Abstract
The life cycle of human papillomaviruses (HPVs) is tightly coupled to the differentiation program of their host epithelial cells. HPV E4 gene expression is first observed in the parabasal layers of squamous epithelia, suggesting that the E4 gene product contributes to the mechanism of differentiation-dependent virus replication, although its biological function remains unclear. We analyzed the effect of HPV type 18 E4 on cell proliferation and found that E4 expression induced cell cycle arrest at the G2/M boundary. The functional region of E4 necessary for the growth arrest activity was located in the central portion of the molecule, and this activity was independent of the E4-mediated collapse of cytokeratin intermediate filament structures.
Human papillomaviruses (HPVs) infect epithelial cells, causing hyperproliferative lesions such as warts and condylomas. Over 70 HPV types have been identified (12). According to their tissue tropism, they are categorized into two major groups, the cutaneous and mucosal HPVs. The mucosal HPVs are further grouped into high-risk and low-risk types. Lesions caused by high-risk HPVs have a propensity to progress to malignant tumors, most prominently cervical carcinomas. In contrast, lesions caused by low-risk HPVs have a much lower risk for malignant progression (51).
The HPV E6 and E7 oncoproteins are known to play key roles in HPV-associated cancer formation (21, 22, 24, 33, 34). This fact is supported by the finding that most HPV-positive cancer cells maintain the expression of E6 and E7 (4, 52). E6 forms a complex with p53 and, in combination with the cellular ubiquitin ligase E6AP, induces the degradation of p53 through the proteasome (25, 48, 49, 60). E7 binds to and functionally inactivates pRB (20, 35). Both p53 and pRB play critical roles as regulators of the cell cycle and apoptosis. By inhibiting these and other regulatory mechanisms, HPV E6 and E7 allow for the accumulation of genetic mutations and the survival of mutated cells (8, 42, 61). E6 and E7 expression also contributes to the immortalization of infected cells; E6 can enhance telomerase activity through an unknown mechanism (29, 57), whereas E7 inhibits a p16ink4A-dependent pathway that limits cellular proliferation in epithelial cells (28). It is generally believed that these functions of the viral oncoproteins contribute in important ways to HPV-induced cancer formation. However, it is likely that additional viral genes also contribute to the establishment of hyperproliferative potentially precancerous lesions caused by high-risk HPVs. In addition, the relatively low incidence of malignant progression of high-risk HPV-positive lesions indicates that additional mutations of cellular genes may also be necessary for malignant progression.
Studies of the viral life cycle are important for understanding the process of HPV-induced cancer formation. HPV infects the basal layer cells of cutaneous or mucosal membranes. The HPV genome is maintained in an episomal state and at a low copy number in basal cells. The basal cells divide, and the descendant cells move to the upper layers of the epithelium and undergo a program of terminal differentiation. The HPV genome is amplified to a high copy number in differentiated cells, late genes are expressed, and virus particle formation is observed. This tight association between viral replication and the host cell differentiation program is a prominent feature of the HPV life cycle. Several reports have described the regulatory mechanisms of viral replication and how these are connected to the cellular differentiation program. Certain transcription factors, including YY1, skn-1, and CDP, have been implicated in differentiation-dependent HPV gene expression (1-3, 30, 38, 63).
The expression profile of the HPV E4 gene is clearly linked to cellular differentiation status (6, 9, 10, 13, 14, 36, 39). The E4 open reading frame is located in the region of early viral genes, such as E1 and E2, even though other studies have suggested that its expression pattern is more characteristic of a late gene. E4 expression is first detected in the parabasal layers, where vegetative HPV DNA replication is initiated. The E4 protein is encoded by a spliced E1∧E4 mRNA and is expressed as a fusion protein with the N terminus of the E1 protein. It has been reported that in HPV type 1 (HPV1)-infected warts, the E4 protein accumulates at high levels and constitutes approximately 20% of the total proteins (6, 13). Since the expression of E4 is tightly linked to host cell differentiation, it likely plays an important role in the viral life cycle (13, 26). Several biological activities of E4 have been reported; the best defined is its association with cytokeratins and the concomitant destabilization of cytokeratin networks (17, 41, 44, 47, 54). It has been proposed that this activity of E4 may contribute to the efficient transmission of HPV, as progeny virus is shed within terminally differentiated epithelial squamae (17). These studies, however, do not rule out the possibility that E4 may have additional biological activities that also contribute to the viral life cycle.
In this report, high-risk HPV-derived E4 proteins were expressed in cultured cells, and their effect on cell growth was analyzed. E4 expression suppressed cell growth and arrested cell cycle progression at the G2/M boundary. No apoptosis was observed, however. Moreover, this activity of E4 was independent of its ability to disrupt cytokeratin networks. Our findings suggest that E4 may also contribute to the regulation of the viral life cycle by modulating the host cell division cycle.
MATERIALS AND METHODS
Cell cultures and transfection.
HeLa, CV1, and C33A cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum. DNA transfections were performed by using a standard calcium phosphate precipitation method (37). Cells (2 × 105) were seeded in a 6-cm dish 1 day prior to transfection. Plasmid and carrier DNAs (total, 10 μg) were incubated with 500 μl of HEPES-buffered saline transfection buffer (140 mM NaCl, 0.75 mM Na2HPO4, 25 mM HEPES, 110 mM CaCl2 [pH 6.90]) for 30 min at room temperature and then added to a culture dish. At 20 h after transfection, cells were washed once with phosphate-buffered saline (PBS), and fresh growth medium was added.
Plasmids.
The E1∧E4 cDNA expression plasmid was created by joining the corresponding E1 and E4 sequences by PCR. The information for the HPV16 E1∧E4 (16E4) and HPV18 E1∧E4 (18E4) mRNA structures was obtained from the HPV database (18). The E1∧E4 DNA was cloned into plasmid pCMV-FLAG1 (Stratagene Inc., San Diego, Calif.) to fuse a FLAG epitope tag to the N terminus. The FLAG-E1∧E4 sequence was subsequently transferred to a pCMV4 expression plasmid (37). A series of truncation mutations were introduced by using a PCR-mediated mutagenesis strategy (11); see Fig. 4A for the individual E1∧E4 mutants. A commercially available green fluorescent protein (GFP) expression plasmid, pGreenLantern-1 (Invitrogen Corp., Carlsbad, Calif.), was used to mark transfected cells. For transfection experiments, herring sperm DNA (Roche Diagnostics GmbH, Mannheim, Germany) was used as carrier DNA. The human immunodeficiency virus type 1 (HIV-1) Vpr gene was amplified from an HIV-1 LAI DNA clone (40) by PCR, and a FLAG-Vpr expression plasmid was constructed as described for the FLAG-E1∧E4 expression plasmid.
FIG. 4.
Identification of the region within the 18E4 protein that is necessary for G2/M arrest. (A) Schematic representation of the mutant 18E4 proteins tested. The truncated regions of the FLAG-tagged 18E4 protein are represented by black-gray bars. The motifs and functional regions reported for 16E4 are indicated (45). The corresponding regions in 18E4 are represented by gray boxes, and the amino acid sequence alignment of the conserved regions is presented. (B) Steady-state levels of mutant 18E4 proteins expressed in transfected cells. Both detergent-soluble (s) and non-detergent-soluble (i) fractions were analyzed by SDS-PAGE, followed by immunoblot analysis with an anti-FLAG antibody. The two panels represent short (upper) and long (lower) exposures of the same film. The positions of molecular weight markers (in thousands) are indicated on the left. (C) Cell cycle profiles of HeLa cells expressing the indicated 18E4 mutants. Cells were transfected with 4 μg of an E4 expression plasmid and pGreenLantern-1. FACScan analysis was performed as described in the legend to Fig. 2A. Cr, control; WT, wild type.
Immunofluorescence microscopy and immunoblotting.
The transfected cells were fixed with 3% paraformaldehyde and permeabilized with 0.1% Triton X-100. FLAG-tagged E4 proteins were detected with an anti-FLAG polyclonal antibody (Sigma, St. Louis, Mo.) and Alexa Fluor 488 goat anti-rabbit immunoglobulin G (Molecular Probes, Eugene, Oreg.). Cytokeratins were detected with an anticytokeratin 8/18 monoclonal antibody (MAb) (Progen Biotechnik GmbH, Heidelberg, Germany) and Alexa Fluor 546 goat anti-mouse immunoglobulin G (Molecular Probes). Nuclei were visualized by staining with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI) (Nacalai Tesque, Inc., Kyoto, Japan).
Whole-cell extracts were prepared from transfected cells with radioimmunoprecipitation (RIPA) buffer (150 mM NaCl, 50 mM Tris-HCl [pH 8.0], 0.1% sodium dodecyl sulfate [SDS], 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 mM dithiothreitol, 16 μg of benzamidine HCl/ml, 10 μg of phenanthroline/ml, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, 10 μg of pepstatin A/ml, 1 mM phenylmethylsulfonyl fluoride). A 500-μl portion of RIPA buffer was added directly to a culture dish and incubated for 20 min at 4°C with rocking. The cell lysate was transferred to a microcentrifuge tube and centrifuged at 12,000 × g for 10 min at 4°C. The supernatant was used as the detergent-soluble fraction. The pellet was resuspended in 500 μl of SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer (50 mM Tris-HCl [pH 6.8], 2% SDS, 100 mM dithiothreitol, 10% glycerol, 0.1% bromophenol blue) and used as the non-detergent-soluble fraction. Both fractions were analyzed by SDS-15% PAGE and transferred to a Hybond-P polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Ltd., Little Chalfont, England). An anti-FLAG MAb M5 (Sigma) was used for the detection of FLAG-tagged proteins. For visualization, a chemiluminescence detection reagent (Lumi-Light Western blotting substrate; Roche Diagnostics GmbH) was used.
Flow cytometry.
Cells were cotransfected with E4 or Vpr expression plasmid (2 μg) and GFP expression plasmid pGreenLantern-1 (0.5 μg), fixed with 1% paraformaldehyde-ethanol for 10 min at various times after transfection, and treated with 0.5 mg of RNase A/ml-0.1% Triton X-100 in PBS for 30 min at 37°C. Cells were stained with 0.1 mg of propidium iodide (PI)/ml-PBS and analyzed by flow cytometry (FACScan; Becton Dickinson and Company, Franklin Lakes, N.J.). To selectively analyze transfected cells, only GFP-positive cells were counted for PI staining.
The detection of apoptotic cells was performed with an Annexin-V-FLUOS staining kit (Roche Diagnostics GmbH).
Growth suppression assays.
HeLa cells (2 × 105) transfected with 2 μg of E4 or Vpr expression plasmid, 0.5 μg of pGreenLantern-1, and 7.5 μg of carrier DNA were seeded at a density of 0.5 × 105 cells/6-cm dish at 24 h after transfection. Cell numbers per dish were determined at various times after transfection. Only live cells, determined by trypan blue exclusion, were counted.
RESULTS
18E4 expression induces cellular growth suppression.
The HPV E4 gene product is expressed as an E1∧E4 fusion protein containing the 5 amino-terminal amino acids of E1 at its N terminus (18). To investigate the biological activity of high-risk HPV E1∧E4 proteins, we constructed FLAG-tagged E1∧E4 expression plasmids for HPV16 and HPV18. HeLa cells were transfected with these E1∧E4 expression plasmids, and the growth of the transfected cells was analyzed (Fig. 1A). In each experiment, transfection efficiency was monitored by cotransfection of a GFP expression plasmid, and over 80% of the cells were confirmed to be GFP positive. Cell growth was diminished in 18E4-expressing cells compared to control transfected cells. Similar results were obtained for the expression of an untagged E1∧E4 protein (data not shown).16E4 also showed weak but significant activity for suppressing cell growth. As a positive control for growth suppression, we expressed a FLAG-tagged HIV-1 Vpr regulatory protein which was previously reported to interfere with cellular proliferation (31). The HIV-1 Vpr protein was able to induce growth suppression to a similar extent as 18E4 (Fig. 1A).
FIG. 1.
Growth-inhibitory effect of HPV E4. (A) HeLa cells (2 × 105) were transfected with 2 μg of expression plasmid for 16E4, 18E4, or HIV-1 Vpr. A GFP expression plasmid, pGreenLantern-1 (Cr), was cotransfected to distinguish the transfected cells. GFP-positive cells were counted at the indicated times after transfection. Transfection efficiency was over 80%. The results shown are derived from an experiment performed in triplicate; error bars indicate standard deviations. (B) At 48 h after transfection, cell extracts were prepared with RIPA buffer. Both detergent-soluble (s) and non-detergent-soluble (i) fractions were analyzed by SDS-PAGE, and E4 and Vpr were detected by immunoblot analysis with an anti-FLAG antibody. The two panels represent short (left) and long (right) exposures of the same film. The positions of molecular weight markers (in thousands) are indicated on the left.
Next, we analyzed the levels of expression of the 16E4, 18E4, and HIV-1 Vpr proteins by immunoblot analysis with FLAG epitope-specific antibodies. 18E4 and HIV-1 Vpr were expressed at similar levels, whereas the steady-state levels of 16E4 appeared much lower (Fig. 1B), a finding which might account for its weak growth-inhibitory activity (Fig. 1A). As expected, the Vpr protein was localized to the nucleus (data not shown) and was recovered in the detergent-soluble fraction (Fig. 1B). In contrast, 18E4 and 16E4 were mostly cytoplasmic (data not shown) and were detected in the non-detergent-soluble fraction (Fig. 1B).
18E4 expression induces G2 cell cycle arrest.
We next analyzed whether 16E4 and 18E4 caused growth arrest at a specific phase of the cell cycle. It has been reported that HIV-1 Vpr induces growth arrest at the G2 phase of the cell cycle (23, 27). HeLa cells transfected with the Vpr expression plasmid exhibited an increased proportion of cells in G2/M at 40 h after transfection, consistent with G2 arrest (Fig. 2A). This effect was even more dramatic at later times. Like Vpr-expressing cells, HeLa cells transfected with 18E4 also exhibited a dramatic increase in the G2/M population (Fig. 2A). As expected, HeLa cells transfected with 16E4 showed a less dramatic increase in the G2/M population, particularly at early times after transfection, but this increase became more noticeable at later times (Fig. 2A). The difference between 16E4 and 18E4 is most likely a consequence of the lower steady-state levels of 16E4 in the transfected HeLa cells (Fig. 1B). These results suggest that 18E4 is expressed at higher levels and therefore that its biological activities are more noticeable. Hence, we focused mostly on 18E4 for the rest of our studies. The results suggest that, like the expression of HIV-1 Vpr protein, the expression of high-risk HPV E1∧E4 proteins can induce growth arrest in cells at the G2/M boundary of the cell division cycle.
FIG. 2.
Cell cycle analysis of E4- and Vpr-expressing cells. (A) HeLa cells were transfected with 2 μg of Vpr or the indicated E4 expression plasmid in combination with pGreenLantern-1. Cells were collected at the indicated times after transfection, and the DNA contents were analyzed by flow cytometry (FACScan). Nuclei were stainedwith PI. Only the population of transfected GFP-positive cells was counted. Closed and open arrows indicate peaks corresponding to G1 and G2/M phases, respectively. The ratio of G2/M to G1 is shown in each panel. (B) CV1 and C33A cells were transfected with an 18E4 expression plasmid, and the cell cycle profiles were analyzed as described for panel A. Cr, control. (C) Induction of apoptosis by E4 or Vpr expression, as determined by Annexin V staining at 50 h after transfection. The samples were costained with PI for the detection of necrotic cells. Apoptotic cells are positive for Annexin V staining (FL1-H) and negative for PI staining (FL2-H).
The HeLa cell line is a cervical cancer cell line that contains integrated copies of HPV18, and the HPV18 E6 and E7 oncoproteins are expressed in these cells (5, 50). High-risk HPV E6 and E7 proteins are known to subvert G1/S as well as G2/M checkpoint control, raising the possibility that these viral oncoproteins may somehow mask additional effects of E4 expression (19, 32, 53, 58, 59, 62). To clarify this issue, we analyzed the effects of E4 expression in two other, HPV-negative epithelium-derived cell lines: C33A, an HPV-negative human cervical carcinoma cell line, and CV1, a monkey kidney-derived cell line. 18E4 induced G2 arrest in both cell lines, indicating that the G2/M growth arrest effect observed was independent of E6 and E7 functions (Fig. 2B).
In addition to inducing G2 arrest, Vpr is also known to elicit an apoptotic response (55, 56). Vpr expression increased the Annexin V-positive cell population in HeLa cells. The samples were costained with PI to distinguish apoptotic cells from necrotic cells. Vpr-induced Annexin V-positive cells were negative for PI staining, indicating that Vpr induced apoptotic cell death (Fig. 2C). In contrast to the expression of Vpr, the expression of 18E4 did not increase the incidence of apoptosis in HeLa cells. It has been reported that the mechanisms by which Vpr induces G2 arrest and apoptosis are linked (55). Since 18E4 induced an increase in the G2/M population almost as efficiently as HIV-1 Vpr (Fig. 1) but exhibited no proapoptotic activity, these results suggest that the mechanism of G2 cell cycle arrest induction by E1∧E4 is different from that of Vpr.
Under normal conditions, cell cycle arrest at the G2/M boundary is induced in response to DNA damage and unfavorable environmental conditions. If high-risk HPV E1∧E4 expression induces similar G2 arrest, then cells will be maintained at the G2 phase without DNA synthesis or nuclear division. Microscopic observations of 18E4-transfected HeLa and CV1 cells at 70 h after transfection revealed that the cells and the nuclei were frequently enlarged. Especially with HeLa cells, cells containing several gigantic nuclei and micronuclei were observed (Fig. 3). These results suggest that 18E4-expressing cells may continue to synthesize DNA and undergo aberrant nuclear division. This notion is supported by the FACScan results shown in Fig. 2A, where a cell population with a greater than 4N DNA content was observed upon expression of 18E4 in HeLa cells.
FIG. 3.
Morphology of E4-expressing cells. The cells were transfected with 4 μg of an 18E4 expression plasmid, fixed with 50% acetone-methanol at 70 h after transfection, and stained with methylene blue. Nuclei were visualized by DAPI staining. Control cells were transfected with the empty expression plasmid.
The central region of the 18E4 protein is necessary for G2/M cell cycle arrest.
Next, we mapped the domain(s) in the 18E4 protein that is required for the G2 arrest effect. Thus far, most studies on E4 proteins have been performed with HPV1 and HPV16 (18). Mapping studies with16E4 have indicated that the N-terminal region is required for an association with cytokeratin, whereas the C-terminal region is involved in the collapse of the cytokeratin intermediate filament (IF) network as well as E4 oligomerization (16, 43, 45). The functional motifs of 18E4 were extrapolated by sequence comparison with 16E4 and are shown in Fig. 4A. Given the importance of the N- and C-terminal domains of 16E4, we constructed mutant 18E4 expression plasmids with serial deletions at the N and C termini.
E4 is a relatively small protein, consisting of approximately 100 amino acids, and deletions introduced into such a small protein may severely affect protein stability. Therefore, steady-state levels of the mutant 18E4 proteins were analyzed prior to functional analyses. HeLa cells were transfected with the mutant FLAG-18E4 plasmids, and steady-state levels of the E4 proteins in both detergent-soluble and non-detergent-soluble fractions were determined by immunoblot analysis with an anti-FLAG MAb (Fig. 4B). Only amino-terminal mutant NΔ20 was expressed at levels similar to those of wild-type 18E4 protein. Steady-state levels of amino-terminal mutants NΔ5 and NΔ10 were reduced but, like the NΔ20 mutant, these mutants showed distributions in detergent-soluble and non-detergent-soluble fractions similar to those of wild-type 18E4 protein. Steady-state levels of the NΔ30 mutant and all the C-terminal deletion mutants were dramatically reduced, and these proteins were mainly detected in the detergent-soluble fraction, suggesting that the intracellular localization of these mutant proteins is altered compared to that of wild-type 18E4 protein.
To analyze which region(s) of E4 may be essential for the induction of G2 arrest, we expressed the mutant proteins in HeLa cells and analyzed cell cycle distribution by FACScan analysis at 50 h posttransfection. Except for the expression of the NΔ30 and CΔ60 mutants, the expression of each of the other mutant 18E4 proteins induced a marked increase in the G2/M population similar to that seen with wild-type 18E4 protein (Fig. 4C). The effect of CΔ70 was relatively weak, but this result is difficult to interpret due to the low level of expression of this mutant. When we transfected larger amounts of expression plasmids, we observed a more dramatic increase in the G2/M population with CΔ60 but not with NΔ30 (data not shown). However, from these results it is not possible to discriminate whether NΔ30 lacks the essential domain to induce G2 arrest or whether the level of expression of the mutant protein was too low to exhibit its G2 arrest effect. Nevertheless, these results indicate that the domain of 18E4 essential to induce G2/M arrest is minimally contained within amino acids 21 to 59 of 18E4.
Cytokeratin association is dispensable for E4-mediated G2 arrest.
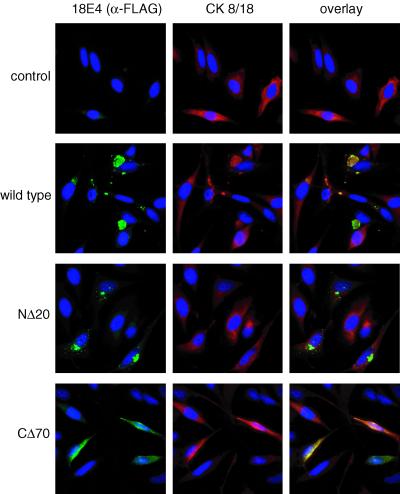
In contrast to wild-type 18E4 and the NΔ5, NΔ10, and NΔ20 mutants of 18E4, which were detected in the non-detergent-soluble fraction, the CΔ70 and CΔ80 mutants were found mostly in the detergent-soluble fraction (Fig. 4C). Since each of these mutants was similarly active in inducing G2/M arrest, it appears unlikely that the ability of E4 to form non-detergent-soluble complexes is linked to its growth-inhibitory function. To analyze this notion in more detail, we performed an immunofluorescence analysis of cytokeratin 8/18 and E4 in HeLa cells transfected with a control vector or a vector expressing wild-type 18E4 or the NΔ20 or CΔ70 mutant (Fig. 5). Cytoplasmic aggregates presumably corresponding to the non-detergent-soluble E4 material were detected in cells expressing wild-type 18E4. Cytokeratin 8/18 staining of normal cells revealed a filamentous network throughout the cytoplasm. In 18E4-expressing cells, the staining pattern for cytokeratin 8/18 was markedly different, suggesting that the IF network might have collapsed, presumably due to 18E4 expression. In support of this notion, cytokeratin 8/18 and 18E4 colocalized in cytoplasmic aggregates, as was previously reported for 16E4 (17). Although the NΔ20 mutant formed aggregates similar to those seen with wild-type 18E4, there was less dramatic colocalization with cytokeratin 8/18-positive structures. The CΔ70 mutant displayed a diffuse cytoplasmic and perinuclear staining pattern, similar to that of cytokeratin 8/18 in normal cells. The cytokeratin IF network was maintained intact in CΔ70-expressing cells. These results are consistent with studies on 16E4 that mapped the domains for multimerization to the C terminus and the ability to interact with cytokeratins to the N terminus (45). These results indicate that neither the ability of E4 to form oligomeric aggregates nor its association with cytokeratins is required for the ability of E4 to arrest cells in G2/M. The failure of the CΔ70 mutant to induce the formation of E1∧E4 aggregates and/or collapse of the cytokeratin network may be attributable to the low levels of expression of this mutant. Regardless, since this mutant still efficiently induced G2/M arrest, the formation of E1∧E4 aggregates and/or collapse of the cytokeratin network are not necessary prerequisites for the E1∧E4-mediated induction of G2/M growth arrest.
FIG. 5.
Immunofluorescence analysis of 18E4 proteins and cytokeratin 8/18 in transfected cells. Cells transfected with wild-type, NΔ20, and CΔ70 18E4 expression plasmids (5 μg) were fixed at 48 h after transfection. E4 proteins (left panels) and cytokeratin (CK) (middle panels) were detected by using anti-FLAG and anticytokeratin 8/18 antibodies, respectively. Nuclei (right panels) were visualized by DAPI staining.
DISCUSSION
The life cycle of papillomaviruses is tightly associated with the differentiation program of epithelial cells via mechanisms that remain to be fully investigated. The ability of HPVs to induce hyperproliferation of epithelial cells is an important aspect of the oncogenic activities of high-risk HPVs. To fully understand the molecular mechanisms that contribute to this activity of HPVs, it is important to investigate the cross talk between HPVs and their epithelial host cells on a molecular level. The expression of the E4 gene product is coupled to the keratinocyte differentiation program, and it has been proposed that E4 contributes to the viral life cycle by interacting with cytokeratins and inducing collapse of the IF network (17, 43). This process presumably facilitates the egress of progeny HPVs when they are shed within the terminally differentiated keratinocyte squamae. In this report, we have analyzed the effect of E4 expression on cell growth. These studies revealed that 18E4 can induce G2/M growth arrest. Since this effect was observed in HPV18-positive and -negative cells, the E6 and E7 oncoproteins do not interfere with this activity of E4. The growth arrest activity of 18E4 was independent its ability to induce collapse of the cytokeratin IF network.
Both 16E4 and 18E4 induced G2/M arrest in HeLa cells, suggesting that growth inhibition may be an activity shared by other HPV E4 proteins. Compared to that of 18E4, the activity of 16E4 appeared weaker, but this finding may be a consequence of the lower levels of 16E4 expression in HeLa cells. Additional experiments will be necessary to define the molecular targets of 18E4 that mediate this G2/M arrest.
The 18E4 protein was as efficient as HIV-1 Vpr in suppressing the growth of HeLa cells. However, in contrast to that of Vpr, 18E4-mediated growth suppression was not accompanied by apoptosis. Since the p53 pathway in HeLa cells is functionally compromised by the expression of HPV18 E6, Vpr-induced apoptosis is likely independent of p53. Although the mechanism is largely unknown, it has been suggested that the abilities of Vpr to induce G2/M arrest and apoptosis may be functionally related (55). Since no apoptosis was observed in 18E4-expressing cells, it may be concluded that HIV-1 Vpr-induced G2/M arrest and 18E4-induced G2/M arrest are mediated by different mechanisms. Alternatively, it is possible that 18E4 contains additional antiapoptotic activities that prevent G2/M-arrested cells from being eliminated by apoptosis (46).
18E4-expressing HeLa cells showed marked nuclear enlargement and multinucleation, and FACScan analysis revealed the appearance of a hyperploid cell population. This finding suggests that a small percentage of 18E4-expressing HeLa cells, after arresting at the G2/M boundary for various amounts of time, will undergo additional rounds of DNA synthesis and nuclear division in the absence of cellular division. It is interesting that high-risk HPV E6 and E7 can also induce aberrant DNA synthesis in G2/M-arrested epithelial cells (58). Moreover, functional disruption of p53 or p21cip1/WAF1 by high-risk HPV E6 also causes DNA rereplication in G2/M-arrested cells (7). Hence, it is possible that the observed multinucleation and the appearance of hyperploid cells are a manifestation of compromised G2/M checkpoint control caused by HPV18 E6 and/or E7 proteins that are synthesized from the integrated HPV18 genomes in HeLa cells. HPV E4 gene expression in an HPV-associated lesion is first detected in the parabasal layers, and it is expected that the other viral genes, including those for E6 and E7, are coexpressed in these cells. Hence, the cooperative interaction between E4-expressing cells and E6- and E7-expressing cells that likely causes the endoreduplication and multinucleation observed in 18E4-expressing HeLa cells may be physiologically significant.
Our studies showed that the N- and C-terminal domains of 18E4 fulfill functions similar to those in 16E4. The N-terminal domain contributes to the ability of E4 to interact with cytokeratins, whereas the C-terminal domain is necessary for E4 to form cytoplasmic aggregates and to cause the collapse of the cytokeratin IF network structure (43, 45). The C-terminal domain of E4 is also the site of interaction for a recently identified putative RNA helicase, E4-DBP (15). Our analysis showed that the C- and N-terminal domains were largely dispensable for the growth-suppressing activity of E4. Our mapping experiments revealed that a sequence of approximately 40 amino acid residues located in the central region of E4 may constitute a novel domain necessary for E4-mediated G2/M growth arrest. Additional mutagenesis experiments with this domain will be necessary to map the structural determinants of this novel activity of E4 in greater detail and to perform a targeted search for cellular factors that can interact with this region of E4.
Acknowledgments
We thank Atsue Ueda for technical assistance and manuscript preparation. We are grateful to Karl Münger (Harvard Medical School) for critical review of the manuscript.
This research was supported in part by grants to H.S. from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
REFERENCES
- 1.Ai, W., J. Narahari, and A. Roman. 2000. Yin yang 1 negatively regulates the differentiation-specific E1 promoter of human papillomavirus type 6. J. Virol. 74:5198-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai, W., E. Toussaint, and A. Roman. 1999. CCAAT displacement protein binds to and negatively regulates human papillomavirus type 6 E6, E7, and E1 promoters. J. Virol. 73:4220-4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen, B., A. Hariri, M. R. Pittelkow, and M. G. Rosenfeld. 1997. Characterization of Skn-1a/i POU domain factors and linkage to papillomavirus gene expression. J. Biol. Chem. 272:15905-15913. [DOI] [PubMed] [Google Scholar]
- 4.Baker, C. C., W. C. Phelps, V. Lindgren, M. J. Braun, M. A. Gonda, and P. M. Howley. 1987. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J. Virol. 61:962-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boshart, M., L. Gissmann, H. Ikenberg, A. Kleinheinz, W. Scheurlen, and H. zur Hausen. 1984. A new type of papillomavirus DNA: its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 3:1151-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitburd, F., O. Croissant, and G. Orth. 1987. Expression of human papillomavirus type 1 E4 gene products in warts, p. 115-122. In B. M. Steinberg, J. L. Brandsma, and L. B. Taichman (ed.), Papillomavirus: cancer cells. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 7.Bunz, F., A. Dutriauux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497-1501. [DOI] [PubMed] [Google Scholar]
- 8.Butz, K., C. Denk, A. Ullmann, M. Scheffner, and F. Hoppe-Seyler. 2000. Induction of apoptosis in human papillomavirus positive cancer cells by peptide aptamers targeting the viral E6 oncoprotein. Proc. Natl. Acad. Sci. USA 97:6693-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow, L. T., M. Nasseri, S. M. Wolinsky, and T. R. Broker. 1987. Human papillomavirus type 6 and 11 mRNAs from genital condylomata acuminata. J. Virol. 61:2581-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow, L. T., S. S. Reilly, T. R. Broker, and L. B. Taichman. 1987. Identification and mapping of human papillomavirus type 1 RNA transcripts recovered from plantar warts and infected epithelial cell cultures. J. Virol. 61:1913-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cormack, B. 1987. Mutagenesis of cloned DNA, p. 8.5.1-8.5.9. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 12.de Villiers, E. M. 1994. Human pathogenic papillomavirus types: an update. Curr. Top. Microbiol. Immunol. 184:1-12. [DOI] [PubMed] [Google Scholar]
- 13.Doorbar, J., D. Campbell, R. J. Grand, and P. H. Gallimore. 1986. Identification of the human papilloma virus-1a E4 gene products. EMBO J. 5:355-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doorbar, J., I. Coneron, and P. H. Gallimore. 1989. Sequence divergence yet conserved physical characteristics among the E4 proteins of cutaneous human papillomaviruses. Virology 172:51-62. [DOI] [PubMed] [Google Scholar]
- 15.Doorbar, J., R. C. Elston, S. Napthine, K. Raj, E. Medcalf, D. Jackson, N. Coleman, H. M. Griffin, P. Masterson, S. Stacey, Y. Mengistu, and J. Dunlop. 2000. The E1E4 protein of human papillomavirus type 16 associates with a putative RNA helicase through sequences in its C terminus. J. Virol. 74:10081-10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doorbar, J., S. Ely, N. Coleman, M. Hibma, D. H. Davies, and L. Crawford. 1992. Epitope-mapped monoclonal antibodies against the HPV16E1-E4 protein. Virology 187:353-359. [DOI] [PubMed] [Google Scholar]
- 17.Doorbar, J., S. Ely, J. Sterling, C. McLean, and L. Crawford. 1991. Specific interaction between HPV-16 E1-E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature 352:824-827. [DOI] [PubMed] [Google Scholar]
- 18.Doorbar, J., and G. Myers. 1996. The E4 protein, p. III58-III80. In G. L. Myers et al. (ed.), The human papillomaviruses. 1996 Compendium. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 19.Duensing, S., L. Y. Lee, A. Duensing, J. Basile, S. Piboonniyom, S. Gonzalez, C. P. Crum, and K. Munger. 2000. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc. Natl. Acad. Sci. USA 97:10002-10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 21.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1991. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J. Virol. 65:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawley-Nelson, P., K. H. Vousden, N. L. Hubbert, D. R. Lowy, and J. T. Schiller. 1989. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 8:3905-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson, J. B., M. A. Bedell, D. J. McCance, and L. A. Laimins. 1990. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J. Virol. 64:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huibregtse, J. M., M. Scheffner, and P. M. Howley. 1991. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus type 16 or 18. EMBO J. 10:4129-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jareborg, N., and S. Burnett. 1991. Immunofluorescent detection of bovine papillomavirus E4 antigen in the cytoplasm of cells permissive in vitro for viral DNA amplification. J. Gen. Virol. 72:2269-2274. [DOI] [PubMed] [Google Scholar]
- 27.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 29.Klingelhutz, A. J., S. A. Foster, and J. K. McDougall. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79-82. [DOI] [PubMed] [Google Scholar]
- 30.Kukimoto, I., and T. Kanda. 2001. Displacement of YY1 by differentiation-specific transcription factor hSkn-1a activates the P670 promoter of human papillomavirus type 16. J. Virol. 75:9302-9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macreadie, I. G., L. A. Castelli, D. R. Hewish, A. Kirkpatrick, A. C. Ward, and A. A. Azad. 1995. A domain of human immunodeficiency virus type 1 Vpr containing repeated H(S/F)RIG amino acid motifs causes cell growth arrest and structural defects. Proc. Natl. Acad. Sci. USA 92:2770-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, L. G., G. W. Demers, and D. A. Galloway. 1998. Disruption of the G1/S transition in human papillomavirus type 16 E7-expressing human cells is associated with altered regulation of cyclin E. J. Virol. 72:975-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matlashewski, G., J. Schneider, L. Banks, N. Jones, A. Murray, and L. Crawford. 1987. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. EMBO J. 6:1741-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munger, K., W. C. Phelps, V. Bubb, P. M. Howley, and R. Schlegel. 1989. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 63:4417-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasseri, M., R. Hirochika, T. R. Broker, and L. T. Chow. 1987. A human papilloma virus type 11 transcript encoding an E1-E4 protein. Virology 159:433-439. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura, A., T. Ono, A. Ishimoto, J. J. Dowhanick, M. A. Frizzell, P. M. Howley, and H. Sakai. 2000. Mechanisms of human papillomavirus E2-mediated repression of viral oncogene expression and cervical cancer cell growth inhibition. J. Virol. 74:3752-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Connor, M. J., W. Stunkel, C. H. Koh, H. Zimmermann, and H. U. Bernard. 2000. The differentiation-specific factor CDP/Cut represses transcription and replication of human papillomaviruses through a conserved silencing element. J. Virol. 74:401-410. [PMC free article] [PubMed] [Google Scholar]
- 39.Palefsky, J. M., B. Winkler, J. P. Rabanus, C. Clark, S. Chan, V. Nizet, and G. K. Schoolnik. 1991. Characterization of in vivo expression of the human papillomavirus type 16 E4 protein in cervical biopsy tissues. J. Clin. Investig. 87:2132-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peden, K. 1991. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1 LAI. Virology 185:661-672. [DOI] [PubMed] [Google Scholar]
- 41.Pray, T. R., and L. A. Laimins. 1995. Differentiation-dependent expression of E1-E4 proteins in cell lines maintaining episomes of human papillomavirus type 31b. Virology 206:679-685. [DOI] [PubMed] [Google Scholar]
- 42.Reznikoff, C. A., C. Belair, E. Savelieva, Y. Zhai, K. Pfeifer, T. Yeager, K. J. Thompson, S. DeVries, C. Bindley, M. A. Newton, et al. 1994. Long-term genome stability and minimal genotypic and phenotypic alterations in HPV16 E7-, but not E6-, immortalized human uroepithelial cells. Genes Dev. 8:2227-2240. [DOI] [PubMed] [Google Scholar]
- 43.Roberts, S., I. Ashmole, L. J. Gibson, S. M. Rookes, G. J. Barton, and P. H. Gallimore. 1994. Mutational analysis of human papillomavirus E4 proteins: identification of structural features important in the formation of cytoplasmic E4-cytokeratin networks in epithelial cells. J. Virol. 68:6432-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts, S., I. Ashmole, G. D. Johnson, J. W. Kreider, and P. H. Gallimore. 1993. Cutaneous and mucosal human papillomavirus E4 proteins form intermediate filament-like structures in epithelial cells. Virology 197:176-187. [DOI] [PubMed] [Google Scholar]
- 45.Roberts, S., I. Ashmole, S. M. Rookes, and P. H. Gallimore. 1997. Mutational analysis of the human papillomavirus type 16 E1-E4 protein shows that the C terminus is dispensable for keratin cytoskeleton association but is involved in inducing disruption of the keratin filaments. J. Virol. 71:3554-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogel-Gaillard, C., F. Breitburd, and G. Orth. 1992. Human papillomavirus type 1 E4 proteins differing by their N-terminal ends have distinct cellular localizations when transiently expressed in vitro. J. Virol. 66:816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogel-Gaillard, C., G. Pehau-Arnaudet, F. Breitburd, and G. Orth. 1993. Cytopathic effect in human papillomavirus type 1-induced inclusion warts: in vitro analysis of the contribution of two forms of the viral E4 protein. J. Investig. Dermatol. 101:843-851. [DOI] [PubMed] [Google Scholar]
- 48.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 49.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz, E., U. K. Freese, L. Gissmann, W. Mayer, B. Roggenbuck, A. Stremlau, and H. zur Hausen. 1985. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 314:111-114. [DOI] [PubMed] [Google Scholar]
- 51.Shah, K. V., and P. M. Howley. 1996. Papillomavirus, p. 2077-2109. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology, 3rd ed. Lippincott-Raven Press, Ltd., New York, N.Y.
- 52.Shirasawa, H., Y. Tomita, S. Sekiya, H. Takamizawa, and B. Simizu. 1987. Integration and transcription of human papillomavirus type 16 and 18 sequences in cell lines derived from cervical carcinomas. J. Gen. Virol. 68:583-591. [DOI] [PubMed] [Google Scholar]
- 53.Slebos, R. J., M. H. Lee, B. S. Plunkett, T. D. Kessis, B. O. Williams, T. Jacks, L. Hedrick, M. B. Kastan, and K. R. Cho. 1994. p53-dependent G1 arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc. Natl. Acad. Sci. USA 91:5320-5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sterling, J. C., J. N. Skepper, and M. A. Stanley. 1993. Immunoelectron microscopical localization of human papillomavirus type 16 L1 and E4 proteins in cervical keratinocytes cultured in vivo. J. Investig. Dermatol. 100:154-158. [DOI] [PubMed] [Google Scholar]
- 55.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 71:5579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Chen. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoppler, H., D. P. Hartmann, L. Sherman, and R. Schlegel. 1997. The human papillomavirus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J. Biol. Chem. 272:13332-13337. [DOI] [PubMed] [Google Scholar]
- 58.Thomas, J. T., and L. A. Laimins. 1998. Human papillomavirus oncoproteins E6 and E7 independently abrogate the mitotic spindle checkpoint. J. Virol. 72:1131-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson, D. A., G. Belinsky, T. H. Chang, D. L. Jones, R. Schlegel, and K. Munger. 1997. The human papillomavirus-16 E6 oncoprotein decreases the vigilance of mitotic checkpoints. Oncogene 15:3025-3035. [DOI] [PubMed] [Google Scholar]
- 60.Werness, B. A., A. J. Levine, and P. M. Howley. 1990. Association of human papillomavirus type 16 and 18 E6 proteins with p53. Science 248:76-79. [DOI] [PubMed] [Google Scholar]
- 61.White, A. E., E. M. Livanos, and T. D. Tlsty. 1994. Differential disruption of genomic integrity and cell cycle regulation in normal human fibroblasts by the HPV oncoproteins. Genes Dev. 8:666-677. [DOI] [PubMed] [Google Scholar]
- 62.Xiong, Y., D. Kuppuswamy, Y. Li, E. M. Livanos, M. Hixon, A. White, D. Beach, and T. D. Tlsty. 1996. Alteration of cell cycle kinase complexes in human papillomavirus E6- and E7-expressing fibroblasts precedes neoplastic transformation. J. Virol. 70:999-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yukawa, K., K. Butz, T. Yasui, H. Kikutani, and F. Hoppe-Seyler. 1996. Regulation of human papillomavirus transcription by the differentiation-dependent epithelial factor Epoc-1/skn-1a. J. Virol. 70:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]