Abstract
1. The uptake of the inhibitors N-ethylmaleimide (NEM) and 1-fluoro-2,4-dinitrobenzene (DNFB) by human red cells has been correlated with the inhibition of glucose exit.
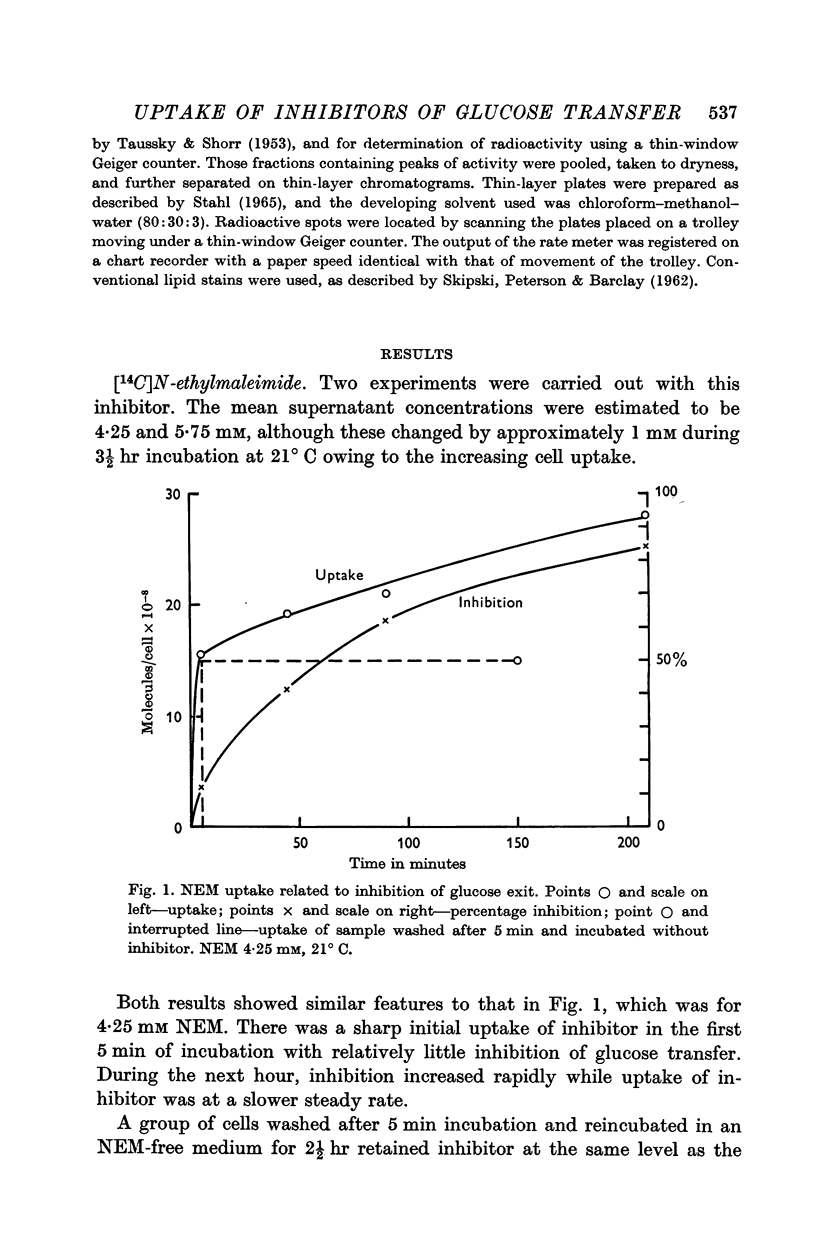
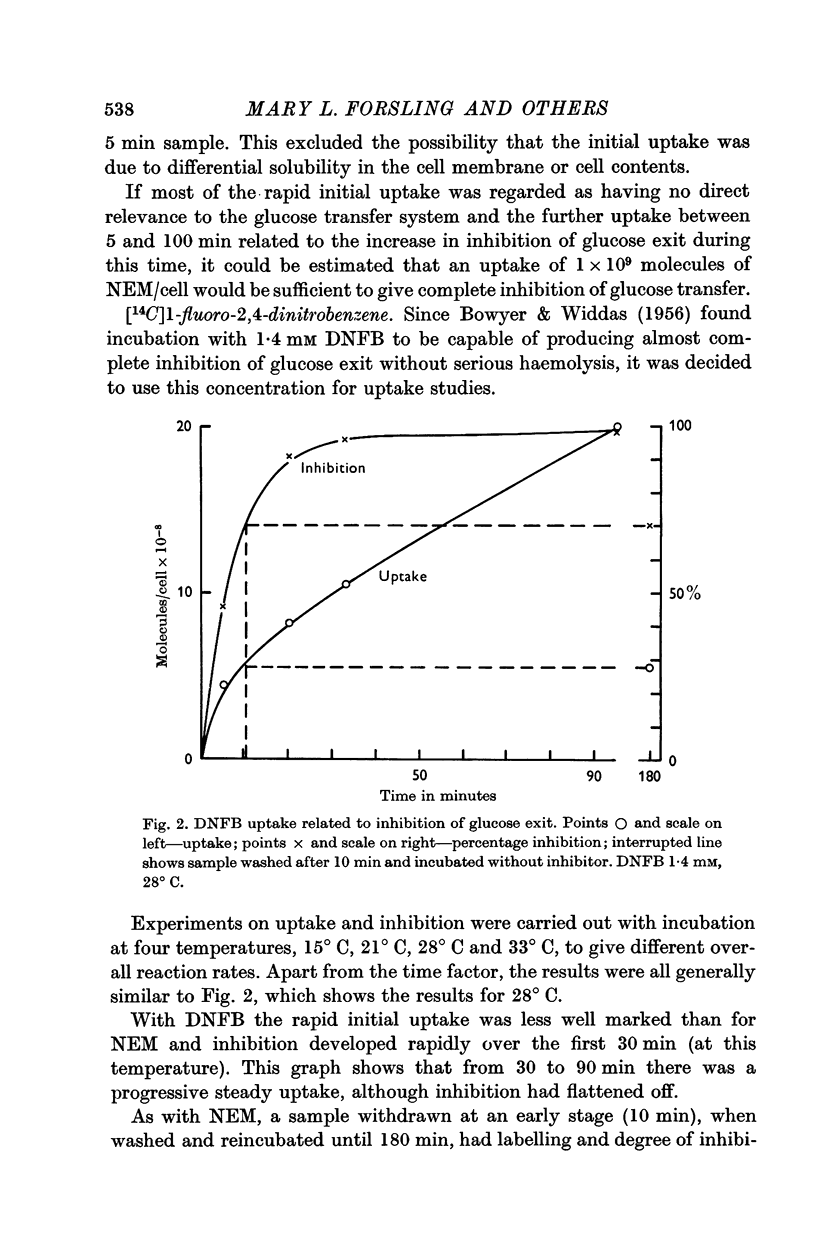
2. With both inhibitors there was an initial rapid uptake by the cells with little inhibition; this was followed by a phase when inhibition was developing rapidly but uptake continued at a steady rate even after the development of inhibition had flattened off.
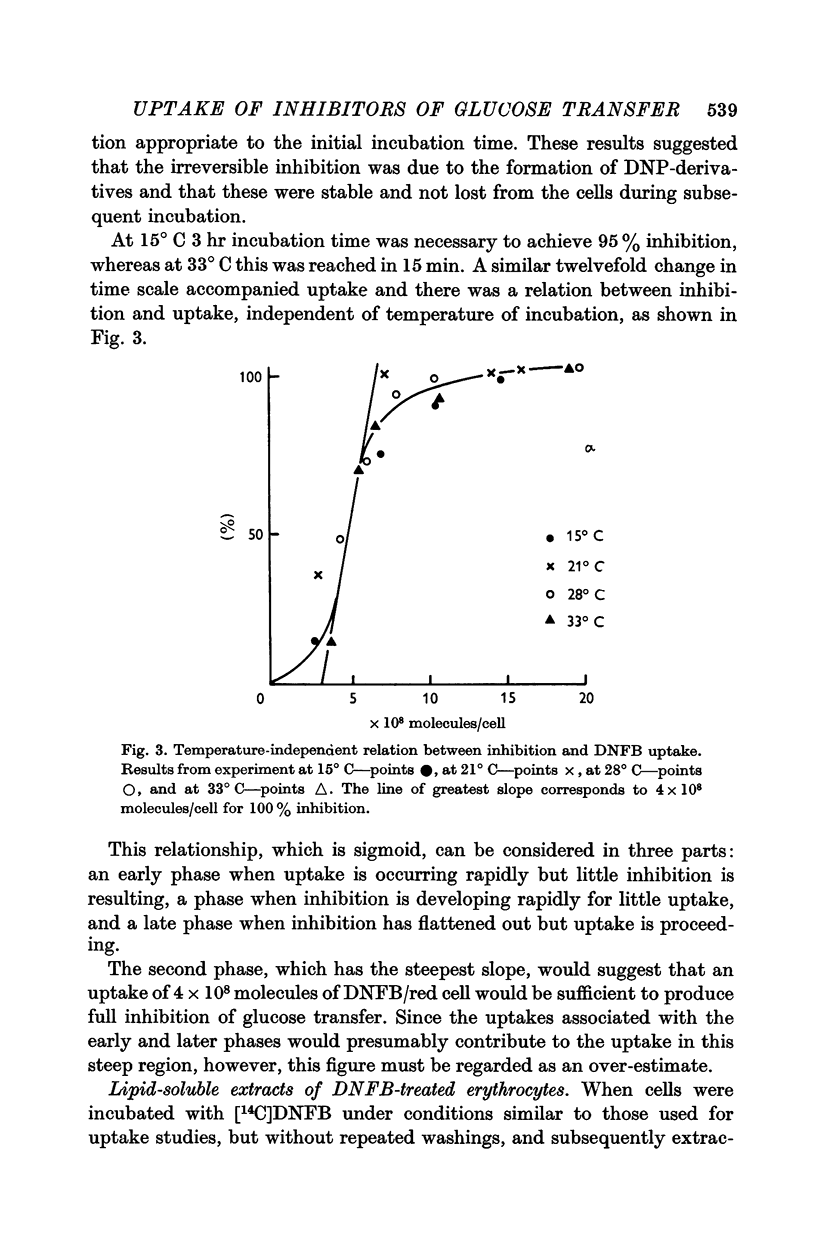
3. The rate of uptake of DNFB during the rapid development of inhibition corresponded to about 4 × 108 molecules/cell for 100% inhibition, irrespective of the temperature of incubation. This cannot be used as an estimate of the number of glucose transfer sites in the cell membrane because of the lack of specificity.
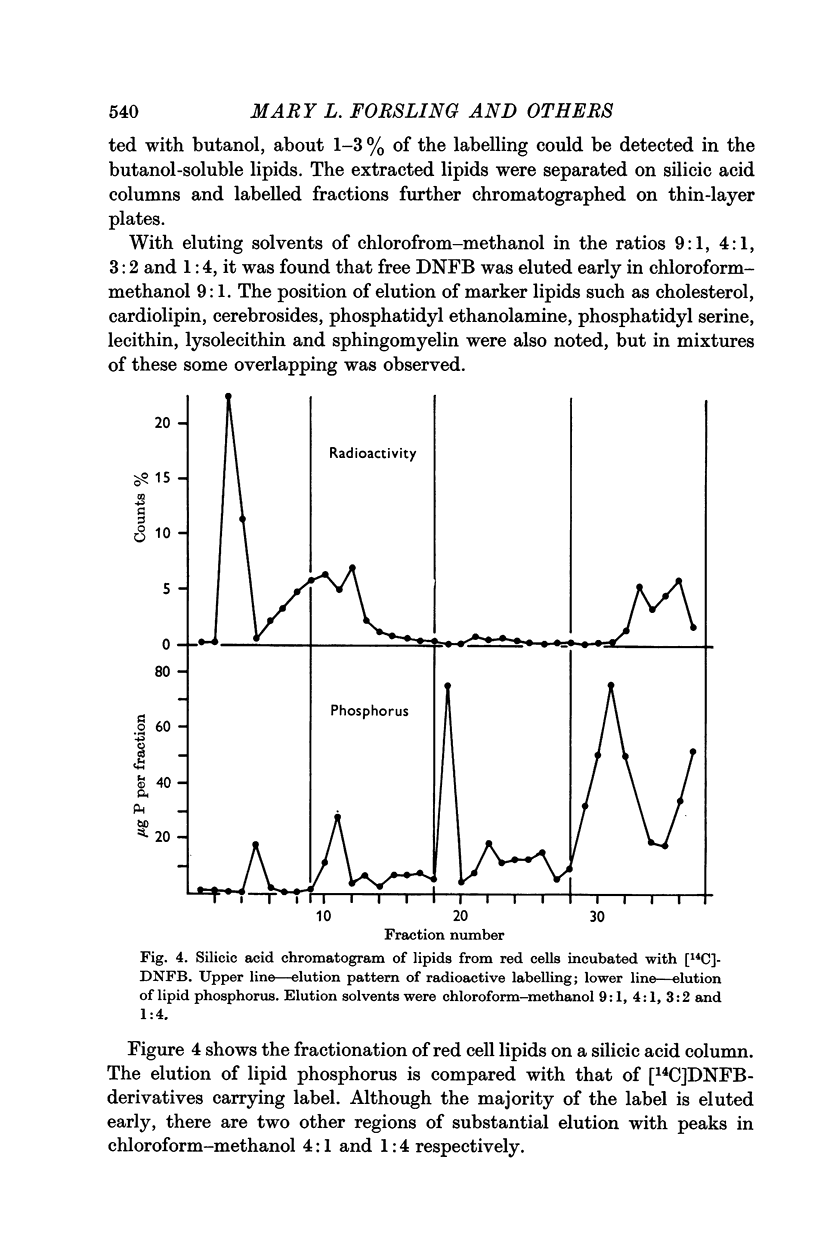
4. In an examination of lipids from red cells incubated with [14C]DNFB, labelling associated with lipids was eluted with peaks in chloroform—methanol 4:1 and 1:4 respectively. Thus, although DNFB is normally regarded as a protein reagent, involvement of lipids in the transfer of glucose could not be excluded.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOWYER F., WIDDAS W. F. The action of inhibitors on the facilitated hexose transfer system in erythrocytes. J Physiol. 1958 Apr 30;141(2):219–232. doi: 10.1113/jphysiol.1958.sp005969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON A. C., WIDDAS W. F. INHIBITION OF THE GLUCOSE PERMEABILITY OF HUMAN ERYTHROCYTES BY N-ETHYL MALEIMIDE. J Physiol. 1963 Oct;168:644–659. doi: 10.1113/jphysiol.1963.sp007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAHAN D. J., DITTMER J. C., WARASHINA E. A column chromatographic separation of classes of phospholipides. J Biol Chem. 1957 Oct;228(2):685–700. [PubMed] [Google Scholar]
- HUNTER F. R. PERMEABILITY OF ERYTHROCYTES TO SUGARS. III. A FURTHER ANALYSIS OF THE EFFECT OF TANNIC ACID, MERCURY AND TRITON X-100. J Cell Physiol. 1964 Feb;63:39–54. doi: 10.1002/jcp.1030630105. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G., HABICH K. I., HESS H. S., HUDSON M. R. PHOSPHOLIPID-SUGAR COMPLEXES IN RELATION TO CELL MEMBRANE MONOSACCHARIDE TRANSPORT. Science. 1964 Feb 28;143(3609):955–957. doi: 10.1126/science.143.3609.955. [DOI] [PubMed] [Google Scholar]
- Mawdsley D. A., Widdas W. F. A lipid-soluble glucose complex extractable from human erythrocyte ghosts. J Physiol. 1967 Apr;189(2):75P–76P. [PubMed] [Google Scholar]
- SEN A. K., WIDDAS W. F. Variations of the parameters of glucose transfer across the human erythrocyte membrane in the presence of inhibitors of transfer. J Physiol. 1962 Mar;160:404–416. doi: 10.1113/jphysiol.1962.sp006855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]


