Abstract
The influenza A virus nonstructural NS1 protein is known to modulate host cell gene expression and to inhibit double-stranded RNA (dsRNA)-mediated antiviral responses. Here we identify NS1 as the first viral protein that antagonizes virus- and dsRNA-induced activation of the stress response-signaling pathway mediated through Jun N-terminal kinase.
Cells respond to a variety of stress conditions such as UV irradiation and endotoxin exposure by activation of Jun N-terminal kinase (JNK) (also termed stress-activated protein kinase [SAPK]), which in turn phosphorylates and thereby upregulates the activity of transcription factors of the AP-1 family (21, 44). JNK activation is also critical in the innate response to acute viral infections, because JNK-dependent AP-1 factors transactivate several antiviral and proinflammatory cytokine genes following infection. Importantly, the AP-1 heterodimer c-Jun::ATF-2 cooperates with the transcription factors NF-κB and IRF to transactivate the beta interferon (IFN-β) gene (40, 43). Secretion of IFN-β is the initial event that triggers the expression of several IFN-dependent gene products with antiviral activity (7, 15, 38). One of the main triggering agents of this reaction is thought to be double-stranded RNA (dsRNA) produced during viral replication, because synthetic dsRNA is capable of activating JNK and the IFN system (5, 18, 31). However, neither the activating structures in viral RNAs nor their intracellular localization has been precisely identified.
It has been proposed that the influenza A virus NS1 protein is a viral regulator of gene expression that inhibits pre-mRNA processing and nucleocytoplasmic export of cellular mRNAs and enhances translation (1, 4, 6, 9, 10, 25, 28, 33, 34, 46). On the other hand, the NS1 protein, which has RNA-binding activity (16), was also shown to antagonize antiviral dsRNA-dependent enzymes and processes. These include activation of protein kinase R (PKR) (2, 17, 29) and of the transcription factors NF-κB, IRF-3, and IRF-7 (37, 39, 42). As a result, the NS1 protein effectively restrains IFN-α/β functions in infected cells (12). Recently, we and others have shown that influenza A virus replication stimulates the transcriptional activity of nuclear AP-1 factors and their target genes via the MKK4/7-JNK pathway (22, 27, 30). Moreover, these studies suggested that induction of AP-1 factors is detrimental to virus production, because expression of dominant-negative mutants of JNK or its substrate c-Jun increased viral titers (30). Thus, the question was raised whether the NS1 protein might affect this cellular response.
Influenza virus-induced JNK and c-Jun activation is upregulated in the absence of NS1 protein.
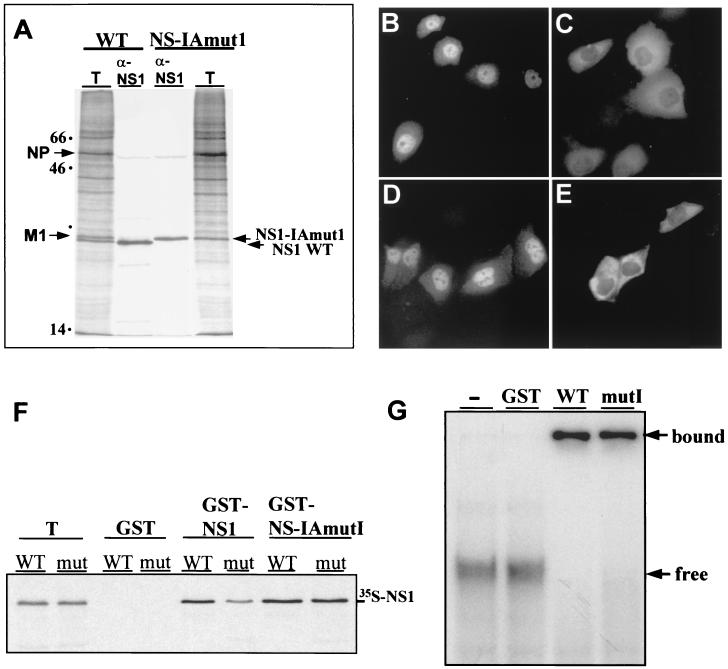
To study the role of the NS1 protein in JNK activation, we compared the influenza A/PR/8/34 wild-type (WT) virus with its two isogenic variants delNS1 and NS- IAmut1, which were generated by the ribonucleoprotein transfection method (12, 42). The delNS1 virus completely lacks the NS1 gene, whereas NS-IAmut1 expresses an NS1 protein of wild-type length (230 amino acids) with five amino acid replacements at positions 181 to 185 (LIGGL to KQRRS). These mutations mediate a shift from a nuclear to a predominantly cytoplasmic NS1 localization in infected and transfected cells and slightly slow migration on sodium dodecyl sulfate (SDS)- polyacrylamide gels (Fig. 1A to E). The introduced amino acid changes do not appear to alter the general structure of the viral NS1 protein because they do not affect its dimerization as judged by binding of the NS-IAmutI protein to corresponding glutathione S-transferase (GST)-NS1 mutant or WT fusion proteins (Fig. 1F). Also, the mutation did not reduce the ability of the NS1 protein to form stable complexes with dsRNA (Fig. 1G). Currently we cannot tell whether the altered intracellular localization of the NS-IAmutI protein is the consequence of an enhanced activity of the proposed nuclear export signal at NS1 amino acids 138 to 147 (26) or, rather, is due to cytoplasmic retention. However, replication of the NS-IAmutI virus seems not to be compromised by the mutation since the variant could be grown to titers of up to 10 8 PFU/ml in embryonated chicken eggs, which is comparable to WT growth (data not shown).
FIG. 1.
Expression, localization, dimerization, and dsRNA- binding of NS1 proteins encoded by influenza A/PR/8/34 WT and NS-IAmut1 viruses. (A) MDCK cells were infected with WT or NS-IAmut1 viruses. After a 5-h incubation at 37°C, the cells were metabolically labeled with [35S]methionine for 1 h and lysed in RIPA buffer. The NS1 proteins were immunoprecipitated from total-cell lysates with rabbit anti-NS1 serum and analyzed by SDS-gel electrophoresis and autoradiography as described previously (45). The positions of the M1, NP, and NS1 proteins are indicated on the left and right. T, 10% of cell lysate used for immunoprecipitation. (B to E) Intracellular NS1 localization was determined by indirect immunofluorescence analysis of HeLa cells infected with WT (B) or NS-IAmutI (C) virus. Cells were fixed and permeabilized, and the NS1 proteins were detected by the use of rabbit anti-NS1 serum and anti-rabbit immunoglobulin G (IgG)- fluorescein isothiocyanate (FITC) conjugate as described elsewhere (45). The localization of NS1 proteins in the absence of virus infection was determined in MDCK cells after Lipofectamine 2000 (Invitrogen)-mediated transfection with mammalian pcDNA3.1-Myc/His expression plasmids (Invitrogen) encoding NS1 WT (D) or IAmut1 (E) protein. Cells were prepared for microscopic analysis, and the NS1 proteins were visualized by indirect immunofluorescence using the Myc-tag specific antibody 9E10 (Santa Cruz Biotechnology) and secondary FITC-conjugated anti-mouse IgG. The cells were viewed under a Nikon Axiophot 300 microscope equipped with an epifluorescence attachment. Images were recorded by a SPOT RT digital camera and processed using Adobe Photoshop 5.0 software. (F) To assess dimerization of NS1 proteins, equal amounts of GST, GST-NS1, and GST-NS-IAmut1 proteins were immobilized on glutathione-agarose beads and reacted with NS1 WT or NS-IAmut1 (mut) proteins that were synthesized in coupled transcription-translation reactions in the presence of [35S]methionine as described previously (45). The precipitated proteins were separated by SDS-gel electrophoresis and visualized by autoradiography. T, 10% of the total amount used for precipitation. (G) Binding of GST-NS1 fusion proteins (0.4 μM) to dsRNA (approximately 0.5 nM) derived from complementary 32P-labeled transcripts of the polylinker region of plasmid pGEM-11zf (Promega) was examined as described previously (28). Protein-RNA complexes formed in the absence or presence of GST, GST-NS1 WT and GST-NS-IAmutI proteins were separated from free RNA by gel electrophoresis on a nondenaturing 10% polyacrylamide gel. The positions of free and complexed RNAs are indicated to the right.
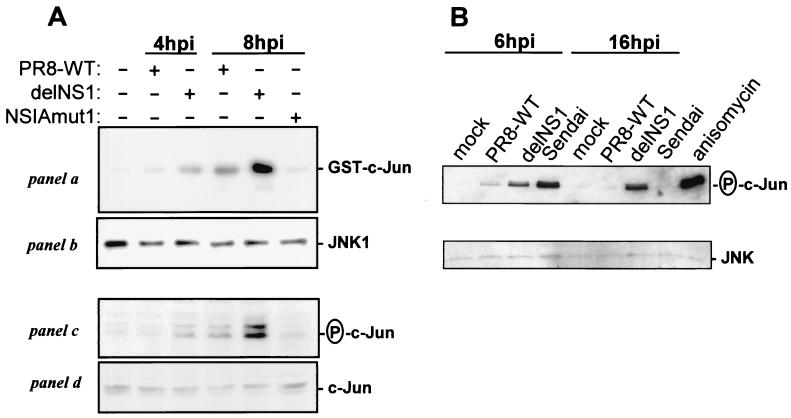
Permissive Vero cells were infected with WT and mutant viruses, and cell lysates were analyzed for JNK activation and phosphorylation of its downstream effector c-Jun at 4 and 8 h postinfection (Fig. 2A). While JNK activity and c-Jun phoshorylation increased only moderately on infection with the WT and NS-IAmut1 viruses, these parameters were strongly enhanced by the delNS1 virus. These results suggest that expression of nuclear and/or cytoplasmic NS1 proteins opposes signaling through the JNK pathway. Furthermore, we conclude that JNK activation is not mediated by an autocrine I IFN-α/β loop, since Vero cells are deficient in IFN production (8). Essentially the same effects of NS1 expression during influenza A virus infection on c-Jun phosphorylation were observed with retarded kinetics in A549 lung epithelial cells (Fig. 2B) and in human HEK293 cells (data not shown), suggesting that these observations are of general importance in influenza virus infections of epithelial cells. Interestingly, active JNK was still present in delNS1 virus-infected A549 cells at 16 h postinfection while JNK activity was back to basal levels in Sendai virus-infected cells.
FIG. 2.
Viral NS1 expression inhibits activation of JNK and phosphorylation of c-Jun. (A) Vero cells were mock infected or infected with PR8 WT, delNS1, or NS-IAmut1 viruses at a multiplicity of infection (MOI) of 5 and lysed 4 or 8 h postinfection (hpi) as described previously (30). Endogenous JNK1 was immunoprecipitated from lysates representing equal number of cells with JNK- specific antiserum (Santa Cruz), and washed immune complexes were analyzed for JNK activity by using recombinant GST-cJun(1-135) as a substrate as described previously (30) (panel a). Equal loadings with precipitated kinase were verified by immunoblot detection with JNK-1 antiserum (panel b). The same lysates were subjected to SDS-polyacylamide gel electrophoresis and analyzed by immunoblotting with antisera specific for phospho-c-Jun and total c-Jun (Cell Signaling) (panels c and d). (B) Human A549 lung epithelial cells were either mock infected, or infected with PR8 WT or delNS1 virus at an MOI of 1. Total-cell extracts were made at 6 and 16 h postinfection, and total JNK was precipitated overnight at 4°C with 2 μg of c-Jun fusion protein coupled to glutathione- agarose beads. Kinase assays were performed for 30 min at 30°C in the presence of 100 μM ATP. The incubated proteins were separated by SDS-gel electrophoresis and analyzed by immunoblotting using phospho-c-Jun-specific antibody (upper panel). In addition, each sample was subjected to anti-JNK immunoblot analysis to determine the total level of JNK protein. A549 cells that were stimulated for 20 min with the common JNK activator anisomycin or infected with Sendai virus for 6 or 16 h were included as positive controls.
The viral NS1 protein downregulates AP-1-dependent gene expression.
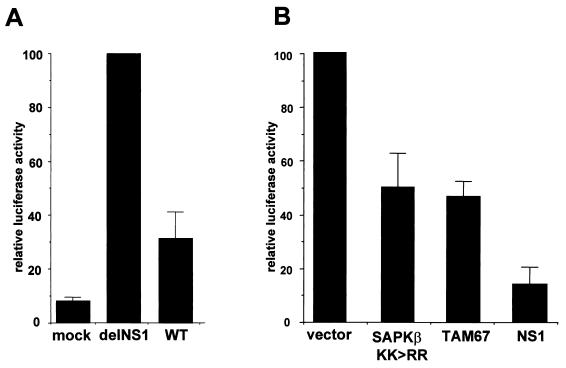
To evaluate the role of NS1 in regulating JNK-dependent gene expression, we studied the induction of the IFN-β reporter plasmid p125-Luc (47) in response to infection by PR8 WT or delNS1 virus (Fig. 3A). This comparison showed that NS1 expression reduced viral promoter activation in our experimental setting by approximately threefold. Thus, virus-induced hyperphosphorylation of c-Jun in the absence of NS1 (Fig. 2) correlated with enhanced transcriptional activity of AP-1 factors. We confirmed that JNK and c-Jun are involved in delNS1-mediated IFN-β promoter activation by transfecting cells, prior to infection, with empty vector or plasmids expressing either dominant-negative JNK (SAPKβ KK>RR) or dominant-negative c-Jun (TAM67) (Fig. 3B). Coexpression of the two mutants decreased reporter gene activity, demonstrating that activation of the IFN-β promoter in delNS1 virus- infected cells requires activation of the JNK pathway. Inhibition was less pronounced than in cells expressing the NS1 protein (Fig. 3B). This outcome is consistent with additional inhibitory effects of NS1 on NF-κB and IRF-3 activity in IFN-β induction (39, 42), while the JNK and c-Jun mutants act only on a single pathway. Given the large number of genes that are regulated by AP-1 factors, the NS1 protein is likely to suppress activation not only of the IFN-β promoter but also of other target genes.
FIG. 3.
Inhibition of JNK/AP-1 signaling impairs delNS1 virus-induced IFN-β promoter activation. (A) MDCK cells (106) were transfected with 100 ng of the IFN-β promoter luciferase reporter plasmid p125-Luc (47). After 24 h, the cells were mock infected or infected with influenza PR8 WT or delNS virus at an MOI of 1. Cell extracts were prepared at 4 h postinfection in reporter lysis buffer (Promega) and assayed for luciferase activity. For a comparison, enzyme activity induced by delNS1 virus was arbitrarily set to 100%. Average values determined in three independent experiments are shown. (B) Cells were transfected with p125-Luc together with 4 μg of empty pKRSPA vector (vector) or expression plasmids encoding dominant negative JNK/SAPK (SAPKβ KK>>RR), dominant-negative c-Jun (TAM67) (30), or NS1 protein. Cells were infected with delNS1 virus at an MOI of 1, and 4 h later they were lysed and luciferase activity was determined as described for panel A. Error bars indicate standard deviations.
The NS1 protein inhibits dsRNA-mediated JNK activation.
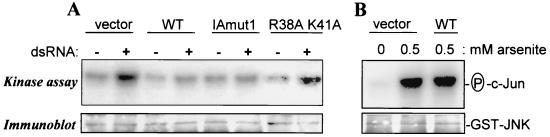
We finally examined whether NS1 expression would regulate JNK and AP-1 activation by dsRNA or other known stimuli. Cells were transfected with a plasmid encoding a GST-JNK fusion protein, together with NS1 expression constructs or empty vector. Subsequently, the cells were mock treated or stimulated with synthetic dsRNA and analyzed for JNK activity. Figure 4 shows that both the predominantly nuclear NS1 WT and the predominantly cytosolic IAmut1 protein almost completely prevented JNK activation (compare lanes vector with lanes WT and IAmut1). These results are consistent with the observations made during virus infections (Figs. 1 and 2). These findings were unexpected because influenza virus replication during which dsRNA might be generated by hybridization of genomic and antigenomic RNA strands is most probably a nuclear event (23). Possibly, this instead indicates that NS1 inhibits JNK activation by association with intramolecularly base-paired regions in viral RNAs that are exported to the cytoplasm. Such an interaction has been demonstrated previously (32). Accordingly, expression of the NS1 protein carrying the R38A/K41A double mutation that attenuates binding to dsRNA (39, 41) did not prevent JNK activation (Fig. 4A, lanes R38AK41A). This finding gives additional support to the conclusion that the NS1 protein antagonizes JNK activation by virtue of its dsRNA-binding activity. However, we cannot rule out the possibility that interactions between NS1 and the cellular protein(s) involved in dsRNA-mediated activation of JNK play a role in the prevention of JNK activation. The specificity of the NS1 WT in repressing virus- and dsRNA-dependent responses was shown by its inability to inhibit JNK activation by the classical inducer sodium arsenite (Fig. 4B).
FIG. 4.
NS1 protein expression inhibits dsRNA- but not arsenite-induced activation of c- Jun. (A) MDCK cells were transfected with a plasmid expressing GST-tagged JNK/SAPKβ together with either empty vector (lanes vector) or derivatives thereof expressing NS1 WT, NS-IAmut1, or the NS1-R38A/K41A RNA-binding mutant protein (lanes WT, IAmut1, and R38A K41A, respectively). At 24 h later, the cells were left untreated or stimulated with synthetic dsRNA (50 μg/ml) for 6 h and extracts were prepared as described previously (30). JNK activity was assessed in the lysates by immune complex kinase assays using GST-c-Jun(1-135) as a substrate (30). The amount of GST-JNK in each sample was determined by immunoblotting with GST-specific antiserum. (B) In parallel reactions, we analyzed c-Jun phosphorylation in cells that were transfected with empty or NS1 expression vector and were mock treated (lane 0) or stimulated with 0.5 mM sodium arsenite, a common JNK activator, for 30 min (lanes 0.5).
Influenza virus infection and dsRNA treatment trigger specific signal transduction cascades that culminate in posttranscriptional activation of AP-1 and other transcription factors (11, 30). This leads to the expression of antiviral (e.g., IFN-α/β), proinflammatory (e.g., tumor necrosis factor alpha and interleukin-6), and chemotactic (e.g., RANTES) cytokines that combine to shape the innate and adaptative immune response of mammals toward the intruding virus (3, 20). Activation of JNK and c-Jun have also been linked to the induction of apoptosis (reviewed in reference 36), which is known to occur during influenza virus infection (22, 35). Thus, inhibition of JNK-dependent responses by the NS1 protein is in the interest of efficient viral replication and spread and is likely to influence viral pathogenicity. We suggest that the lack of JNK inhibition contributes to the strong attenuation of the delNS1 virus in IFN-competent hosts and to its increased propensity to induce apoptosis (12, 48).
The present characterization of NS1 as a specific antagonist of virus- and dsRNA-induced activation of JNK and its downstream targets is, to our knowledge, the first report of such a property of a viral protein. We speculate that other viruses express gene products that are functionally equivalent to NS1. This hypothesis is supported by the recent finding that the rotavirus VP8* protein can block TRAF2-dependent JNK activation, although it is not known if this occurs during virus infection (24). JNK activity is regulated by phosphorylation through the dual-specificity kinases MKK4 and MKK7, which are controlled by farther upstream regulators (13). PKR and 2′,5′-oligoadenylate synthetase have been implicated as potential dsRNA and/or viral RNA sensors that signal for JNK activation, but alternative pathways appear to exist (14, 18, 19). Based on the failure to inhibit JNK activation by chemotoxic stress and due to its dsRNA-binding capability, the NS1 protein is likely to exert its inhibitory function through its dsRNA-binding properties. Mechanistically, this would resemble the effect of NS1 in preventing the activation of NF-κB and IRF-3 (39, 42), although those factors may be controlled by at least partially different upstream sensors. For instance, genetic knockout of PKR ablated activation of NF-κB but not of JNK or IRF-3 in response to virus infections in mouse fibroblasts (5, 37). Whether the NS1-mediated inhibition of the JNK pathway is mediated by mere sequestration of dsRNA or by targeting of cellular dsRNA- sensor molecules through binding to shared dsRNA molecules will be a matter of future investigations. Taken together, our results characterize the NS1 protein as an antagonist of virus- and dsRNA-induced activation of JNK and AP-1. The precise identification of all the factors and pathways that participate in the induction of cytokine and chemokine genes in response to influenza virus infection remains an important challenge for future research.
Acknowledgments
S.L., X.W., and C.E. contributed equally to this work.
We thank Takashi Fujita (Tokyo, Japan) for providing plasmid p125-Luc.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (S.L., O.P., and S.P.) and the NIH (A.G.-S.).
REFERENCES
- 1.Aragon, T., S. de la Luna, I. Novoa, L. Carrasco, J. Ortin, and A. Nieto. 2000. Eukaryotic translation initiation factor 4GI is a cellular target for NS1 protein, a translational activator of influenza virus. Mol. Cell. Biol. 20:6259-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann, M., A. Garcia-Sastre, E. Carnero, H. Pehamberger, K. Wolff, P. Palese, and T. Muster. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 74:6203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biron, C. A., and G. C. Sen. 2001. Interferons and other cytokines, p. 321-351. In D. M. Knipe, P. M. Howley, D. E. Griffin et al. (ed.), Fields virology, 4th ed. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 4.Chen, Z., Y. Li, and R. M. Krug. 1999. Influenza A virus NS1 protein targets poly(A)- binding protein II of the cellular 3′-end processing machinery. EMBO J. 18:2273-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu, W. M., D. Ostertag, Z. W. Li, L. Chang, Y. Chen, Y. Hu, B. Williams, J. Perrault, and M. Karin. 1999. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity 11:721-731. [DOI] [PubMed] [Google Scholar]
- 6.de la Luna, S., P. Fortes, A. Beloso, and J. Ortin. 1995. Influenza virus NS1 protein enhances the rate of translation initiation of viral mRNAs. J. Virol. 69:2427-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz, M. O., S. Ziemin, M. M. Le Beau, P. Pitha, S. D. Smith, R. R. Chilcote, and J. D. Rowley. 1988. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc. Natl. Acad. Sci. USA 85:5259-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enami, K., T. A. Sato, S. Nakada, and M. Enami. 1994. Influenza virus NS1 protein stimulates translation of the M1 protein. J. Virol. 68:1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortes, P., A. Beloso, and J. Ortin. 1994. Influenza virus NS1 protein inhibits pre- mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J. 13:704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Sastre, A. 2001. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology 279:375-384. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 13.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen- activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211-218. [DOI] [PubMed] [Google Scholar]
- 14.Goh, K. C., M. J. deVeer, and B. R. Williams. 2000. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 19:4292-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 16.Hatada, E., and R. Fukuda. 1992. Binding of influenza A virus NS1 protein to dsRNA in vitro. J. Gen. Virol. 73:3325-3329. [DOI] [PubMed] [Google Scholar]
- 17.Hatada, E., S. Saito, and R. Fukuda. 1999. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J. Virol. 73:2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iordanov, M. S., J. M. Paranjape, A. Zhou, J. Wong, B. R. Williams, E. F. Meurs, R. H. Silverman, and B. E. Magun. 2000. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol. Cell. Biol. 20:617-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iordanov, M. S., J. Wong, J. C. Bell, and B. E. Magun. 2001. Activation of NF-κB by double-stranded RNA (dsRNA) in the absence of protein kinase R and RNase L demonstrates the existence of two separate dsRNA-triggered antiviral programs. Mol. Cell. Biol. 21:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julkunen, I., T. Sareneva, J. Pirhonen, T. Ronni, K. Melen, and S. Matikainen. 2001. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 12:171-180. [DOI] [PubMed] [Google Scholar]
- 21.Karin, M. 1995. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270:16483-16486. [DOI] [PubMed] [Google Scholar]
- 22.Kujime, K., S. Hashimoto, Y. Gon, K. Shimizu, and T. Horie. 2000. p38 mitogen- activated protein kinase and c-jun-NH 2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J. Immunol. 164:3222-3228. [DOI] [PubMed] [Google Scholar]
- 23.Lamb, R. A., and R. M. Krug. 2001. Orthomyxoviridae: the viruses and their replication, p. 1487-1531. In D. M. Knipe, P. M. Howley, D. E. Griffin et al. (ed.), Fields virology, 4th ed. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 24.LaMonica, R., S. S. Kocer, J. Nazarova, W. Dowling, E. Geimonen, R. D. Shaw, and E. R. Mackow. 2001. VP4 differentially regulates TRAF2 signaling, disengaging JNK activation while directing NF-kappa B to effect rotavirus-specific cellular responses. J. Biol. Chem. 276:19889-19896. [DOI] [PubMed] [Google Scholar]
- 25.Li, Y., Z.-Y. Chen, W. Wang, C. C. Baker, and R. M. Krug. 2001. The 3′-end- processing factor CPSF is required for the splicing of single-intron pre-mRNAs in vivo. RNA 7:920-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Y., Y. Yamakita, and R. M. Krug. 1998. Regulation of a nuclear export signal by an adjacent inhibitory sequence: the effector domain of the influenza virus NS1 protein. Proc. Natl. Acad. Sci. USA 95:4864-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, C., S. G. Zimmer, Z. Lu, R. E. Holland, Jr., Q. Dong, and T. M. Chambers. 2001. The involvement of a stress-activated pathway in equine influenza virus-mediated apoptosis. Virology 287:202-213. [DOI] [PubMed] [Google Scholar]
- 28.Lu, Y., X. Y. Qian, and R. M. Krug. 1994. The influenza virus NS1 protein: a novel inhibitor of pre-mRNA splicing. Genes Dev. 8:1817-1828. [DOI] [PubMed] [Google Scholar]
- 29.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the eIF-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig, S., C. Ehrhardt, E. R. Neumeier, M. Kracht, U. R. Rapp, and S. Pleschka. 2001. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J. Biol. Chem. 276:10990-10998. [PubMed] [Google Scholar]
- 31.Majde, J. A. 2000. Viral double-stranded RNA, cytokines, and the flu. J. Interferon Cytokine Res. 20:259-272. [DOI] [PubMed] [Google Scholar]
- 32.Marion, R. M., T. Zurcher, S. de la Luna, and J. Ortin. 1997. Influenza virus NS1 protein interacts with viral transcription-replication complexes in vivo. J. Gen. Virol. 78:2447-2451. [DOI] [PubMed] [Google Scholar]
- 33.Nemeroff, M. E., S. M. Barabino, Y. Li, W. Keller, and R. M. Krug. 1998. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′ end formation of cellular pre-mRNAs. Mol. Cell 1:991-1000. [DOI] [PubMed] [Google Scholar]
- 34.Salvatore, M., C. F. Basler, J. P. Parisien, C. M. Horvath, S. Bourmakina, H. Zheng, T. Muster, P. Palese, and A. Garcia-Sastre. 2002. Effects of influenza A virus NS1 protein on protein expression: the NS1 protein enhances translation and is not required for shutoff of host protein synthesis. J. Virol. 76:1206-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz-Cherry, S., R. M. Krug, and V. S. Hinshaw. 1998. Induction of apoptosis by influenza virus. Semin. Virol. 8:491-495. [Google Scholar]
- 36.Shaulian, E., and M. Karin. 2001. AP-1 in cell proliferation and survival. Oncogene 20:2390-400. [DOI] [PubMed] [Google Scholar]
- 37.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA- dependent protein kinase R or IκB kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 276:8951-8957. [DOI] [PubMed] [Google Scholar]
- 38.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 39.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thanos, D., and T. Maniatis. 1995. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell 83:1091-1100. [DOI] [PubMed] [Google Scholar]
- 41.Wang, W., K. Riedel, P. Lynch, C. Y. Chien, G. T. Montelione, and R. M. Krug. 1999. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5:195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]
- 44.Wisdom, R. 1999. AP-1: one switch for many signals. Exp. Cell Res. 253:180-185. [DOI] [PubMed] [Google Scholar]
- 45.Wolff, T., R. E. O'Neill, and P. Palese. 1996. Interaction cloning of NS1-I, a human protein that binds to the nonstructural NS1 proteins of influenza A and B viruses. J. Virol. 70:5363-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolff, T., R. E. O'Neill, and P. Palese. 1998. NS1-binding protein (NS1-BP): a novel human protein that interacts with the influenza A virus nonstructural NS1 protein is relocalized in the nuclei of infected cells. J. Virol. 72:7170-7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhirnov, O. P., T. E. Konakova, T. Wolff, and H. D. Klenk. 2002. NS1 protein of influenza A virus down-regulates apoptosis. J. Virol. 76:1617-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]