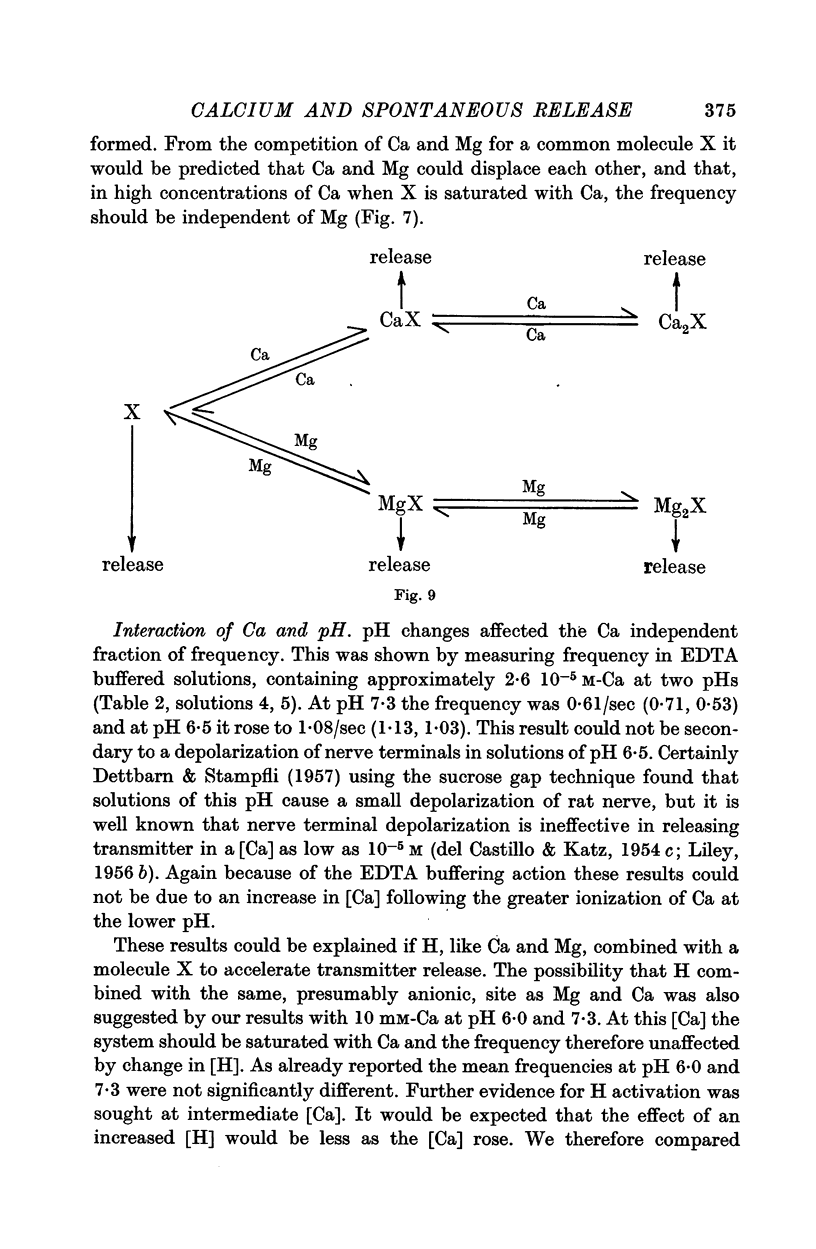
5. Mg could accelerate m.e.p.p. frequency in the absence of added Ca. The interactions of Ca and Mg upon m.e.p.p. frequency indicated that Ca and Mg competed for the same sites.
6. Raising the [H+] of the bathing medium accelerated m.e.p.p. frequency. This effect was thought to be exerted partly by combination with the same receptor sites as Ca and Mg and partly by variation of the ionization of the CaCl2 of the bathing solution.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., CURTIS D. R. THE EXCITATION OF THALAMIC NEURONES BY ACETYLCHOLINE. Acta Physiol Scand. 1964 May-Jun;61:85–99. doi: 10.1111/j.1748-1716.1964.tb02945.x. [DOI] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., STARK L. The effect of calcium ions on the motor end-plate potentials. J Physiol. 1952 Apr;116(4):507–515. doi: 10.1113/jphysiol.1952.sp004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DETTBARN W. D., STAMPFLI R. Die Wirkung von 2, 4-Dinitrophenol auf das Membranpotential der markhaltigen Nervenfaser. Helv Physiol Pharmacol Acta. 1957;15(1):25–37. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Influence of ionic environment on acetylcholine release from the motor nerve terminals. Acta Physiol Scand. 1966 May;67(1):34–42. doi: 10.1111/j.1748-1716.1966.tb03284.x. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FENN W. O., GILBERT D. L. Calcium equilibrium in muscle. J Gen Physiol. 1957 Jan 20;40(3):393–408. doi: 10.1085/jgp.40.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILBERT D. L. Magnesium equilibrium in muscle. J Gen Physiol. 1960 Jul;43:1103–1118. doi: 10.1085/jgp.43.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. The origin of the post-tetanic hyperpolarization of mammalian motor nerve terminals. J Physiol. 1966 May;184(2):335–352. doi: 10.1113/jphysiol.1966.sp007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Competition between sodium and calcium ions in transmitter release at mammalian neuromuscular junctions. J Physiol. 1966 Jul;185(1):95–123. doi: 10.1113/jphysiol.1966.sp007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. Post-activation changes at the mammalian neuromuscular junction. Nature. 1959 Dec 19;184(Suppl 25):1945–1947. doi: 10.1038/1841945a0. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Effects of the intracellular Ca ion concentration upon the excitability of the muscle fiber membrane of a barnacle. J Gen Physiol. 1966 Mar;49(4):807–818. doi: 10.1085/jgp.49.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Loyning Y. The effects of hypoxia on neuromuscular transmission in a mammalian preparation. J Physiol. 1966 Jul;185(1):205–223. doi: 10.1113/jphysiol.1966.sp007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAMBRINI J., BENOIT P. R. ACTION DU CALCIUM SUR LA JONCTION NEURO-MUSCULAIRE CHEZ LA GRENOUILLE. C R Seances Soc Biol Fil. 1964;158:1454–1458. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H., STAMPFLI R. Die Depolarisation durch Calcium-Mangel und ihre Abhängigkeit von der Kalium-Konzentration. Helv Physiol Pharmacol Acta. 1957;15(2):200–211. [PubMed] [Google Scholar]



