Abstract
Recent studies have shown that the accumulation of multiple mutations associated with nucleoside reverse transcriptase inhibitor (NRTI) resistance may be grouped as multi-NRTI resistance (MNR) complexes. In this study, we have examined the viral fitness of recombinant viruses carrying the reverse transcriptase (RT) of a human immunodeficiency virus type 1 (HIV-1) primary isolate harboring mutations comprising the MNR 69 insertion complex. Different RT mutants were prepared in the sequence context of either the wild-type RT sequence of the HIV-1BH10 isolate or the sequence found in a clinical HIV-1 isolate with the MNR 69 insertion mutation. As expected, in the presence of zidovudine, recombinant viruses harboring the MNR RT from the patient were more fit than wild-type viruses. However, in the absence of drug, the virus with the RT from the original clinical isolate (SS) was more fit than (i) the wild-type virus with an engineered serine insertion between residues 69 and 70 (T69SSS) and (ii) the recombinant virus with the MNR RT where the insertion was removed (2S0S). These results suggest that RT insertions, in the right sequence context (i.e., additional mutations contained in the MNR 69 insertion complex), enhance NRTI resistance and may improve viral fitness. Thus, comparing complex mutation patterns with viral fitness may help to elucidate the role of uncharacterized drug resistance mutations in antiretroviral treatment failure.
Multidrug-resistant (MDR) human immunodeficiency virus type 1 (HIV-1) strains, with reduced susceptibilities to antiretroviral drugs from two or more classes, are now commonly found in extensively treated patients (13, 27). A recent study has shown that at least 50% of HIV-positive individuals in the United States are infected with drug-resistant variants (D. D. Richman, 2nd HIV DRP Symp. Antivir. Drug Resist., p. 51, 2001.), which may have a profound effect on suboptimal treatment responses, reduced viral fitness, and the potential for transmission of drug-resistant virus (5, 20). Among MDR viruses, resistance to multiple nucleoside analogue reverse transcriptase inhibitors (NRTI), or multi-NRTI resistance (MNR), can be developed by at least three main pathways: (i) accumulation of mutations associated with cross-resistance to NRTI, previously called zidovudine or thymidine analogue resistance mutations, recently referred to as multi-NRTI-associated mutations (e.g., M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E); (ii) selection of the key 151 M mutation, followed by the mutations A62V, V75I, F77L, and F116Y, denominated the 151 complex; and (iii) the 69 insertion complex, consisting of a mutation at codon 69 (typically Ser), followed by an insertion of two or more amino acids (e.g., Ser-Ser, Ser-Arg, or Ser-Gly) and generally accompanied by multi-NRTI-associated mutations (13, 15, 36; http://www.iasusa.org). To date, it is still unclear why viruses choose one or the other resistance pathway, although the molecular events leading to selection of specific drug-resistant variants are probably determined by the viral genetic background.
Although with a relatively low prevalence (1 to 3%), several studies have identified heavily treated patients carrying HIV-1 isolates with the MNR 69 insertion complex (1, 2, 6, 10, 11, 19, 24, 31, 34, 37-40, 41). Viral isolates harboring these mutations often show moderate to high-level resistance to all NRTI (11, 19, 23-25, 34, 37, 39, 41). This insertion is located in the β3-β4 hairpin loop, at the fingers subdomain of HIV-1 reverse transcriptase (RT) (39, 41). The biochemical properties of the wild-type HIV-1BH10 RT are not significantly altered upon introduction of the dipeptide insertion (4, 23). However, the insertion appears to be critical in enhancing AZT resistance in the sequence context of the MDR RT, containing additional NRTI resistance-related mutations (23). Drug susceptibility assays have shown that in the presence of the zidovudine (AZT) resistance mutations M41L, L210W, and T215Y, the insertion confers a moderate to large increase in resistance to NRTI (23).
In the absence of antiretroviral therapy, HIV-1 strains containing drug resistance mutations have a reduced fitness compared to the wild-type virus (9). However, this impairment on viral fitness is generally compensated with secondary mutations (3, 8, 16, 26). Multiple studies have reported impaired enzyme function and reduced viral fitness of HIV-1 isolates harboring mutations conferring resistance to protease and RT inhibitors (PI and RTI, respectively) (for reviews, see references 8, 26, and 29). Several others have assessed the in vitro fitness of MNR viruses (18, 22, 29). Fitness studies with viruses resistant to multiple NRTI have shown that HIV-1 isolates harboring the MNR-151 complex display higher fitness than the wild-type virus in the absence of drug (18, 22). On the other hand, viral dynamics studies suggest that viruses carrying the MNR 69 insertion complex have a clear selective disadvantage compared with HIV-1 variants lacking the insertion (6, 21, 41). In this work, we have analyzed the impact on viral fitness of an insertion of two amino acids (Ser-Ser) between residues 69 and 70 of HIV-1 RT through the use of a dual infection/competition assay (30). The results reveal the role of the viral genetic background on HIV-1 fitness and how the RT sequence context (i.e., additional mutations) may play a role in improving the replication capacity of viruses harboring these insertions.
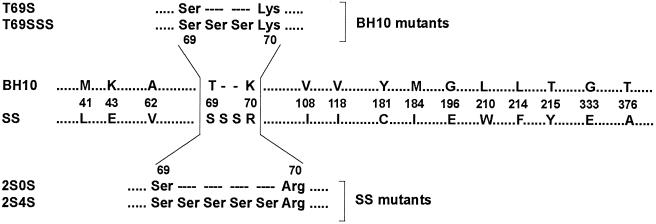
Plasma samples were obtained from a highly treated HIV-infected individual as part of a previous study that screened HIV-1-infected patients for the presence of virus with an amino acid insertion between codons 69 and 70 of HIV-1 RT (7). This HIV-infected 38-year old man had been extensively treated with several RTI and PI, which included a first period of AZT monotherapy (1992 to 1996) and a current regimen combining five antiretroviral drugs (i.e., dideoxyinosine, stavudine, nevirapine, saquinavir, and nelfinavir). At the time of sample collection, the plasma viral load was 29,420 HIV-1 RNA copies/ml, with a CD4+ cell count of 144 cells/μl (7, 23). Viral RNA was extracted from the plasma and the RT-coding region was RT-PCR amplified and cloned as previously described (6). Sequencing of the HIV-1 pol gene revealed multiple mutations in the protease and RT, many of them associated with resistance to PI and RTI (23). In addition to the insertion of two serines between residues 69 and 70, 43 additional mutations scattered throughout the entire RT-coding sequence were identified (6, 23), including 15 amino acid substitutions related to RTI resistance (36) (Fig. 1).
FIG. 1.
Amino acid sequence differences between RTs from wild-type (BH10) and clinical (SS) HIV-1 mutants. Only those amino acid differences found at codons associated with resistance to RTIs (36) are indicated. Six different plasmid constructions were generated containing RT sequences from the wild-type strain (BH10) and the sequence found in the clinical isolate (SS) (see text for details). The amino acid sequences around positions 69 and 70 of mutants used in this study having insertions, deletions, or substitutions are shown above and below the alignment.
The role of this dipeptide insertion and its RT genetic background in HIV-1 replication was evaluated by using a series of HIV-1 variants obtained through recombination of the analyzed RT-coding region with an RT-deleted HXB2-D clone (17, 23). Recombinant constructs included viruses harboring either the RT sequence of a wild-type HIV-1BH10 strain (termed BH10) or the RT found in an MDR clinical isolate (designated as SS). Two mutant RTs were prepared by replacing the Thr-69 with Ser (T69S), or by making this substitution and inserting two additional serines between codons 69 and 70 of the wild-type HIV-1BH10 RT (T69SSS) (Fig. 1). Mutant derivatives of the SS RT include 2S0S, which lacked the Ser-Ser insertion, and 2S4S, which contained four serines instead of two between codons 69 and 70 (Fig. 1). Recombinant viruses recovered from transfection experiments, carrying the appropriate nucleotide sequence, were propagated in MT-4 cells.
Peripheral blood mononuclear cells (PBMC) from HIV-seronegative blood donors were obtained by Ficoll-Hypaque density gradient centrifugation of heparin-treated venous blood. Prior to HIV-1 infection the cells were stimulated with 2 μg of phytohemagglutinin (PHA; Gibco BRL) per ml for 3 to 4 days and maintained in RPMI 1640-2 mM l-glutamine medium (Cellgro) supplemented with 10% fetal bovine serum (Cellgro), 10 mM HEPES buffer (Cellgro), 1 ng of interleukin-2 (IL-2)/ml (Gibco BRL), 100 U of penicillin/ml, and 100 μg of streptomycin/ml (both from Cellgro). Two syncytium-inducing and two non-syncytium-inducing HIV-1 primary isolates (A-92UG029 and E-CMU06, and A-92RW009 and C-92BR025, respectively) were obtained from the AIDS Research and Reference Reagent Program to be used as controls in the growth competition experiments. All viral stocks (i.e., recombinant virus and primary isolates) were expanded in PHA-stimulated, IL-2-treated PBMC. Tissue culture doses for 50% infectivity (TCID50) were determined for each isolate in triplicate with serially diluted supernatants of each viral propagation. RT activity in culture supernatants on day 8 of culture was used to calculate TCID50 values using the Reed and Muench method (32). Titers were expressed as infectious units per milliliter (IU/ml).
In previous studies, we and others had shown that the insertion per se of two serines between amino acids 69 and 70 of the HIV-1 RT did not confer significant resistance to NRTI (19, 23, 41). Thus, the wild-type RT (BH10) as well as mutants T69S and T69SSS were all highly susceptible to AZT (Table 1). On the other hand, all recombinant viruses with clinical (SS) background showed resistance to AZT (Table 1) and to other NRTI (i.e., dideoxycytosine, dideoxyinosine, lamivudine, and stavudine) (23). Interestingly, the removal of the two serines (2S0S) or the addition of two extra serines (2S4S) in the SS RT sequence context produced 5-fold and >200-fold reductions in the 50% inhibitory concentration (IC50) value for AZT, respectively (Table 1). These results suggest that both (i) the insertion of two serines and (ii) the correct sequence background are necessary to achieve the high-level resistance to AZT found in the RT obtained from the clinical isolate (SS), as depicted in the MNR 69 insertion complex.
TABLE 1.
Susceptibility to AZT and relative fitness of recombinant HIV-1 constructs
| Construct group | RT | AZT IC50 (μM)a | Total relative fitness (% relative to wild type)b
|
|
|---|---|---|---|---|
| No drug | AZT, 6 nM | |||
| BH10 mutants | T69S | 2.4 × 10−3 (0.4×) | 2.53 (89.4) | 1.41 (49.8) |
| T69SSS | 2.2 × 10−3 (0.3×) | 1.56 (55.1) | 1.19 (42.1) | |
| BH10 | 6.6 × 10−3 | 2.83 (100) | 1.71 (60.4) | |
| SS mutants | SS | 5.17 (786×) | 1.86 (65.7) | 4.37 (154) |
| 2S0S | 0.94 (143×) | 1.04 (36.7) | 4.37 (154) | |
| 2S4S | 0.024 (3.6×) | 0.82 (28.9) | 4.13 (146) | |
Based on data taken from reference 23. IC50 values represent the mean of two to four tests, each one performed in sextuplicate. The fold increase in IC50 relative to the wild-type HIV-1BH10 virus control is shown in parentheses.
Total relative fitness is the average of four relative fitness values, corresponding to the four competitions of each viral construction with each of four HIV-1 control strains (see text for details). Viral fitness was calculated relative to that of the wild-type HIV-1BH10 control (100%).
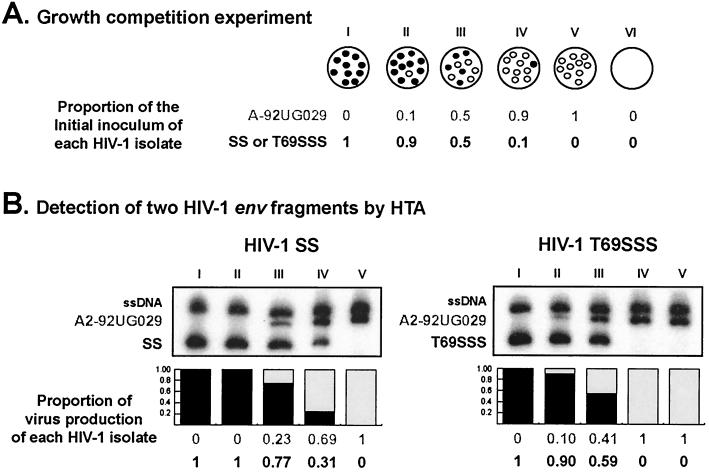
Dual infection/competition experiments were performed in PBMC from one donor as previously described (30) in order to determine the viral fitness of these recombinant HIV-1 strains. Briefly, the dual infection/competition assay involved three separate dual infections with two HIV-1 isolates at different multiplicities of infection (MOIs; expressed as infectious units per cell) (Fig. 2A). Each recombinant HIV-1 variant was added to growth competition experiments along with each of four control HIV-1 primary isolates (i.e., A-92UG029, E-CMU06, A-92RW009, and C-92BR025) under two different conditions: in the absence of drug and in the presence of 6 nM AZT. We used this AZT concentration since it was the determined IC50 value for the wild-type HIV-1BH10 strain (Table 1). One milliliter of these virus mixtures was incubated with 106 PBMC for 2 h at 37°C, 5% CO2. Subsequently, the cells were washed three times with phosphate-buffered saline and then resuspended in complete medium (106 cells/ml). Cells were washed and fed with complete medium twice a week. New PHA-IL-2-prestimulated PBMC from the same donor were added weekly to replenish viable cells from cultures. Supernatants and two aliquots of cells were harvested at day 15, resuspended in dimethyl sulfoxide-fetal bovine serum, and then stored at −80°C for subsequent analysis.
FIG. 2.
Schematic representation of growth competition experiments and HTA detection for dual infections. (A) Dual infections with a pair of HIV-1 isolates were performed at three different MOIs (proportion of viruses in wells II, III, and IV). Wells I and V correspond to positive controls for both viruses. An uninfected culture (well VI) was used as a negative control. (B) HTA results from growth competition experiments involving two recombinant HIV-1 strains (SS and T69SSS) and the HIV-1A-92UG029 control. Final ratios of each HIV-1 variant in the dual infection were measured by HTA. Nested env PCR products from the competitions were denatured and annealed to a subtype B probe (HIV-1B-SF162). The proportion of virus production of each HIV-1 isolate is shown below the autoradiographs. To derive relative fitness values for each recombinant HIV-1 variant, the final amount of each virus produced from the dual infection was divided by the initial proportion of the virus in the inoculum, as previously described (30). The total relative fitness is the average of four relative fitness values, corresponding to the four competitions of each recombinant virus with each of four controls (i.e., A1, HIV-1A-92RW009; C, HIV-1C-92BR025; A2, HIV-1A-92UG029; and E, HIV-1E-CMU06). See text for experimental details. ssDNA, single-stranded DNA from the probe. A-92UG029, SS, and T69SSS correspond to DNA heteroduplexes.
Proviral DNA was extracted from lysed PBMC using the QIAamp DNA blood kit (Qiagen) and then PCR amplified using a set of external [envB (14)-ED14 (12) (the gp120-coding region of env, ≈1.7 kb)] and nested [E80-E105 (35) (C2-C4 env region, 0.66 kb)] primers. Both external and nested PCRs were carried out in a 100-μl reaction mixture as previously described (30). Nested PCR products were isolated in agarose gels, purified using the QIAquick PCR purification kit (Qiagen), and then analyzed using heteroduplex tracking analysis (HTA) (30) (Fig. 2B). Briefly, the same genomic region (env C2-C4) was PCR amplified from the HIV-1B-SF162 strain for use as a DNA probe. For this amplification, the E80 primer was radiolabeled using T4 polynucleotide kinase and 2 μCi of [γ-32P]ATP. Reaction mixtures containing DNA annealing buffer (100 mM NaCl, 10 mM Tris-HCl [pH 7.8], 2 mM EDTA), 10 μl of unlabeled PCR-amplified DNA from the competition culture, and approximately 0.1 pmol of radioactive probe DNA were denatured at 95°C for 3 min and then rapidly annealed on wet ice. After 30 min on ice, the DNA heteroduplexes were resolved on Tris-borate-EDTA buffer 5% nondenaturing polyacrylamide gels (30:0.8 acrylamide:bisacrylamide) for 2.5 h at 200 V. Gels were dried, exposed to X-ray film (Eastman Kodak Co., Rochester, N.Y.), and scanned for analysis using 1D Image Analysis software (Kodak). To estimate the viral fitness of the recombinant viruses, the final ratio of the two viruses produced from each of the three dual infections (MOI ratios of 10:1, 1:1, and 1:10) were determined by HTA and compared to production in the monoinfections as previously described (30). Briefly, a relative fitness value for each virus was obtained from the average of the three independent dual infections (i.e., production of individual HIV-1 strain in a dual infection, divided by its initial proportion in the inoculum). The ratio of relative fitness values of each HIV-1 variant in the competition is a measure of the fitness difference between both HIV-1 strains (i.e., recombinant and control viruses) (30). Finally, total relative fitness was calculated as the average of four relative fitness values, corresponding to competitions between each recombinant HIV-1 variant and each one of the four HIV-1 control strains (30). Examples of two HIV-1 dual infections and detection of HIV-1 env fragments by HTA are shown in Fig. 2.
Growth kinetics curves in the absence of AZT (based on HIV-1 p24 antigen and RT activity determinations in MT-4 cells and PBMC, respectively) were similar for all recombinant viruses (data not shown). However, some differences were observed when dual infection experiments were carried out in the absence or in the presence of 6 nM AZT. Figure 3A shows the fitness difference of recombinant viruses relative to the four HIV-1 control strains used. Recombinant viruses carrying a wild-type BH10 RT outcompeted the control strains in growth competition experiments in the absence of drug, while viruses with an MNR RT derived from the clinical isolate totally outcompeted the control strains in the presence of 6 nM AZT (Fig. 3A). Based on these growth competition experiments and fitness difference values, we were able to calculate the total relative fitness of each recombinant virus in these two different environments (i.e., no drug and 6 nM AZT) (Table 1). Although not statistically significant, the total relative fitness of all recombinant viruses carrying wild-type BH10 RT was higher in the absence of drug than when AZT was present in the cell culture (relative fitness ranges of 1.56 to 2.83 and 1.19 to 1.71, respectively) (Table 1). Conversely, relative fitness values were significantly lower for all three recombinant viruses carrying an MNR SS RT in the absence of drug than with those obtained in the presence of AZT (relative fitness ranges of 0.82 to 1.86 and 4.13 to 4.37, respectively; P < 0.001, Mann-Whitney test) (Table 1). These results are in agreement with previous studies revealing that an accumulation of drug resistance mutations has a debilitating effect on HIV-1 replication (and, consequently, viral fitness) while conferring a selective advantage over the wild-type virus in the presence of antiretroviral drugs (reviewed in references 26 and 29).
FIG. 3.
Viral fitness of recombinant HIV-1 variants in the absence (No drug) or presence (6 nM AZT) of drug selection. (A) Fitness differences were derived from the three dual infections in each competition with a control strain (i.e., A1, HIV-1A-92RW009; C, HIV-1C-92BR025; A2, HIV-1A-92UG029; and E, HIV-1ECMU06). Even though our equation for fitness difference always produces a positive value, these values were plotted as negative when the recombinant virus was less fit than the control strain, to facilitate data interpretation (30). Thus, a positive or negative fitness difference corresponds to a recombinant HIV-1 strain being more or less fit than the HIV-1 control isolate, respectively. (B) Total relative fitness of each recombinant HIV-1 strain, with wild-type (BH10) or clinical isolate (SS) RT background, calculated relative to the viral fitness of the wild-type HIV-1BH10 control (100%).
Total relative fitness values for each recombinant HIV-1 variant were compared with the viral fitness of recombinant virus having the RT of the wild-type HIV-1BH10 strain (BH10). The fitness of each recombinant virus was then expressed as a percentage of the wild-type virus fitness (taken as 100%) (Fig. 3B). A rank order of viral fitness among the recombinant viruses in the absence or presence of drug was determined. As expected, in the presence of AZT, recombinant viruses bearing the MNR SS RT showed higher fitness than viruses carrying a wild-type BH10 RT (i.e., SS = 2S0S > 2S4S ≫ WT > T69S > T69SSS) (Table 1; Fig. 3B). In addition, there was a correlation between the corresponding AZT IC50 values and the relative fitness of recombinant viruses determined in the presence of AZT (r = 0.98, P = 0.003; Pearson product moment). However, in the absence of drug, the order for viral fitness was wild type > T69S > SS ≥ T69SSS > 2S0S > 2S4S (Table 1; Fig. 3B). Interestingly, the virus with the RT from the original clinical HIV-1 isolate (SS) was slightly more fit than the wild-type virus with the engineered serine insertion (T69SSS) (65.7 and 55.1% of the wild-type BH10 control, respectively) (Table 1; Fig. 2 and 3B). Moreover, the introduction of a dipeptide insertion in a wild-type RT background (T69SSS) was not sufficient to confer AZT resistance (Table 1), although viral fitness was significantly reduced in this mutant (Table 1; Fig. 3B). On the other hand, deleting the two serine insertions from the MNR background (2S0S virus) did not have a large impact on AZT resistance, but viral fitness in the absence of drug was considerably reduced (36.7%; Table 1 and Fig. 3B). From these results, it is clear that development of high-level resistance to AZT and optimal viral fitness requires both the insertion at codons 69 and 70 and additional mutations found in the SS RT sequence (i.e., MNR 69 insertion complex) whose identification remains to be investigated.
Our results indicate that the insertion between codons 69 and 70 of the viral RT does not confer a selective advantage in the absence of drug. This observation is consistent with its low prevalence in HIV-infected individuals (approximately 1 to 3%) (1, 2, 6, 24, 39, 40, 41) and with the fluctuating nature of the genomes harboring the insertion, which disappears quickly after AZT treatment is interrupted (6, 21). Estimates obtained from a theoretical model considering viral dynamics in vivo showed that the relative fitness of the insertion mutant in the absence of therapy was less than 84%, compared to wild-type virus (21). Interestingly, insertions at codons 69 and 70 have not been detected in antiretroviral therapy-naïve patients, suggesting that they could bear a selective disadvantage in the absence of compensatory mutations. On the other hand, in extensively treated HIV-infected individuals, insertion-containing multi-NRTI mutants were able to maintain high viral loads in the presence of antiretroviral therapy (21). A recent study has identified three patients infected with viruses harboring the MNR 69 insertion complex mutation (1). In these case, the insertion persisted in both plasma HIV-1 RNA and proviral DNA in all patients after a 1-year follow-up, despite several changes in antiretroviral regimens, while exhibiting sustained virologic failure (1). These results, as well as several studies indicating the presence of these mutants in heavily treated individuals (1, 2, 6, 7, 10, 11, 24, 34, 37-39, 41), indicate that these viruses are selected and replicate efficiently despite the fitness loss expected from drug resistance mutations.
Altogether, our results suggest that RT insertions may improve the fitness of viruses harboring the Ser-Ser insertion at codons 69 and 70 of the RT-coding region within the MNR 69 insertion complex (i.e., 69SSS in a background of NRTI resistance mutations). This would help explain why insertion mutations have only been found in this particular RT sequence context. Hence, both drug resistance and viral fitness play a role in selection of HIV-1 RT mutations. The emergence of specific mutations is often highly dependent on the baseline sequence as well as on the sequential selection of compensatory mutations that contribute to viral fitness (28, 33; J. Weber, H. Valdez, H. R. Rangel, B. Chakraborty, E. Connick, K. Smith, A. Landay, D. R. Kuritzkes, M. M. Lederman, and M. E. Quiñones-Mateu, 9th Conf. Retrovir. Opportunistic Infect., p. 266, 2002). Additional studies will be necessary to evaluate the role of drug resistance mutations on viral fitness and the clinical significance of this relationship on HIV-1 pathogenesis and drug failure.
Acknowledgments
Research performed at the Cleveland Clinic Foundation (M.E.Q.-M.) was supported by research grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH) (5-KO1-HL67610-02), and the NIH Center for AIDS Research (AI36219) at Case Western Reserve University. Grants 01/0067-01 (to L.M.-A.) and 01/0067-02 (to M.A.M.) from the Spanish Fondo de Investigación Sanitaria and grant 36207/01 (to L.M.-A. and M.A.M.) from the Fundación para la Investigación y Prevención del SIDA en España are acknowledged. Research at Badalona (M.A.M.) was also supported by funds of Fundació irsiCaixa. The Centro de Biología Molecular “Severo Ochoa” is a recipient of an institutional grant from Fundación Ramón Areces.
REFERENCES
- 1.Andreoletti, L., L. Weiss, A. Si-Mohamed, C. Piketty, T. Prazuck, G. Calamy, J. E. Malkin, M. Matta, F. X. Mbopi-Keou, F. Clavel, M. D. Kazatchkine, and L. Belec. 2002. Multidrug-resistant HIV-1 RNA and proviral DNA variants harboring new dipeptide insertions in the reverse transcriptase pol gene. J. Acquir. Immune Defic. Syndr. 29:102-104. [DOI] [PubMed] [Google Scholar]
- 2.Balotta, C., M. Violin, L. Monno, P. Bagnarelli, C. Riva, G. Facchi, A. Berlusconi, M. Lippi, S. Rusconi, M. Clementi, M. Galli, G. Angarano, and M. Moroni. 2000. Prevalence of multiple dideoxynucleoside analogue resistance (MddNR) in a multicenter cohort of HIV-1-infected Italian patients with virologic failure. J. Acquir. Immune Defic. Syndr. 24:232-240. [DOI] [PubMed] [Google Scholar]
- 3.Berkhout, B. 1999. HIV-1 evolution under pressure of protease inhibitors: climbing the stairs of viral fitness. J. Biomed. Sci. 6:298-305. [DOI] [PubMed] [Google Scholar]
- 4.Boyer, P. L., J. Lisziewicz, F. Lori, and S. H. Hughes. 1999. Analysis of amino insertion mutations in the fingers subdomain of HIV-1 reverse transcriptase. J. Mol. Biol. 286:995-1008. [DOI] [PubMed] [Google Scholar]
- 5.Brenner, B. G., J. P. Routy, M. Petrella, D. Moisi, M. Oliveira, M. Detorio, B. Spira, V. Essabag, B. Conway, R. Lalonde, R. P. Sekaly, and M. A. Wainberg. 2002. Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection. J. Virol. 76:1753-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briones, C., A. Mas, G. Gomez-Mariano, C. Altisent, L. Menendez-Arias, V. Soriano, and E. Domingo. 2000. Dynamics of dominance of a dipeptide insertion in reverse transcriptase of HIV-1 from patients subjected to prolonged therapy. Virus Res. 66:13-26. [DOI] [PubMed] [Google Scholar]
- 7.Briones, C., and V. Soriano. 1999. Different outcome in the first two patients with an HIV-1 multinucleoside drug-resistant T69SSS insertion in Spain. Antivir. Ther. 4:125-127. [PubMed] [Google Scholar]
- 8.Clavel, F., E. Race, and F. Mammano. 2000. HIV drug resistance and viral fitness. Adv. Pharmacol. 49:41-66. [DOI] [PubMed] [Google Scholar]
- 9.Coffin, J. M. 1995. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483-489. [DOI] [PubMed] [Google Scholar]
- 10.De Antoni, A., A. Foli, J. Lisziewicz, and F. Lori. 1997. Mutations in the pol gene of human immunodeficiency virus type 1 in infected patients receiving didanosine and hydroxyurea combination therapy. J. Infect. Dis. 176:899-903. [DOI] [PubMed] [Google Scholar]
- 11.de Jong, J. J., J. Goudsmit, V. V. Lukashov, M. E. Hillebrand, E. Baan, R. Huismans, S. A. Danner, J. H. ten Veen, F. de Wolf, and S. Jurriaans. 1999. Insertion of two amino acids combined with changes in reverse transcriptase containing tyrosine-215 of HIV-1 resistant to multiple nucleoside analogs. AIDS 13:75-80. [DOI] [PubMed] [Google Scholar]
- 12.Delwart, E. L., E. G. Shpaer, J. Louwagie, F. E. McCutchan, M. Grez, H. Rübsamen-Waigmann, and J. I. Mullins. 1993. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science 262:1257-1261. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine, E., K. Van Vaerenbergh, A.-M. Vandamme, and D. Schmitt. 1999. Multidrug resistant human immunodeficiency virus type 1. AIDS Rev. 1:231-237. [Google Scholar]
- 14.Gao, F., L. Yue, S. Craig, C. L. Thornton, D. L. Robertson, F. E. McCutchan, J. A. Bradac, P. M. Sharp, B. H. Hahn, and WHO Network for HIV Isolation and Characterization. 1994. Genetic variation of HIV type 1 in four World Health Organization-sponsored vaccine evaluation sites: generation of functional envelope (glycoprotein 160) clones representative of sequence subtypes A, B, C, and E. AIDS Res. Hum. Retrovir. 10:1359-1368. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch, M. S., F. Brun-Vezinet, R. T. D'aquila, S. M. Hammer, V. A. Johnson, D. R. Kuritzkes, C. Loveday, J. W. Mellors, B. Clotet, B. Conway, L. M. Demeter, S. Vella, D. M. Jacobsen, and D. D. Richman. 2000. Antiretroviral drug resistance testing in adult HIV-1 infection: recommendations of an International AIDS Society—USA panel. JAMA 283:2417-2426. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch, M. S., B. Conway, R. T. D'aquila, V. A. Johnson, F. Brun-Vezinet, B. Clotet, L. M. Demeter, S. M. Hammer, D. M. Jacobsen, D. R. Kuritzkes, C. Loveday, J. W. Mellors, S. Vella, and D. D. Richman. 1998. Antiretroviral drug resistance testing in adults with HIV infection: implications for clinical management. International AIDS Society—USA Panel. JAMA 279:1984-1991. [DOI] [PubMed] [Google Scholar]
- 17.Kellam, P., and B. A. Larder. 1994. Recombinant virus assay: a rapid, phenotypic assay for assessment of drug susceptibility of human immunodeficiency virus type 1 isolates. Antimicrob. Agents Chemother. 38:23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosalaraksa, P., M. F. Kavlick, V. Maroun, R. Le, and H. Mitsuya. 1999. Comparative fitness of multi-dideoxynucleoside-resistant human immunodeficiency virus type 1 (HIV-1) in an in vitro competitive HIV-1 replication assay. J. Virol. 73:5356-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larder, B. A., S. Bloor, S. D. Kemp, K. Hertogs, R. L. Desmet, V. Miller, M. Sturmer, S. Staszewski, J. Ren, D. K. Stammers, D. I. Stuart, and R. Pauwels. 1999. A family of insertion mutations between codons 67 and 70 of human immunodeficiency virus type 1 reverse transcriptase confer multinucleoside analog resistance. Antimicrob. Agents Chemother. 43:1961-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little, S. J., E. S. Daar, R. T. D'aquila, P. H. Keiser, E. Connick, J. M. Whitcomb, N. S. Hellmann, C. J. Petropoulos, L. Sutton, J. A. Pitt, E. S. Rosenberg, R. A. Koup, B. D. Walker, and D. D. Richman. 1999. Reduced antiretroviral drug susceptibility among patients with primary HIV infection. JAMA 282:1142-1149. [DOI] [PubMed] [Google Scholar]
- 21.Lukashov, V. V., R. Huismans, M. F. Jebbink, S. A. Danner, R. J. De Boer, and J. Goudsmit. 2001. Selection by AZT and rapid replacement in the absence of drugs of HIV type 1 resistant to multiple nucleoside analogs. AIDS Res. Hum. Retrovir. 17:807-818. [DOI] [PubMed] [Google Scholar]
- 22.Maeda, Y., D. J. Venzon, and H. Mitsuya. 1998. Altered drug sensitivity, fitness, and evolution of human immunodeficiency virus type 1 with pol gene mutations conferring multi-dideoxynucleoside resistance. J. Infect. Dis. 177:1207-1213. [DOI] [PubMed] [Google Scholar]
- 23.Mas, A., M. Parera, C. Briones, V. Soriano, M. A. Martinez, E. Domingo, and L. Menendez-Arias. 2000. Role of a dipeptide insertion between codons 69 and 70 of HIV-1 reverse transcriptase in the mechanism of AZT resistance. EMBO J. 19:5752-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masquelier, B., E. Race, C. Tamalet, D. Descamps, J. Izopet, C. Buffet-Janvresse, A. Ruffault, A. S. Mohammed, J. Cottalorda, A. Schmuck, V. Calvez, E. Dam, H. Fleury, F. Brun-Vezinet, and the ANRS AC11 Resistance Study Group. 2001. Genotypic and phenotypic resistance patterns of human immunodeficiency virus type 1 variants with insertions or deletions in the reverse transcriptase (RT): multicenter study of patients treated with RT inhibitors. Antimicrob. Agents Chemother. 45:1836-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, M. D., N. A. Margot, P. D. Lamy, M. D. Fuller, K. E. Anton, A. S. Mulato, and J. M. Cherrington. 2001. Adefovir and tenofovir susceptibilities of HIV-1 after 24 to 48 weeks of adefovir dipivoxil therapy: genotypic and phenotypic analyses of study GS-96-408. J. Acquir. Immune Defic. Syndr. 27:450-458. [DOI] [PubMed] [Google Scholar]
- 26.Nijhuis, M., S. Deeks, and C. Boucher. 2001. Implications of antiretroviral resistance on viral fitness. Curr. Opin. Infect. Dis. 14:23-28. [DOI] [PubMed] [Google Scholar]
- 27.Omrani, A. S., and D. Pillay. 2000. Multi-drug resistant HIV-1. J. Infect. 41:5-11. [DOI] [PubMed] [Google Scholar]
- 28.Precious, H. M., H. F. Gunthard, J. K. Wong, R. T. D'aquila, V. A. Johnson, D. R. Kuritzkes, D. D. Richman, and A. J. Leigh Brown. 2000. Multiple sites in HIV-1 reverse transcriptase associated with virological response to combination therapy. AIDS 14:31-36. [DOI] [PubMed] [Google Scholar]
- 29.Quiñones-Mateu, M. E., and E. J. Arts. 2001. HIV-1 fitness: implications for drug resistance, disease progression, and global epidemic evolution, p. 134-170. In C. Kuiken, B. Foley, B. Hahn, P. Marx, F. McCutchen, J. Mellors, S. Wolinsky, and B. Korber (ed.), HIV sequence compendium 2001. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 30.Quiñones-Mateu, M. E., S. C. Ball, A. J. Marozsan, V. S. Torre, J. L. Albright, G. Vanham, G. G. van der, R. L. Colebunders, and E. J. Arts. 2000. A dual infection/competition assay shows a correlation between ex vivo human immunodeficiency virus type 1 fitness and disease progression. J. Virol. 74:9222-9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rakik, A., M. Ait-Khaled, P. Griffin, D. A. Thomas, M. Tisdale, J. P. Kleim, et al. 1999. A novel genotype encoding a single amino acid insertion and five other substitutions between residues 64 and 74 of the HIV-1 reverse transcriptase confers high-level cross-resistance to nucleoside reverse transcriptase inhibitors. J. Acquir. Immune Defic. Syndr. 22:139-145. [DOI] [PubMed] [Google Scholar]
- 32.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 33.Rose, R. E., Y. F. Gong, J. A. Greytok, C. M. Bechtold, B. J. Terry, B. S. Robinson, M. Alam, R. J. Colonno, and P. F. Lin. 1996. Human immunodeficiency virus type 1 viral background plays a major role in development of resistance to protease inhibitors. Proc. Natl. Acad. Sci. USA 93:1648-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross, L., M. Johnson, N. Graham, M. Shaefer, and M. St. Clair. 1999. The reverse transcriptase codon 69 insertion is observed in nucleoside reverse transcriptase inhibitor-experienced HIV-1-infected individuals, including those without prior or concurrent zidovudine therapy. J. Hum. Virol. 2:290-295. [PubMed] [Google Scholar]
- 35.Sanders-Buell, E., M. Salminen, and F. McCutchan. 1995. Sequencing primers for HIV-1, p. III-15-III-21. In B. Korber, B. D. Walker, J. P. Moore, G. Myers, C. Brander, R. A. Koup, and B. Haynes (ed.), Human retroviruses and AIDS. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 36.Shafer, R. W., K. Dupnik, M. A. Winters, and S. H. Eshleman. 2000. A guide to HIV-1 reverse transcriptase and protease sequencing for drug resistance studies. In C. Kuiken, B. Foley, B. Hahn, P. Marx, F. McCutchen, J. Mellors, S. Wolinsky, and B. Korber (ed.), HIV sequence compendium 2000. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex. [PMC free article] [PubMed]
- 37.Sugiura, W., M. Matsuda, Z. Matsuda, H. Abumi, A. Okano, T. Oishi, K. Moriya, Y. Yamamoto, K. Fukutake, J. Mimaya, A. Ajisawa, M. Taki, K. Yamada, and Y. Nagai. 1999. Identification of insertion mutations in HIV-1 reverse transcriptase causing multiple drug resistance to nucleoside analogue reverse transcriptase inhibitors. J. Hum. Virol. 2:146-153. [PubMed] [Google Scholar]
- 38.Tamalet, C., J. Izopet, N. Koch, J. Fantini, and N. Yahi. 1998. Stable rearrangements of the β3-β4 hairpin loop of HIV-1 reverse transcriptase in plasma viruses from patients receiving combination therapy. AIDS 12:F161-F166. [DOI] [PubMed] [Google Scholar]
- 39.Tamalet, C., N. Yahi, C. Tourres, P. Colson, A. M. Quinson, I. Poizot-Martin, C. Dhiver, and J. Fantini. 2000. Multidrug resistance genotypes (insertions in the β3-β4 finger subdomain and MDR mutations) of HIV-1 reverse transcriptase from extensively treated patients: incidence and association with other resistance mutations. Virology 270:310-316. [DOI] [PubMed] [Google Scholar]
- 40.Van Vaerenbergh, K., K. Van Laethem, J. Albert, C. A. Boucher, B. Clotet, M. Floridia, J. Gerstoft, B. Hejdeman, C. Nielsen, C. Pannecouque, L. Perrin, M. F. Pirillo, L. Ruiz, J. C. Schmit, F. Schneider, A. Schoolmeester, R. Schuurman, H. J. Stellbrink, L. Stuyver, J. Van Lunzen, B. Van Remoortel, E. Van Wijngaerden, S. Vella, M. Witvrouw, S. Yerly, E. De Clercq, J. Destmyer, and A. M. Vandamme. 2000. Prevalence and characteristics of multinucleoside-resistant human immunodeficiency virus type 1 among European patients receiving combinations of nucleoside analogues. Antimicrob. Agents Chemother. 44:2109-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winters, M. A., K. L. Coolley, Y. A. Girard, D. J. Levee, H. Hamdan, R. W. Shafer, D. A. Katzenstein, and T. C. Merigan. 1998. A 6-base pair insert in the reverse transcriptase gene of human immunodeficiency virus type 1 confers resistance to multiple nucleoside inhibitors. J. Clin. Investig. 102:1769-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]