Abstract
Neurons are often assumed to be the principal sites for replication of the infectious agents causing Creutzfeldt-Jakob disease (CJD), scrapie, and bovine spongiform encephalopathy because they express high levels of normal and pathological prion protein (PrP). However, isolated brain cell types have not been evaluated for either infection or gene expression. Microglia purified from CJD-infected mice showed infectivity comparable to that of starting brain homogenate but expressed ∼50-fold less PrP. CJD-infected microglia also displayed morphological changes indicative of cellular activation. To determine the molecular pathways of activation, we evaluated pertinent transcripts, including those linked to inflammation. Semiquantitative reverse transcription-PCR showed a >4-fold increase in cathepsin S, an enzyme important in antigen presentation, the cytokine interleukin-1β, and the chemokine B-lymphocyte chemoattractant. The profile of microglial changes induced by the CJD agent differed substantially from activation induced by bacterial lipopolysaccharide or by β-amyloid, a structure comparable to pathological PrP. These microglial studies emphasize migratory hematopoietic cells in the dispersion, and possibly replication, of the CJD agent. The low PrP levels in these highly infectious and activated cells further support the concept that pathological PrP is the result of infection rather than the infectious agent itself. Because microglia develop a specific pattern of responses to the CJD agent, microglial markers may be exploited in the diagnosis of these spongiform encephalopathies.
Creutzfeldt-Jakob disease (CJD) and scrapie are neurodegenerative diseases caused by infectious agents that are incompletely characterized at the molecular level. The outbreak of bovine spongiform encephalopathy (BSE) and its link to variant CJD in humans makes it important to identify which types of cells carry the agent through the body. Many investigators emphasize neurons in agent spread because these cells accumulate abundant pathological prion protein (PrP), a host protein that aggregates with amyloid properties after infection. Although the contribution of other cell types in the brain, such as microglia, is unknown, such cells are often considered only reactive players. Nevertheless, these infectious agents can be recovered from circulating peripheral white blood cells that have no direct contact with neurons (20).
More recent studies have suggested that B lymphocytes or B-cell-dependent follicular dendritic cells are required for infection from the periphery (4, 35). However, this conclusion is contradicted by positive infection in several different B-cell-null mouse models (28, 39). An alternative route for the agent would be through cells of the myeloid lineage (24). Myeloid cells are phenotypically flexible and can develop macrophage or related dendritic cell characteristics in response to environmental stimuli. We have shown that peripheral myeloid cells can carry and maintain the CJD agent for at least 5 weeks in vitro (28), and subsequent studies have shown that scrapie infectivity can be recovered from acutely isolated dendritic cells (2).
Within the central nervous system, microglia are the major cells of myeloid origin. Microglia respond to brain injury and various types of infections with alterations in morphology, gene expression, and cytokine release (47). Microglial activation also occurs in various rodent models of CJD and scrapie, although the kinetics and magnitude of these responses vary among different agent strains (3, 13, 24). In at least one CJD model, profound and sustained microglial responses precede typical neurodegenerative changes by 100 days (24). This early microglial activation is followed by the sequential development of molecular changes culminating in the eventual deposition of PrP and vacuolar degeneration. A different CJD agent strain in another species also showed comparable microglial responses prior to PrP pathology (3). Thus, microglia may have a major role in the relatively early spread of the infectious agent and may also be critical for the later development of PrP pathology and vacuolar change.
To determine whether microglia carry the CJD agent, we assayed infectivity in microglial and nonmicroglial cell populations isolated from CJD-infected mouse brains. We also characterized the microglial response to CJD infection at the molecular level by semiquantitative reverse transcription (RT)-PCR analysis, paying particular attention to transcripts related to inflammation and immunity. Our results demonstrate that microglia have reasonably high levels of infectivity. Moreover, infectious microglia displayed changes in morphology and inflammation-related transcripts when compared to microglia from uninfected controls. Interestingly, this phenotype of activation by the CJD agent was distinct from the pattern elicited by the nonspecific inflammatory activator lipopolysaccharide (LPS).
MATERIALS AND METHODS
Immunomagnetic separation of microglia for infectivity assay.
Mice infected with the FU strain of CJD (22) were anesthetized with carbon dioxide when clinical signs appeared and perfused with physiological saline. Meninges were removed, and brains were diced and disrupted with 1 mg of collagenase II (Sigma, St. Louis, Mo.) per ml in RPMI for 1 h at 37°C. After DNA was digested with DNase I at 25 μg/ml for 5 min at 37°C, cells were pelleted at 500 × g for 5 min, resuspended in microglial medium (MM; RPMI 1640 with 5% fetal bovine serum, 10 mM HEPES, 1 mM sodium pyruvate, 50 U of penicillin per ml, and 50 μg of streptomycin per ml), and filtered through 100-μm nylon mesh. Cell suspensions were mixed with isotonic Percoll (Amersham Pharmacia Biotech, Piscataway, N.J.) to a density of ∼1.012 g/ml, layered onto a cushion of 1.088-g/ml Percoll, and centrifuged at 500 × g for 20 min at 22°C.
Cells from the interface were washed with MM and resuspended in PBE (phosphate-buffered saline with 1% bovine serum albumin and 5 mM EDTA). Aliquots taken at this stage were designated the Input cells. Microglial separation was done with anti-mouse CD11b microbeads and MS+ columns (Miltenyi Biotech, Auburn, Calif.). Magnetically labeled cell suspensions (0.5 ml) were applied to the column, and the initial flowthrough was collected, followed by five 0.5-ml PBE buffer washes of the column. Retained cells were eluted in 1 ml of PBE after magnet removal. This positive selection was then repeated on a fresh column.
After two rounds of positive selection, microglia (MGL+) were pelleted, resuspended in MM at 105 cells/μl, and stored at −70°C for inoculation. The negative cell population (Other) was pooled from the flowthrough and washes of both columns. Typical yields were 5 × 105 cells per brain in both the MGL+ and Other cell populations. Purity of these cells was monitored for CD45 by flow cytometry, because magnetic separation interfered with the later detection of CD11b.
Microglial and astrocyte cultures.
For short-term culture and RNA analyses, a slightly different cell protocol was adapted to increase microglial yield and viability (12, 31). The basic procedure described above was used, with the inclusion of 0.25% trypsin in the collagenase solution. After the initial Percoll separation, cells from the interface were applied to a second Percoll gradient consisting of 1.012-, 1.048-, 1.072-, and 1.088-g/ml steps instead of magnetic columns. Cells collected from the 1.048:1.072 interface were washed in MM and seeded at 105 cells/cm2. Following a 16- to 18-h incubation, nonadherent cells such as oligodendrocytes were removed by MM rinses. For stimulation experiments, LPS (serotype O55:B5; Sigma) was included in the medium at 100 ng/ml during the 16- to 18-h culture.
To compare astrocyte expression, we also prepared neonatal murine astroglial cultures by following established protocols (30). Briefly, neonatal animals (less than 24 h old) were decapitated; brains without meninges were triturated and passed through 100-μm and 75-μm nylon mesh. The resulting cell suspensions were pelleted and resuspended in Dulbecco's modified Eagle's medium with 10% fetal calf serum and seeded at densities equivalent to one mouse brain per 25-cm2 tissue culture flask. Cells were grown for 7 to 10 days, after which contaminating microglia and oligodendrocytes were removed by shaking for 16 h at 250 rpm at 37°C. Adherent astrocytes were grown for an additional day before collection. Astrocyte cultures contained >99% glial fibrillary acidic protein (GFAP)-positive cells, in contrast to microglia.
Flow cytometry and immunofluorescence.
About 105 cells were resuspended in staining medium (SM; phosphate-buffered saline with 2% fetal calf serum, 1 mM EDTA, and 0.1% NaN3) containing Fc Block (1:200; Pharmingen, Los Angeles, Calif.) to prevent nonspecific binding of antibodies to microglial Fc receptors. Cells were then incubated for 20 min on ice with primary antibody, washed, and analyzed on a Coulter MCL-XL flow cytometer. Antibodies to CD45 and CD11b were also from Pharmingen. Matched isotype standards were included in every experiment to measure background staining levels. For immunofluorescent microscopy, cells were fixed in 1% formalin in phosphate-buffered saline for 15 min at 22°C and stained with CD11b as described above. For GFAP staining, cells were fixed and permeabilized in 1% formalin containing 0.1% Triton X-100 and then stained with rabbit anti-mouse GFAP (Dako, Carpinteria, Calif.) and Alexa 488-conjugated goat anti-rabbit immunoglobulin secondary antibody (Molecular Probes, Eugene, Oreg.).
Western blotting and protease-resistant PrP assay.
Cell aliquots or brain homogenates were mixed with lysis buffer (150 mM NaCl, 50 mM Tris [pH 7.5], 0.5% Triton X-100, 0.5% sodium deoxycholate) for 15 min on ice and spun at 10,000 × g for 1 min at 4°C to remove nuclei. Acrylamide gels, blotting, and chemiluminescence were done as previously described (23). Antibodies included rabbit anti-PrP (1:200; Immuno-Biological Laboratories, Gunma, Japan), rabbit anti-GFAP (1:3,000; Dako), mouse anti-α-tubulin (1:2,000; Sigma), and mouse antineurofilament light chain (1:800; Sigma). Protein assays used the DC protein assay kit (Bio-Rad, Hercules, Calif.), and protease-resistant PrP was detected as previously described (23).
Animal inoculation.
Cells were diluted in 2% normal mouse brain homogenate to ∼5 × 103 cells/μl and passed repeatedly through a 26-gauge needle. Immediately after mixing, 30 μl was injected intracerebrally into tga20 transgenic mice (11), a kind gift of Charles Weissmann. CJD was diagnosed by progressive clinical signs and confirmed by immunohistochemistry and Western blotting as previously described (23).
RT-PCR analysis.
RNA isolation and reverse transcription (500 ng of total RNA per reaction) were done as described previously (3). Aliquots (0.5 μl) of this first-strand cDNA were amplified and biotinylated under standard PCR conditions with 0.2 mM each of the four deoxynucleoside triphosphates and 0.4 mM biotin-14-dCTP (Invitrogen). PCR cycles included 30 s at 95°C, 1 min of annealing, and 1 min at 72°C. The number of cycles for detection within the exponential phase of amplification was determined empirically and is listed in Table 1 along with annealing temperatures and primer sequences. Biotinylated PCR products were separated on 2.5% agarose gels, transferred to nylon membranes (Biodyne B; Pierce, Rockford, Ill.), and detected with the BrightStar Biodetect kit (Ambion, Austin, Tex.). Densitometry and expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as previously described (3).
TABLE 1.
Cycle numbers, annealing temperatures, and primer sequences for RT-PCR analysis
| Gene | No. of cycles | Temp (°C) | Forward primer | Reverse primer |
|---|---|---|---|---|
| BLC | 25 | 60 | TCAGCACAGCAACGCTGCTTCT | CTGGAGCTTGGGGAGTTGAAGA |
| Cathepsin | 18 | 64 | CAACTGCAGAGAGACCTACCCTGG | AGAGGAAGAAGGAGGAATGGCTGG |
| CCR5 | 26 | 65 | AAGAGAAGGTGAGACATCCGTTCCC | AAACTTCCTGTTCTCCTGTGGACCG |
| CD40 | 26 | 66 | GCGCGGCCGCTGCCCTGCATGGTGTCTTTGC | GCGGGATCCTGCACTGGGCTCTGTCTTGGC |
| CD68 | 20 | 60 | CAACAAAACCAAGGTCCAGGGA | CCAATGATGAGAGGCAGCAAGA |
| c-fms | 20 | 60 | TGAAGGTGGCTGTGAAGATGC | TCACTTGAAGAAGTCGAGACAGGC |
| GAPDH | 20 | 60 | GACCTCAACTACATGGTCTACAT | TGGTTCACACCCATCACAAACAT |
| GFAP | 28 | 64 | GAGGGCCAAAGCCTCAAGGAGGAGA | CTTTACCACGATGTTCCTCTTGAGGTG |
| IL-1β | 21 | 63 | TGCAAGTGTCTGAAGCAGCTATGG | GGTGGGTGTGCCGTCTTTCATTACA |
| IP-10 | 22 | 60 | GCTGCAACTGCATCCATATCGA | TTGGCTAAACGCTTTCATTAAATTC |
| MIP-1α | 23 | 60 | GAAGAGTCCCTCGATGTGGCTA | CCCTTTTCTGTTCTGCTGACAAG |
| MIP-1β | 25 | 60 | CCACAATAGCAGAGAAACAGCAAT | AACCCCGAGCAACACCATGAAG |
| Neurofilament | 28 | 66 | GCTGGAGAAGCGCATCGACAGCC | GACATCAAGTAGGAGCTGCTCTGC |
| PrP | 26 | 64 | CTGGCTGCTGGCCCTCTTTGTG | GGTGGTGACCGTGTGCTGCTTG |
| TNF-α | 22 | 60 | GCTGGAAGACTCCTCCCAGGTA | ATGATCCGCGACGTGGAACTG |
RESULTS
Characterization of microglia isolated from CJD-infected brains.
We purified microglial cells from the brains of mice with clinical disease (∼130 days postinoculation), with immunomagnetic separation columns that select for CD11b+ cells. The negative flowthrough cells from the columns were CD11b− and are designated here as the Other population. Positively selected microglia from the brain parenchyma expressed low levels of CD45, a receptor tyrosine phosphatase expressed by all leukocytes, in addition to CD11b. This distinguishes them from peripheral leukocytes, which express high levels of CD45.
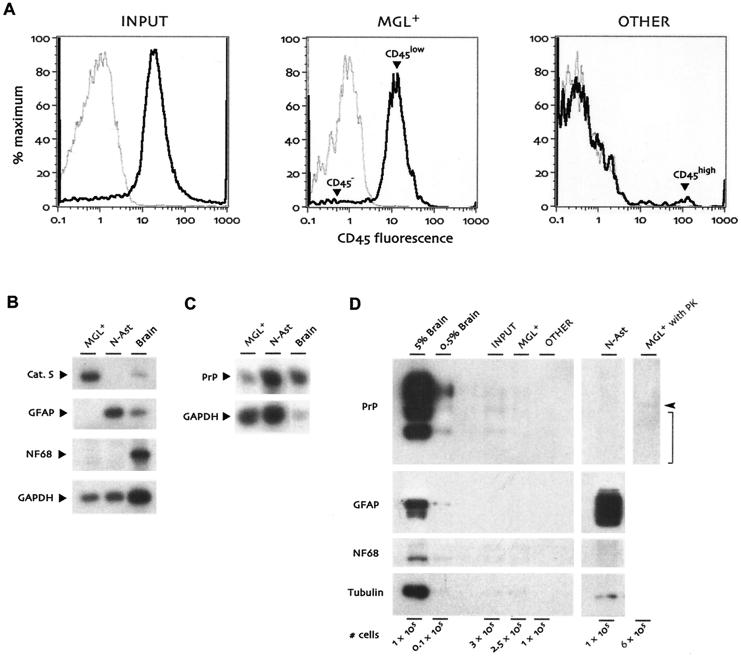
To evaluate the purification protocol, we examined cell surface CD45 (shown as black lines in Fig. 1A) by flow cytometry in comparison with an irrelevant matched isotype control antibody (gray lines). Prior to purification (Input), most cells were already positive for CD45 (Fig. 1A, left panel). Selection for CD11b+ microglia (MGL+) with the magnetic columns yielded ∼95% CD45low microglia, with a few CD45− cells (arrowheads, Fig. 1A, middle panel). The CD11b− flowthrough from the column purification (Other) demonstrated substantial enrichment for CD45− cells, with only slight contamination by CD45high cells, probably lymphocytes from residual blood (arrowhead, Fig. 1A, right panel). Because there were no obvious CD45low cells, this other population was essentially devoid of microglia.
FIG. 1.
Characterization of cell populations isolated from end-stage CJD-infected mouse brains. (A) Flow cytometry histograms for CD45 (black lines) and an isotype control antibody (gray lines). Cells prior to CD11b magnetic column purification (Input, left panel) were already 80 to 85% CD45+. The positively selected MGL+ population (middle panel) was ∼95% CD45low microglia (arrowhead) after selection for CD11b+ cells, with very few CD45− nonleukocytic cells (arrowhead). The CD11b− flowthrough population (Other, right panel) was ∼90% CD45− with a few CD45high cells, probably lymphocytes from residual blood (arrowhead). CD11b− CD45high cells were not present in MGL+ and were not readily visible in the input population due to their very small numbers prior to column separation. (B) RT-PCR for microglial, astrocytic, and neuronal markers. RNAs from MGL+ cells, normal astrocyte cultures (N-Ast), and whole brain were analyzed in parallel for cathepsin S (Cat. S), GFAP, and neurofilament light chain (NF68). (C) PrP expression levels compared in microglia, astrocytes, and whole brain by semiquantitative RT-PCR. The amount of brain RNA in this assay was reduced so that all samples would be in the exponential phase of PrP amplification in parallel. (D) Analysis of cell populations on Western blots. The indicated number of cells from the Input, MGL+, and Other populations were compared with neonatal astrocytes as well as aliquots from 5% and 0.5% brain homogenates. Cell numbers shown for brain homogenates are based on an estimate of 109 cells per g of brain. Compared with brain, cells displayed little PrP or neurofilament light chain (NF68) expression, and GFAP could be seen only with extremely long film exposure, at least 50-fold reduced relative to that of starting whole brain. α-Tubulin was detected on the same blot for comparison between tubulin-rich neurons and microglial cells, which are relatively tubulin-poor. The final lane shows virtually undetectable levels of protease-resistant PrP (expected position indicated by bracket) in CJD-infected microglia that had been digested with proteinase K (PK). The arrowhead indicates the migration of proteinase K, which cross-reacts with the PrP antiserum.
The purity of the microglial population and the separation from other cell types were also determined by independent examination of mRNA. Semiquantitative RT-PCR assays showed that MGL+ cells were positive for cathepsin S, a myeloid marker, but were negative for GFAP and for the neuron-specific neurofilament light chain even after 28 cycles of PCR amplification (Fig. 1B). This sensitive assay would also detect ribosome-associated mRNAs from neuronal or astrocytic membranes that might nonspecifically adhere to microglia. Normal astrocytes showed neither cathepsin S nor neurofilament expression. In comparison, unfractionated brain RNA was positive for all of these transcripts. Because the expression of PrP transcripts in microglia of the brain parenchyma has not been well resolved, we compared microglial PrP mRNA expression to that of normal astrocytes and whole brain. RT-PCR indicated low but detectable PrP mRNA in microglia, with ∼50-fold-lower steady-state expression relative to whole brain and ∼8-fold less than that found in isolated normal neonatal astrocytes (Fig. 1C).
We further evaluated PrP expression with Western blots for protein. PrP was easily detected in brain homogenates but could only be seen in microglial populations with extremely long film exposures. Starting brain homogenates contained ∼50-fold more PrP than that found in MGL+ by densitometry with normalization to amount of protein, and an even greater difference based on cell number (see below). MGL+ cells also had less PrP than the unpurified input population by ∼5-fold, indicating that nonspecific PrP contamination of microglia was reduced by the separation procedure. Finally, additional protein analyses showed GFAP and neurofilament only in the appropriate cell types and confirmed that microglia were essentially devoid of contaminating astrocytic and neuronal cell fragments (Fig. 1D). The negligible neuronal contamination of microglia was also confirmed by their rather low levels of tubulin (Fig. 1D), a molecule that is highly enriched in neuronal cell bodies and processes.
Infectivity of microglia.
On bioassay, purified microglia were infectious. The MGL+ population induced clinical signs of CJD in tga20 indicator mice at ∼76 days after inoculation (Table 2). A high input of CJD-infected brain homogenate (1%, or 10−2) resulted in an insignificantly shorter incubation of 71 days in the same indicator mice (P = 0.305). However, brain diluted to 10−4 showed a significantly longer incubation time (106 days) from MGL+ cells (P < 0.001). Animals inoculated with normal brain homogenates failed to show disease for >550 days. The incubation time difference of 17 days per log of infectivity in tga20 mice was remarkably consistent with more extensive experiments previously showing 15 days per log in CD-1 mice (22).
TABLE 2.
Comparison of infectivity in whole brain and sorted cell populationsa
| Inoculum | Mean time to terminal disease (days) ± SEM | No. of CJD- positive mice/no. inoculated | No. of cells injectedb |
|---|---|---|---|
| Brain dilution | |||
| 10−2 | 71.25 ± 2.4* | 10/10 | 3.0 × 105 |
| 10−4 | 105.6 ± 4.3 | 7/7 | 3.0 × 103 |
| Cells | |||
| Input | 82.6 ± 1.6* | 5/5 | 1.4 × 104 |
| MGL+ | 76.5 ± 3.6* | 10/10 | 5.0 × 104 |
| Other | 85.0 ± 5.0*,** | 7/7 | 3.9 × 104 |
Infectivity bioassay results after inoculation into tga20 mice. According to the incubation time difference between the two brain dilutions, each log dilution of infectivity extends the incubation period by 17 days. Starting normal brain showed no signs of infectivity at >550 days. *, significantly different (P < 0.001) from 10−4 dilution by analysis of variance; **, significantly different (P < 0.01) from 10−2 dilution by analysis of variance.
Brain cell number based on 109 cells per g of brain.
These incubation time differences with serial dilutions were used to interpolate relative infectious doses in unknown samples. A dose of 5 × 104 microglia was equivalent to the infectivity of a threefold-higher number of cells in 30 μl of a 0.5% brain homogenate (1.5 × 105 cells, based on an estimate of 109 cells per g of brain). Furthermore, on Western blots (Fig. 1D), 2 × 105 MGL+ cells had 20-fold less PrP than 104 cell equivalents from brain, or a 400-fold PrP difference per cell between these two cell types. Thus, low PrP levels in microglia did not predict the high infectivity levels that were indistinguishable from starting brain.
We also examined microglial levels of protease-resistant PrP, which, according to the prion hypothesis, should directly reflect the amount of infectivity. However, protease-resistant PrP was only a miniscule constituent of the total PrP in CJD-infected microglia and was barely visible on Western blots even with higher cell loads (Fig. 1D). This is consistent with observations of only a small subset of microglia containing detectable pathological PrP by immunocytochemistry (24). These discrepancies between infectious titers and any form of PrP have now been reproduced in several laboratories during cell fractionation and in various animal models of transmissible spongiform encephalopathy (7, 19, 23, 24, 26, 37, 42). Moreover, 10,000-fold differences in infectivity have been observed between two CJD strains in which protease-resistant PrP levels were only 10-fold discordant (22). The protease-resistant PrP molecules from these two divergent CJD strains also exhibit identical band patterns on protein gels, contrasting with the notion that the protease-resistant PrP band pattern is a reliable indicator of strain-specific agent properties (44).
Mice inoculated with Input cells developed clinical signs of CJD in ∼83 days (Table 2). This 8-day-longer incubation time relative to that for purified microglia was most likely due to the 3.5-fold-lower cell number in the inoculum. An additional preliminary experiment showed that inoculation of equal numbers of Input and MGL+ cells also yielded the same incubation time (data not shown). The Other cell population, which was devoid of microglia, showed a 10-day-longer incubation period than MGL+ cells. The Other fraction was statistically the only population significantly different from the 10−2 brain dilution (Table 2). Further subfractionation of Other cells may delineate the CD11b− cells that preferentially carry the CJD agent. In any case, infectivity was found in a variety of nonneuronal cell types, not unlike the variety of leukocytic cells that carry these agents peripherally (2, 4, 20, 28, 35).
Although we cannot completely exclude the possibility that some nonspecific contaminant was responsible for MGL+ infectivity or that the CJD agent associated with these cells during tissue disruption, the magnetic columns yielded final cell populations without debris (synaptosomes and cellular processes) visible by microscopy. Sensitive RT-PCR and Western blot detection of neuronal and glial markers as noted above further indicated that fragments of these cells were not contained in the microglial population. Moreover, microglial uptake of agent during processing would be unlikely, because the cells were kept on ice and our isolated microglia did not display any macrophage-like phagocytosis in control experiments with fluorescent microbeads (data not shown). Obviously, protease-resistant PrP was also not adhering to or being taken up by these infectious cells.
Microglia display morphological and molecular activation.
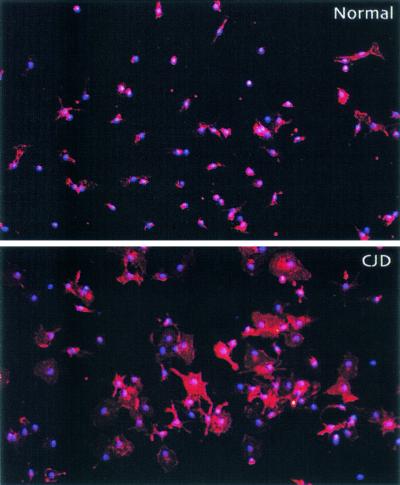
In further studies, we enhanced yields of viable cells by using density gradients and overnight culture for adherent cells. This approach yielded purities comparable to those of the magnetically separated microglia populations (∼95% CD11b+). Such viable cells should be more physiologically representative of the microglial population in vivo than cells acutely stressed during isolation. In four separate experiments, CJD-infected mouse brains yielded ∼4-fold more microglia than controls (1.60 × 106 ± 0.49 × 106 versus 0.36 × 106 ± 0.08 × 106 per brain; P < 0.05). Normal and CJD-infected microglia were of the same purity by CD11b staining. Remarkably, CJD-infected microglia were much larger and displayed more processes relative to cells from control animals, probably a reflection of their activation in CJD (Fig. 2). These morphological changes in cell size and process ramification were similar to those seen in vivo, progressing from the relatively early stages of infection (24).
FIG. 2.
Immunofluorescence of normal and CJD-derived microglial cultures. Representative microscope fields showing staining for CD11b (phycoerythrin fluorescence; red) in overnight cultures of microglia (200× magnification). Virtually all cells in the cultures expressed CD11b; nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue).
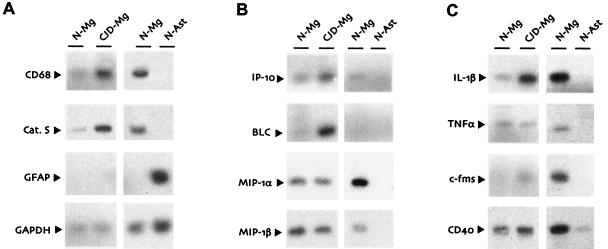
RT-PCR studies further confirmed this activation. We first evaluated cathepsin S and CD68, two transcripts important in the functions of macrophages and dendritic cells and not found in cells of neuroectodermal origin (neurons, astrocytes, and oligodendrocytes). RT-PCR experiments were quantitated by densitometry, with normalization to levels of GAPDH, a transcript unaffected by CJD (23). Steady-state levels of mRNA for the protease cathepsin S, as readily seen on representative RT-PCR blots, were increased about 10-fold in CJD-infected microglia (Fig. 3A). This cathepsin S upregulation was ∼2.5-fold less than that found in previous Northern blots of whole-brain RNA (3), possibly due to a lower differential sensitivity of RT-PCR or the physiologic state of the viable cells after separation and short-term culture. However, our RT-PCR was calculated on a per-cell basis and did not reflect the increased microglial cell numbers found in CJD-infected brains. Thus, the cathepsin S mRNA levels were in fair agreement with our previous Northern blot data. Transcript levels of CD68, a membrane glycoprotein possibly involved in macrophage cholesterol metabolism (38), were also upregulated two- to threefold per cell in CJD-infected microglia (Fig. 3A). Whether regulation of cholesterol and plasma membrane function by CD68 plays a role in CJD remains to be determined.
FIG. 3.
Semiquantitative RT-PCR analysis of normal (N-Mg) and CJD-infected (CJD-Mg) microglia, as well as neonatal normal astrocytes (N-Ast). Representative blots of biotinylated RT-PCR products for microglial markers and control transcripts (A), chemokines (B), and other inflammatory mediators (C). GFAP was also examined to confirm the absence of astrocytes in the microglial cultures, and GAPDH was always included as a standard for differences in RNA loading. Separate preparations of normal microglia were analyzed in parallel with astrocytes or CJD-infected microglia in each experiment.
Chemokines and cytokines elevated in CJD by RT-PCR.
To further understand and evaluate the specificity of microglial changes evoked by the CJD agent, we examined steady-state mRNA levels of several more generally expressed genes that are typically involved in inflammatory processes. A common response of microglia (and other cells) to various infectious agents is the production of chemokines, small secreted ligands that serve as chemoattractants for various types of leukocytes. Because some of these molecules could also relate to the migration of microglia noted in certain CJD models, we examined the chemokines interferon-inducible protein 10 (IP-10), B-lymphocyte chemoattractant (BLC), and macrophage inflammatory proteins (MIP) 1α and 1β.
RT-PCR for both IP-10 and BLC demonstrated increased mRNA levels in CJD-infected microglia (Fig. 3B), consistent with previously described results in scrapie-infected brains (36). However, we detected no differences in MIP-1α or MIP-1β expression between control and CJD-infected microglial cultures on a per-cell basis (Fig. 3B). These expression patterns remain to be confirmed in whole brain, where increased numbers of microglia may significantly contribute to chemokine expression levels. Additionally, such changes might derive from astrocytic activation. In studies of normal astrocyte cultures, we did not detect any of these chemokines (Fig. 3B). However, we do not know if the undetected transcripts in our normal astrocytes can be induced by CJD infection.
Because interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) control the production of chemokines such as MIP-1α (32), it was also relevant to examine these two underlying factors. IL-1β and TNF-α are commonly elevated in viral encephalitis and are also increased in the later stages of scrapie infection (5, 18, 46). Although the amounts of both IL-1β and TNF-α made by astrocytes were barely detectable, these transcripts were much more abundant in our microglial cultures that had no evident GFAP+ cells (Fig. 3C). Semiquantitative RT-PCR analysis further demonstrated a fourfold upregulation of IL-1β but not TNF-α in CJD-infected microglia compared to normal microglia (Fig. 3C). Thus, microglial IL-1β, which upregulates IP-10 in cultured microglia (17), could contribute significantly to the enhanced expression of IP-10 in infected brains. The lack of concurrent TNF-α upregulation in CJD-infected microglia may also explain the failure of IL-1β to induce MIP-1α in these cells.
Changes in CJD-infected microglia are distinct from those caused by amyloid and LPS.
With CJD infection, PrP is induced to form beta-pleated amyloid structures (14). Similar amyloid structures formed by other membrane proteins are also found in other neurodegenerative disorders such as Alzheimer's disease. This raises the question of whether changes observed in CJD-infected microglia were due to amyloid alone. Although PrP amyloid effects were not evaluated here, exposure to Aβ, the similar amyloid structure in Alzheimer's disease, increases levels of the macrophage colony-stimulating factor receptor c-Fms as well as the transmembrane receptor CD40 in microglia (33, 43). We found that levels of c-fms transcripts were upregulated in CJD-infected microglia (Fig. 3C). However, in contrast to studies of Aβ, there was no evidence for increased expression of CD40 in CJD-infected microglia.
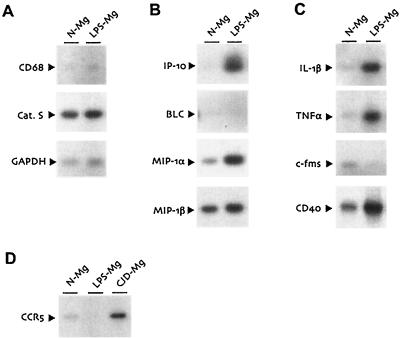
To additionally model less-specific inflammatory changes, we also examined mRNA expression patterns of normal microglia stimulated with bacterial LPS. LPS treatment in vitro is a quintessential method of eliciting inflammatory changes in myeloid cells and here served as a positive control for microglial stimulation. LPS-treated microglia upregulated IP-10 and IL-1β (Fig. 4B). Although these transcripts were also induced in CJD-infected microglia (Fig. 3B), they were elevated to a much lesser extent. LPS, unlike CJD, did not affect cathepsin S, CD68, or BLC transcript levels (Fig. 4A and B). Also in contrast to CJD-infected microglia, LPS treatment upregulated TNF-α and CD40 and interestingly caused a nearly total suppression of c-fms mRNA levels (Fig. 4C). The upregulation of IL-1β and TNF-α in LPS-treated microglia was accompanied by increased MIP-1α mRNA (Fig. 4B), in agreement with previously described effects of LPS on peripheral monocytes (32).
FIG. 4.
Semiquantitative RT-PCR analysis of LPS-stimulated normal microglia. (A to C) Normal microglia cultured overnight in standard medium (N-Mg) or medium supplemented with LPS (LPS-Mg) were analyzed as described for the previous figure. (D) Overnight cultures of normal microglia, LPS-stimulated microglia, and microglia from CJD-infected animals (CJD-Mg) were analyzed for CCR5 expression by RT-PCR.
Finally, we examined the chemokine receptor CCR5 because of its role in microglial trafficking (1). Previous in vivo studies on CJD indicated a robust microglial migration at relatively early stages of infection, as well as increased levels of CCR5 mRNA in whole brain (3, 24). CCR5 expression is also a crucial determinant of microglial infection by human immunodeficiency virus (15, 29). CCR5 transcripts were moderately upregulated in CJD-infected microglia (Fig. 4D), consistent with the increases previously described in vivo (3). In contrast to CJD, LPS treatment of microglia strongly suppressed CCR5 mRNA expression. These data corroborate previous studies on the regulation of CCR5 by LPS (40) and provide further distinction between the consequences of CJD infection and the response to general inflammatory stimuli.
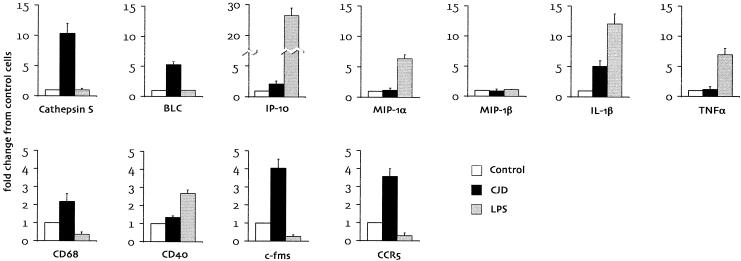
A quantitative profile of microglial mRNA expression in the present study further demonstrated significant differences between LPS-stimulated microglia and CJD-derived microglia (Fig. 5). Some of these differences were manifest as marked differences in mRNA magnitude. Even more dramatic were cases where LPS treatment induced changes that were the opposite of those seen in CJD-infected microglia (i.e., c-fms and CCR5). Based on these results and those of Aβ-stimulated microglia, we conclude that the phenotype of CJD-infected microglia follows a specific pattern that is distinct from the microglial response to a nonspecific inflammatory stimulus or to a common amyloid structure.
FIG. 5.
Quantitative profiles of mRNA expression in microglia from CJD-infected animals (black bars) and normal microglia stimulated with LPS (gray bars). RT-PCR blots for the genes indicated were quantitated by densitometry, with normalization to GAPDH mRNA levels. Results are expressed in terms of fold change from normal unstimulated microglia (white bars) and represent the mean ± standard error of data from at least two independent experiments. Note the different scales on the y axis for the two rows and for IP-10.
DISCUSSION
Large amounts of the CJD agent can be carried by microglia. Furthermore, infected microglia reproducibly displayed a phenotype characterized by morphological activation and a robust pattern of inflammatory molecular changes. Because microglia do not normally survive in long-term culture, as did our previous isolated splenic myeloid cells, we could not do long-term culture to more rigorously rule out nonspecific agent attachment during acute cell fractionation. However, sensitive assays for PrP-rich neuronal components as well as astrocytes did not support the premise that microglial infectivity was derived from the nonspecific adherence of other cellular components. Furthermore, if infectivity were carried by a rare contaminating nonmicroglial cell, then the cells in the other population should be far more infectious (∼100-fold) than was found. Remarkably, microglia were as infectious as starting brain homogenates.
It is often assumed that agents of transmissible spongiform encephalopathy replicate mainly if not exclusively in neurons, but the present studies undermine this notion. Because neither adult neurons nor microglia can be grown isolated in long-term culture, it is not yet possible to determine which cells support agent replication. In any case, the finding of microglial infectivity can explain how these agents transit into and out of the brain prior to the appearance of any pathological changes.
Despite the comparable infectivity of microglia and whole brain, microglial cells had ∼50-fold less PrP by conservative estimates than brain homogenates. Consequently, cells and tissues with relatively low expression of PrP can be effective carriers of the infectious agent. This may no longer be surprising, since many molecular experiments have shown that protease-resistant PrP is a poor predictor of infectious titer (26), and recent experiments with defined cell types demonstrate that cell susceptibility to scrapie is not correlated with PrP levels (10). Our data here further show that microglia were almost 2 logs more infectious than would be predicted based on their PrP expression alone, again suggesting that infection is determined by something other than PrP.
Experimentally the CJD agent has the characteristics of a viral particle (26), and infectivity sediments at the bottom of sucrose gradients, separate from the vast majority of PrP (26, 27, 37, 41). No purified or recombinant PrP has ever been sufficient to induce infection, although many amyloid forms of PrP have been tested in animals (for an example, see reference 16). Interestingly, host PrP expression has a profound effect on the latency and virulence of herpes simplex virus in animals (45), and PrP may be part of a common pathway or a receptor (21) used by several types of viral particles.
Microglia may participate in a chain of cell types that distribute the CJD agent through different tissues. The presence of infectivity in peripheral white blood cells and lymphoid tissues is well known (9, 20), and there is increasing evidence that blood may serve as a conduit for the CJD agent to reach the gut, the lymphoreticular system, and the central nervous system itself (34, 39). Perivascular accumulation of pathological PrP in rodent CJD-infected brains also reveals a potential trail of the CJD agent into the central nervous system from vessels (24, 28). The intimate association of some microglia with the brain vasculature suggests that this may be a site for transfer of agent to these cells. Some microglia also accumulate pathological PrP (24). The mechanism for this is unknown but may be secondary to the uptake of extracellular PrP in a neurodegenerative setting, independent of agent accumulation.
The remarkable migratory capacity of microglia provides a route for agent propagation throughout the brain parenchyma. Even if microglia are not primarily targeted by the CJD agent for replication, they still may serve to transport the agent to other sites where agent propagation may be more productive. Indeed, CJD-infected microglia may be primed for migration through the brain via their enhanced expression of chemoattractant receptors such as c-Fms and CCR5. Migrating microglia may clear the agent, accumulating significant infectivity only at late stages of disease when their clearance capability has been overwhelmed. Interestingly, microglia even in late stage CJD generally do not differentiate to a macrophage lineage, and only rare cells in the brain are positive for the ED1 macrophage antigen (24). In this regard, it will be of interest to determine if microglia carry the agent early in disease.
We are currently investigating the diagnostic sensitivity of our microglial transcripts, because PrP changes are only detectable relatively late after CJD infection and are therefore of limited diagnostic utility. Microglial activation in CJD and scrapie can also lead to a fundamental understanding of progressive neuronal damage. This activation stimulates production of potentially neurotoxic substances such as IL-1, which are often blamed for neurodegenerative changes (6). Interestingly, elevated expression of c-fms, as seen in CJD-infected microglia, is thought to exacerbate neurodegenerative cascades (8, 33). Targeting microglia and linked inflammatory pathways may be useful in CJD treatments for some but not all models of infection (25; unpublished observations).
The variety of agent strains and pathological manifestations in CJD and scrapie are often viewed within the single dimension of PrP. Because CJD-infected microglia displayed a pattern of activation that differed from treatment with the nonspecific inflammatory stimulant LPS as well as that induced by amyloid, microglial analyses offer an opportunity to define new agent-induced mechanisms of disease progression. Our data suggest that microglial activation in CJD is not confined to a single simple pathway but represents a diverse set of responses that may be exploited for diagnosis and, in some cases, therapy. Further studies will likely partition microglial responses into those that are secondary to neuronal damage and amyloidogenesis and those that are specific for direct interaction with the CJD agent.
Because our understanding of the molecular nature of the infectious agent remains incomplete, it is difficult to specify the mechanisms of agent interactions with any cell type at this time. However, it is intriguing that the pattern of microglial activation is consistent with standard viral mechanisms that commandeer myeloid cells for invasion and latency, ultimately leading to secondary destructive changes in the brain.
Acknowledgments
This research was supported by NIH grants NS12674 and NS34569.
REFERENCES
- 1.Albright, A. V., J. Martin, M. O'Connor, and F. Gonzalez-Scarano. 2001. Interactions between HIV-1, gp120, chemokines, and cultured adult microglial cells. J. Neurovirol. 7:196-207. [DOI] [PubMed] [Google Scholar]
- 2.Aucouturier, P., F. Geissmann, D. Darnotte, G. P. Saborio, H. C. Meeker, R. Kascsak, R. I. Carp, and T. Wisniewski. 2001. Infected splenic dendritic cells are sufficient for prion transmission to the central nervous system in mouse scrapie. J. Clin. Investig. 108:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, C. A., Z. Y. Lu, I. Zaitsev, and L. Manuelidis. 1999. Microglial activation varies in different models of Creutzfeldt-Jakob disease. J. Virol. 73:5089-5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, K. L., K. Stewart, D. L. Ritchie, N. A. Mabbott, A. Williams, H. Fraser, W. I. Morrison, and M. E. Bruce. 1999. Scrapie repliction in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nat. Med. 5:1308-1312. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, I. L., M. Eddleston, P. Kemper, M. B. A. Oldstone, and M. V. Hobbs. 1994. Activation of cerebral cytokine gene expression and its correlation with onset of reactive astrocyte and acute-phase response gene expression in scrapie. J. Virol. 68:2383-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combs, C. K., D. E. Johnson, S. B. Cannady, T. M. Lehman, and G. E. Landreth. 1999. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J. Neurosci. 19:929-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czub, M., H. Braig, and H. Diringer. 1988. Replication of the scrapie agent in hamsters infected intracerebrally confirms the pathogenesis of an amyloid-inducing virosis. J. Gen. Virol. 69:1753-1756. [DOI] [PubMed] [Google Scholar]
- 8.Du Yan, S., H. Zhu, J. Fu, S. F. Yan, A. Roher, W. W. Tourtellotte, T. Rajavashisth, X. Chen, G. Godman, D. Stern, and A. M. Schmidt. 1997. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer's disease. Proc. Natl. Acad. Sci. USA 94:5296-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eklund, C. M., R. C. Kennedy, and W. J. Hadlow. 1967. Pathogenesis of scrapie virus infection in the mouse. J. Infect. Dis. 117:15-22. [DOI] [PubMed] [Google Scholar]
- 10.Enari, M., E. Flechsig, and C. Weissmann. 2001. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl. Acad. Sci. USA 98:9295-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer, M., T. Rülicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissman. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 12.Ford, A. L., A. L. Goodsall, W. F. Hickey, and J. D. Sedgwick. 1995. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting: phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 154:4309-4321. [PubMed] [Google Scholar]
- 13.Giese, A., D. R. Brown, M. H. Groschup, C. Feldmann, I. Haist, and H. A. Kretzschmar. 1998. Role of microglia in neuronal cell death and prion disease. Brain Pathol. 8:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldfarb, L. G., and P. Brown. 1995. The transmissible spongiform encephalopathies. Annu. Rev. Med. 46:57-65. [DOI] [PubMed] [Google Scholar]
- 15.He, J., Y. Chen, M. Farzan, H. Choe, A. Ohagen, S. Gartner, J. Busciglio, X. Yang, W. Hofmann, W. Newman, C. R. Mackay, J. Sodroski, and D. Gabuzda. 1997. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 385:645-649. [DOI] [PubMed] [Google Scholar]
- 16.Hill, A., M. Antoniou, and J. Collinge. 1999. Protease-resistant prion protein produced in vitro lacks detectable infectivity. J. Gen. Virol. 80:11-14. [DOI] [PubMed] [Google Scholar]
- 17.Hua, L. L., and S. C. Lee. 2000. Distinct patterns of stimulus-inducible chemokine mRNA accumulation in human fetal astrocytes and microglia. Glia 30:74-81. [DOI] [PubMed] [Google Scholar]
- 18.Kordek, R., V. R. Nerurkar, P. P. Liberski, S. Isaacson, R. Yanagihara, and D. C. Gajdusek. 1996. Heightened expression of tumor necrosis factor alpha, interleukin-1 alpha, and glial fibrillary acidic protein in experimental Creutzfeldt-Jakob disease in mice. Proc. Natl. Acad. Sci. USA 93:9754-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasmezas, C. I., J. P. Deslys, O. Robain, A. Jaegly, V. Beringue, J. M. Peyrin, J. G. Fournier, J. J. Hauw, J. Rossier, and D. Dormont. 1997. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science 275:402-405. [DOI] [PubMed] [Google Scholar]
- 20.Manuelidis, E. E., E. J. Gorgacs, and L. Manuelidis. 1978. Viremia in experimental Creutzfeldt-Jakob disease. Science 200:1069-1071. [DOI] [PubMed] [Google Scholar]
- 21.Manuelidis, L. 1994. The dimensions of Creutzfeldt-Jakob disease. Transfusion 34:915-928. [DOI] [PubMed] [Google Scholar]
- 22.Manuelidis, L. 1998. Vaccination with an attenuated Creutzfeldt-Jakob disease strain prevents expression of a virulent agent. Proc. Natl. Acad. Sci. USA 95:2520-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manuelidis, L., and W. Fritch. 1996. Infectivity and host responses in Creutzfeldt-Jakob disease. Virology 216:46-59. [DOI] [PubMed] [Google Scholar]
- 24.Manuelidis, L., W. Fritch, and Y. G. Xi. 1997. Evolution of a strain of CJD that induces BSE-like plaques. Science 277:94-98. [DOI] [PubMed] [Google Scholar]
- 25.Manuelidis, L., W. Fritch, and I. Zaitsev. 1998. Dapsone to delay symptoms in Creutzfeldt-Jakob disease. Lancet 352:456.. [DOI] [PubMed] [Google Scholar]
- 26.Manuelidis, L., T. Sklaviadis, A. Akowitz, and W. Fritch. 1995. Viral particles are required for infection in neurodegenerative Creutzfeldt-Jakob disease. Proc. Natl. Acad. Sci. USA 92:5124-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manuelidis, L., T. Sklaviadis, and E. E. Manuelidis. 1987. Evidence suggesting that PrP is not the infectious agent in Creutzfeldt-Jakob disease. EMBO J. 6:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manuelidis, L., I. Zaitsev, P. Koni, Z. Y. Lu, R. A. Flavell, and W. Fritch. 2000. Follicular dendritic cells and dissemination of Creutzfeldt-Jakob disease. J. Virol. 74:8614-8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin, J., C. C. LaBranche, and F. Gonzalez-Scarano. 2001. Differential CD4/CCR5 utilization, gp120 conformation, and neutralization sensitivity between envelopes from a microglia-adapted human immunodeficiency virus type 1 and its parental isolate. J. Virol. 75:3568-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy, K., and J. de Vellis. 1980. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85:890-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merz, G. S., V. Schwenk, G. Schuller-Levis, S. Gruca, and H. M. Wisniewski. 1987. Isolation and characterization of macrophages from scrapie-infected mouse brain. Acta Neuropathol. 72:240-247. [DOI] [PubMed] [Google Scholar]
- 32.Muhl, H., and C. A. Dinarello. 1997. Macrophage inflammatory protein-1 alpha production in lipopolysaccharide-stimulated human adherent blood mononuclear cells is inhibited by the nitric oxide synthase inhibitor N(G)-monomethyl-l-arginine. J. Immunol. 159:5063-5069. [PubMed] [Google Scholar]
- 33.Murphy, G. M. J., L. Yang, and B. Cordell. 1998. Macrophage colony-stimulating factor augments beta-amyloid-induced interleukin-1, interleukin-6, and nitric oxide production by microglial cells. J. Biol. Chem. 273:20967-20971. [DOI] [PubMed] [Google Scholar]
- 34.Radebold, K., M. Chernyak, D. Martin, and L. Manuelidis. 2001. Blood-borne transit of CJD from brain to gut at early stages of infection. BMC Infect. Dis. 1:20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raeber, A. J., M. A. Klein, R. Frigg, E. Flechsig, A. Aguzzi, and C. Weissmann. 1999. PrP-dependent association of prions with splenic but not circulating lymphocytes of scrapie-infected mice. EMBO J. 18:2702-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riemer, C., I. Queck, D. Simon, R. Kurth, and M. Baier. 2000. Identification of upregulated genes in scrapie-infected brain tissue. J. Virol. 74:10245-10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riesner, D., K. Kellings, K. Post, H. Wille, H. Serban, D. Groth, M. A. Baldwin, and S. B. Prusiner. 1996. Disruption of prion rods generates 10-nm spherical particles having high α-helical content and lacking scrapie infectivity. J. Virol. 70:1714-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakaguchi, H., M. Takeya, H. Suzuki, H. Hakamata, T. Kodama, S. Horiuchi, S. Gordon, L. J. van der Laan, G. Kraal, S. Ishibashi, N. Kitamura, and K. Takahashi. 1998. Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab. Investig. 78:423-434. [PubMed] [Google Scholar]
- 39.Schlomchik, M. J., K. Radebold, N. Duclos, and L. Manuelidis. 2001. Neuroinvasion by a Creutzfeldt-Jakob disease agent in the absence of B cells and follicular dendritic cells. Proc. Natl. Acad. Sci. USA 98:9289-9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sica, A., A. Saccani, A. Borsatti, C. A. Power, T. N. Wells, W. Luini, N. Polentarutti, S. Sozzani, and A. Mantovani. 1997. Bacterial lipopolysaccharide rapidly inhibits expression of C-C chemokine receptors in human monocytes. J. Exp. Med. 185:969-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sklaviadis, T. K., L. Manuelidis, and E. E. Manuelidis. 1989. Physical properties of the Creutzfeldt-Jakob disease agent. J. Virol. 63:1212-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somerville, R. A., R. C. Oberthur, U. Havekost, F. MacDonald, D. M. Taylor, and A. G. Dickinson. 2002. Characterization of thermodynamic diversity between transmissible spongiform encephalopathy agent strains and its theoretical implications. J. Biol. Chem. 277:11084-11089. [DOI] [PubMed] [Google Scholar]
- 43.Tan, J., T. Town, D. Paris, T. Mori, Z. Suo, F. Crawford, M. P. Mattson, R. A. Flavell, and M. Mullan. 1999. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science 286:2352-2355. [DOI] [PubMed] [Google Scholar]
- 44.Telling, G. C., P. Parchi, S. J. DeArmond, P. Cortelli, P. Montagna, R. Gabizon, J. Mastrianni, E. Lugaresi, P. Gambetti, and S. B. Prusiner. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079-2082. [DOI] [PubMed] [Google Scholar]
- 45.Thackray, A. M., and R. Bujdoso. 2002. PrPc expression influences the establishment of herpes simplex virus type I latency. J. Virol. 76:2498-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams, A. E., A. Van Dam, D. Ritchie, P. Eikelenboom, and H. Fraser. 1994. Immunocytochemical appearance of cytokines, prostaglandin E2 and lipocortin-1 in the central nervous system during the incubation period of murine scrapie correlates with progressive PrP accumulations. Brain Res. 754:171-180. [DOI] [PubMed] [Google Scholar]
- 47.Zielasek, J., and H. P. Hartung. 1996. Molecular mechanisms of microglial activation. Adv. Neuroimmunol. 6:191-222. [DOI] [PubMed] [Google Scholar]