Abstract
Dendritic cells (DCs) play a pivotal role as antigen-presenting cells in the antiviral immune response. Here we show that Hantaan virus (HTNV), which belongs to the Bunyaviridae family (genus Hantavirus) and causes hemorrhagic fever with renal syndrome, productively infects human DCs in vitro. In the course of HTNV infection, DCs did not show any cytopathic effect and viral replication did not induce cell lysis or apoptosis. Furthermore, HTNV did not affect apoptosis-inducing signals that are important for the homeostatic control of mature DCs. In contrast to immunosuppressive viruses, e.g., human cytomegalovirus, HTNV activated immature DCs, resulting in upregulation of major histocompatibility complex (MHC), costimulatory, and adhesion molecules. Intriguingly, strong upregulation of MHC class I molecules and an increased intercellular cell adhesion molecule type 1 expression was also detected on HTNV-infected endothelial cells. In addition, antigen uptake by HTNV-infected DCs was reduced, another characteristic feature of DC maturation. Consistent with these findings, we observed that HTNV-infected DCs stimulated T cells as efficiently as did mature DCs. Finally, infection of DCs with HTNV induced the release of the proinflammatory cytokines tumor necrosis factor alpha and alpha interferon. Taken together, our findings indicate that hantavirus-infected DCs may significantly contribute to hantavirus-associated pathogenesis.
Hantaviruses are enveloped viruses that belong to the Bunyaviridae, a family of tri-segmented negative-sense RNA viruses (26). Hantaviruses are transmitted to humans who inhale aerosolized excreta from chronically infected rodents (66). Infections with certain hantavirus species can manifest in humans as hantavirus pulmonary syndrome (HPS) (51) or hemorrhagic fever with renal syndrome (HFRS) (42). The latter is caused by pathogenic hantavirus species like Hantaan virus (HTNV), Seoul virus, Dobrava virus, and Puumala virus (PUUV). The possibility of therapeutic intervention and vaccine development is currently under investigation (35). To achieve this goal, however, a detailed understanding of the pathogenesis leading to hantavirus-associated diseases is required.
For cellular entry, pathogenic hantaviruses bind to β3 integrin (CD61) (17). Replication takes place in endothelial cells and monocytes/macrophages without causing any direct cytopathic effect (59, 75, 79). Thus, the mechanisms underlying the enhanced vascular permeability that causes HFRS and HPS in humans remain enigmatic. The limited role of direct viral effects in hantavirus-associated pathogenesis suggests that immune-mediated effector mechanisms may be involved. Indeed, several findings support the idea that hantaviruses can induce a vigorous cellular immune response. For example, in patients with acute HFRS, greatly increased numbers of activated CD8 + T cells have been detected (23), resulting in a reversed CD4+/CD8+ T-cell ratio (10). Moreover, kidney biopsy specimens from patients with PUUV infection are characterized by infiltrating CD8+ T lymphocytes (49, 74). Activation of CD8+ T lymphocytes has also been found in patients with HPS (54). In addition, histopathological analysis of specimens derived from HPS patients demonstrated CD8+ T lymphocytes associated with lung endothelial cells (79). In accordance with this description, hantavirus infection of endothelial cells has been shown to trigger the expression of chemokines that attract T cells (72). Finally, the association of certain HLA alleles with a more severe clinical course is suggestive of a role of immunopathology in hantavirus-induced endothelial cell leakage (50).
Activation of antiviral T cells, particularly in a primary response, depends critically on the proper function of monocyte-derived dendritic cells (DCs). Among the first immune cells that encounter viruses after their entry into the human organism are immature DCs which reside in the dermis and epidermis of the skin and mucosa (43). These cells act as sentinels of the immune system in peripheral tissue and are also present in the epithelium and interstitium of the lungs, the portal of entry for hantavirus (38, 52, 58). Immature DCs capture and process viral antigens to form complexes consisting of major histocompatibility complex (MHC) molecules and bound peptides. Signals from damaged tissues or from microbial products trigger the migration of DCs to the draining lymphoid organs. During migration, DCs mature and acquire a characteristic set of surface molecules, thereby becoming the most potent professional antigen-presenting cells of the immune system (4, 71). Mature DCs efficiently activate resting T lymphocytes, which then enter the site of infection to eliminate virus-infected cells. Thus, it is evident that any interaction of viruses with DCs is crucial to the outcome of viral infections and their pathogenesis (6, 33).
A growing number of viruses are known to infect human DCs and interfere with their function by a variety of mechanisms. Among them are human immunodeficiency virus (8, 44), measles virus (14, 19, 68), herpes simplex virus type 1 (36, 64), and human cytomegalovirus (62). These viruses can escape the immune effector components, thereby enhancing their persistence in the host organism. Interestingly, immunochemistry performed on samples derived from HPS patients demonstrated that hantavirus antigen colocalizes with follicular DCs in the spleen and lymph nodes (79). This important finding hinted at hantavirus replication in DCs, although uptake of exogenous viral antigen by DCs without productive infection was an alternative explanation.
In this report we show for the first time that HTNV, a pathogenic hantavirus, can productively infect DCs. Analysis of HTNV-infected DCs revealed that the virus activates DCs, resulting in upregulation of costimulatory, MHC, and adhesion molecules, and induces the release of proinflammatory cytokines. Moreover, HTNV-infected DCs could stimulate T cells as efficiently as could mature DCs. These findings support the hypothesis that in humans, hantaviruses elicit a strong immune response which could be an essential part of the virus-associated pathogenesis.
MATERIALS AND METHODS
Cells.
Vero E6 cells (kindly provided by Å. Lundkvist) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (FCS), 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 4.5 mM l-glutamine. Cultures of human umbilical vein endothelial cells (HUVECs) prepared by the method of Jaffe et al. (25) were grown on gelatin-coated plates and used at the second passage. HUVECs were maintained in MCDB131 (Gibco BRL, Karlsruhe, Germany) supplemented with 10% FCS, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, 1 μg of amphotericin B per ml, 4.5 mM l-glutamine, and 20 μg of endothelial cell growth factor (ECGF) per ml. Medium and FCS were certified endotoxin-free by the manufacturers.
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech, Freiburg, Germany) using buffy coats that were supplied by the German Red Cross, Berlin. In a further step, monocytes were separated from PBMCs by adhesion to plastic for 1 h at 37°C, with contaminating cells being removed by four washes. More than 75% of the resultant cells expressed the CD14 marker. Immature DCs were generated from adhesion-purified monocytes by growth for 6 days in RPMI 1640 plus 10% FCS, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 4.5 mM l-glutamine and supplemented with 800 U of both granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 per ml (IL-4). Where required, mature DCs were generated from these cells by incubation with 1,000 U of tumor necrosis factor alpha (TNF-α) per ml in addition to GM-CSF and IL-4 for 2 days. At the time of infection, immature DCs were CD1a ++ but did not express CD83 whereas mature DCs were CD1a(+) and CD83+.
Fresh blood DCs were isolated from PBMCs by negative selection of T cells, NK cells, and monocytes followed by positive selection of CD4+ DCs (blood DC kit purchased from Miltenyi Biotech) and maintained in the same medium as immature DCs. In addition, DCs were generated from CD34+ stem cells (CD34 + stem cell kit obtained from Miltenyi Biotech) and maintained for 9 days in RPMI 1640 plus 10% FCS, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 4.5 mM l-glutamine and supplemented with 800 U of GM-CSF, 40 U of stem cell factor (SCF) per ml, and 50 U of TNF-α per ml. Recombinant GM-CSF and IL-4 were purchased from ReliaTech, Braunschweig, Germany, whereas SCF and TNF-α were supplied by R&D Systems, Wiesbaden, Germany.
After infection, all DCs were maintained in the presence of the following cytokines: GM-CSF plus IL-4 for immature DCs and peripheral blood DCs; GM-CSF, IL-4, and TNF-α for mature DCs; and GM-CSF, SCF, and TNF-α for CD34+ progenitor cell-derived DCs.
Viruses and infection.
HTNV strain 76-118 was propagated on Vero E6 cells. Supernatant was collected from cell cultures at 14 days postinfection, cleared of cell debris by centrifugation at 2,000 × g, aliquoted, and frozen at −80°C. For certain experiments, concentrated viral stocks were prepared by pelleting virus from infected supernatant at 130,000 × g for 2 h at 4°C. Viral pellets were resuspended in Tris-HCl buffer (pH 7.6) and frozen at −80°C until use. For infection, DCs were incubated either with equal quantities of live virus or with UV-inactivated virus (mock-infected DCs) for 1 h at 37°C. The cells were then washed three times with RPMI 1640 containing 5% heat-inactivated FCS before being resuspended in the appropriate medium at a density of 10 6/ml. Where relevant, one-third of the medium was changed every second day. The titer of virions in the supernatant of HTNV-infected cells was determined by incubation with Vero E6 cells and counting of foci in a chemiluminescence detection assay (20).
Flow cytometry and immunohistochemistry.
For surface immunofluorescence by flow cytometry, cells in suspension were washed once with ice-cold wash solution (phosphate-buffered saline [PBS] with 1% heat-inactivated FCS and 0.05% sodium azide) before being resuspended with the first antibody in ice-cold blocking solution (PBS with 10% heat-inactivated FCS and 0.2% sodium azide) for 1 h. The cells were then washed in ice-cold wash solution, and the staining was repeated with fluorescein isothiocyanate (FITC)-coupled goat anti-mouse secondary antibody. After the final staining step, the cells were washed in ice-cold washing solution and then resuspended in 2 ml of PBS with 1% formaldehyde. The tubes were left at 4°C overnight before being centrifuged, and the cells were resuspended in 200 μl of PBS with 0.2% formaldehyde before being measured.
Flow cytometry was performed on a FACScalibur (Becton Dickinson, Heidelberg, Germany). For immunohistochemistry cells were initially fixed with acetone-methanol (1:1) at −20°C for 10 to 15 min. The slides were then washed three times in PBS and incubated for 15 min at 37°C with blocking solution before monoclonal antibodies were added. Otherwise, staining was performed as described for flow cytometry, except that slides were incubated at 37°C and that isotype-specific secondary antibodies were used.
Determination of phagocytic capacity.
DCs were incubated with FITC-dextran (10 μg/ml) or Lucifer Yellow (4%) for 1 h at either 37 or 4°C. Subsequently, the cells were washed three times and then analyzed by flow cytometry. Receptor-mediated uptake of dextran or macropinocytosis of Lucifer Yellow was determined by measuring the difference between the results at 37 and 4°C. FITC-dextran was provided by Sigma, Deisenhofen, Germany, and Lucifer Yellow was provided by Molecular Probes, Leiden, Netherlands.
Antibodies.
The following antibodies were used for phenotypic analysis of cell markers by flow cytometry: anti-CD11c (clone B-ly6), anti-CD18 (clone 6.7), anti-CDw150 (clone A12), anti-DC SIGN (cloneDCN46), anti αvβ3 (clone23C6), anti-HLA-DR, DP, DQ (clone TÜ39), anti-CD83 (clone HB15e), anti-CD86 (clone IT2.2), anti-CD54 (clone HA58), anti-CD4 (clone RPA-T4), anti-CD11b (clone ICRF44), anti-CD14 (clone M5E2), anti-CD25 (clone M-A251), anti-CD40 (clone LOB7/6), and anti-CD58 (clone L306.4) were purchased from PharMingen, Heidelberg, Germany; anti-CD1a (clone NA1/34) and anti-MHC I (clone W6/32) were obtained from Serotec, Oxford, United Kingdom; anti-CD55 (clone BRIC 110) was purchased from Southern Biotechnology, Birmingham, United Kingdom; anti-CD44 (clone F10-44-2) was purchased from Cymbus Biotechnology, Chandlers Ford, United Kingdom; and anti-CD80 (clone MAB104) and anti-CD106 (clone 1G11) were obtained from Immunotech, Marseilles, France. For detection of viral proteins in immunochemistry and fluorescence-activated cell sorter analysis, the antibody 1C12 specific for the hantavirus N protein (76) was used. Standard enzyme-linked immunosorbent assays that were employed to measure concentrations of alpha interferon (IFN-α), TNF-α, and IL-12 in harvested supernatants from infected cells were obtained from R&D Systems, Wiesbaden, Germany.
Apoptosis.
The degree of apoptosis was determined by a combination of annexin V staining on the cell surface and a propidium iodide assay which measures DNA fragmentation (53). For this purpose, cells were stained initially with FITC-coupled annexin V (Boehringer Mannheim) before being fixed with 4% paraformaldehyde for 10 min. The cells were then washed with PBS and incubated with 0.1% saponin-50 U of RNase A per ml for 30 min before being washed and finally resuspended in PBS containing 5 μg of propidium iodide per ml. In the combined assay, apoptotic cells are annexin V + and show a subdiploid peak of propidium iodide staining due to DNA fragmentation.
Alternatively, cells were fixed with 4% paraformaldehyde for 1 h before permeabilization with 0.1% Triton X-100-0.1% sodium citrate for 10 min. They were subsequently stained for breaks in double-stranded DNA by the TUNEL assay (in situ cell death detection kit; Roche, Mannheim, Germany).
Mixed-lymphocyte reaction.
Immature DCs either infected with HTNV for 4 days or incubated with UV-inactivated virus (mock infected) were incubated with mitomycin C (50 μg/ml) for 20 min at 37°C before being washed four times. Different numbers of these DCs were mixed with 10 5 allogeneic T cells per well in a flat 96-well plate. T cells were purified by depletion of monocytes from PBMCs by adherence to plastic for 1 h at 37°C and subsequent depletion of remaining monocytes and B cells by incubation with goat anti-human immunoglobulin G-coated paramagnetic beads for 15 min at 4°C (Dynal, Hamburg, Germany). Mixed-lymphocyte reaction mixtures were incubated for 5 days before addition of bromodeoxyuridine (BrdU) for 18 h at 37°C. Thereafter, labeled cells were harvested and proliferation was assessed by using an ELISA (Roche) which allows quantitation of BrdU-DNA (61).
RESULTS
DCs are infected by HTNV without loss of viability.
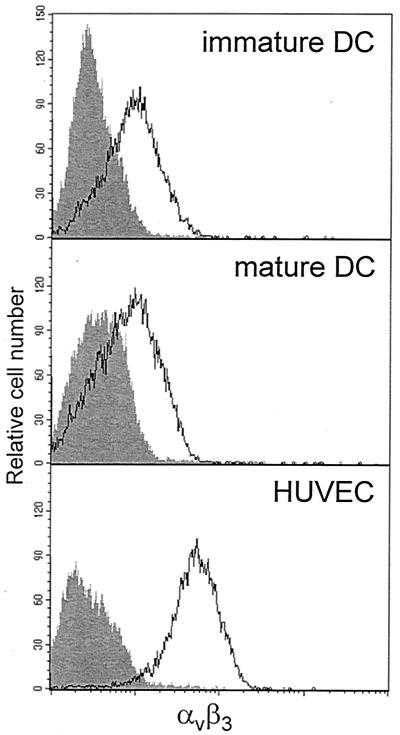
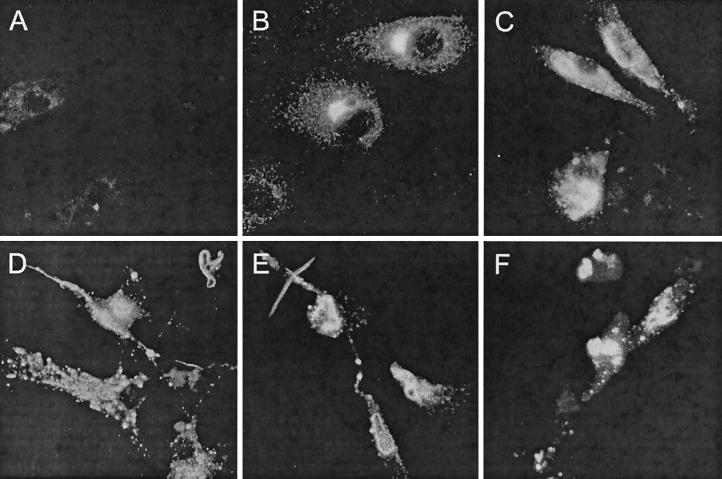
DC-virus interactions profoundly influence the outcome of viral infections for the host. Therefore, we investigated whether HTNV can infect DCs. Since the αvβ3 integrin functions as a receptor for pathogenic hantaviruses (17), we first analyzed its expression on the DC surface. Flow cytometry analysis showed that αvβ3 integrin is present on both immature and mature DCs although at lower levels than on HUVECs (Fig. 1). This suggested that pathogenic hantavirus could enter DCs. Indeed, after 4 days of HTNV infection (multiplicity of infection [MOI] = 0.05), we could detect the viral nucleocapsid (N) protein in HUVECs (80 to 90%) and in monocyte-derived immature (65 to 85%) and mature (35 to 65%) DCs by immunohistochemistry (Fig. 2A to D). In addition, DCs from different sources, e.g., DCs freshly isolated from peripheral blood and DCs derived from CD34+ progenitor cells, could be infected with HTNV (Fig. 2E and F). However, these DC types appeared to be less susceptible to HTNV infection than monocyte-derived immature DCs. After 4 days of infection with HTNV, the viral N protein could be detected in in 40 to 60% of peripheral blood DCs and in 30 to 75% of CD34+ progenitor cell-derived DCs. The pattern of N protein expression in DCs was similar to that found in HTNV-infected HUVECs. In the cytoplasm, punctuated structures and larger twisted threads were visible, which may correspond to membrane compartments in which the process of virus assembly takes place.
FIG. 1.
Expression of αvβ3 integrin on DCs and HUVECs. Monocyte-derived immature DCs, monocyte-derived DCs after maturation with TNF-α, and HUVECs were stained for αvβ3 integrin. Subsequently, the level of expression was analyzed by flow cytometry. The unfilled curves represent αvβ3 integrin expression, whereas the gray filled-in curves show staining of cells with an isotype control. The fluorescence intensity (log scale, four decades) is shown on the x axis, whereas the relative cell number is given on the y axis. The results given are representative of three individual experiments.
FIG. 2.
Detection of viral N protein in HTNV-infected HUVECs and DCs from different sources. Cells were infected with HTNV (MOI = 0.05) and analyzed 4 days postinfection by immunohistochemistry. (A) As a negative control, mock-infected immature DCs were included in the analysis. (B to F) The characteristic distribution of N protein (FITC) is demonstrated in HTNV-infected HUVECs (B), monocyte-derived immature DCs (C), monocyte-derived mature DCs (D), peripheral blood DCs (E), and CD34+ progenitor cell-derived DCs (F). Original magnifications, ×63.
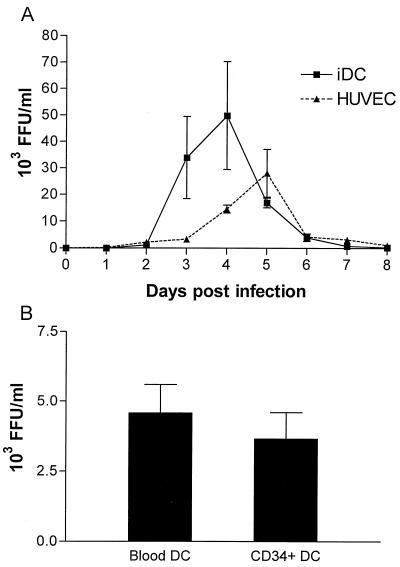
To confirm productive infection, we determined the viral titer in the supernatant of HTNV-infected immature DCs. Figure 3 shows virus production by HUVECs, monocyte-derived DCs, peripheral blood DCs, and CD34+ progenitor cell-derived DCs after infection with HTNV (MOI = 0.05). The number of infectious viral particles released by HUVECs and monocyte-derived immature DCs increased during infection until a peak (30 × 103 to 50 × 103 FFU/ml) was reached on day 5 and day 4 postinfection, respectively (Fig. 3A). However, at later time points, virus yields sharply decreased and only low levels of free virus were detected 8 days after infection. Peripheral blood DCs and CD34+ progenitor cell-derived DCs produced peak titers on day 4 postinfection that were one order of magnitude lower than those observed for monocyte-derived immature DCs (3 × 103 5 × to 103 FFU/ml [Fig. 3B]). Monocyte-derived mature DCs also released virus after HTNV infection with kinetics similar to those of monocyte-derived immature DCs but to a lower extent (data not shown). Thus, DCs from different sources are susceptible to infection with HTNV. Because there is evidence that lung DCs originate from peripheral blood monocytes and show an immature phenotype (52), we used monocyte-derived immature DCs for further analysis.
FIG. 3.
Kinetics of virus production by HTNV-infected HUVECs and DCs from different sources. Cells were infected with HTNV at a MOI of 0.05 for 1 h. Free virions were then removed by thoroughly washing the cells three times. Infected cells were subsequently incubated in culture medium at a density of 1 × 10 6/ml (DCs) or 3 × 10 5/ml (HUVECs). At the time points indicated, supernatant was removed and centrifuged to remove cellular debris and the virus titer was determined by a chemiluminescence detection assay (20) measuring fluorescence-forming units (FFU). (A) The kinetics of virus production by HUVECs and monocyte-derived immature DCs is depicted. (B) The virus titers produced by peripheral blood DCs and CD34+ progenitor cell-derived DCs at 4 days postinfection are given. The results in panel A were calculated from three individual experiments, and the results in panel B are from two individual experiments.
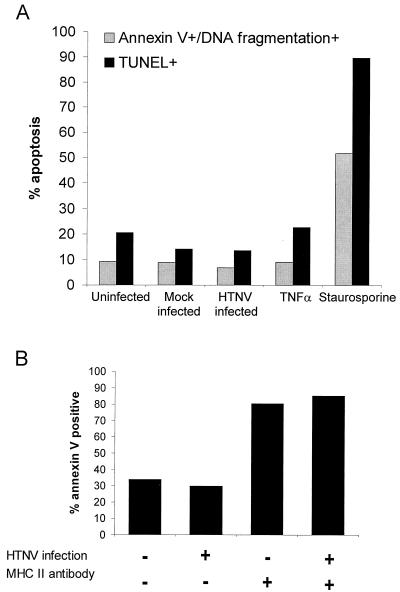
We next determined the morphology and viability of immature DCs after HTNV infection. Similarly to HTNV-infected HUVECs, we did not observe cytopathic effects by microscopic analysis at any time point during the course of infection. In flow cytometry analysis, infected immature DCs showed only a slight increase in size but no change in granularity after infection (data not shown). In addition, at the peak of viral replication the percentage of immature DCs showing both annexin V staining and DNA fragmentation was not increased despite extensive apoptosis in response to staurosporine. Likewise, the percentage of cells with DNA breaks as detected by the TUNEL assay did not change after infection (Fig. 4A). HTNV-infected HUVECs also showed no signs of apoptosis as revealed by annexin V staining and the TUNNEL assay (data not given). Thus, viral replication did not trigger apoptosis in DCs or in HUVECs. Given the maturation stimulus delivered by HTNV to DCs, it was of interest whether death signals via MHC class II molecules could drive virus-infected DCs into apoptosis. This mechanism is important for the homeostatic control of mature DCs (5). Figure 4B shows that HTNV-infected mature DCs were as susceptible to MHC class II-mediated apoptosis as were control mature DCs that had been treated with UV-inactivated virus. These experiments verified that HTNV infection did not significantly alter the morphology, viability, and homeostasis of DCs.
FIG. 4.
Degree of apoptosis in DCs. (A) The percentage of apoptotic cells in immature DCs on day 4 after infection with HTNV (MOI = 1) was analyzed and compared to that in uninfected, mock-infected, and TNF-α-treated immature DCs. Cells treated with staurosporine (5 μg/ml) served as a positive control. Apoptosis was assessed by staining for annexin V and DNA fragmentation or by the TUNEL assay. (B) Mature DCs were incubated with 10 μg of anti-MHC class II monoclonal antibody (clone TÜ39) or isotype control antibody per ml. After 6 h, the degree of apoptosis was determined by staining with FITC-labeled annexin V. The percentage of annexin V-positive cells is given. Representative results of three independent experiments are shown.
DCs upregulate the expression of immunologically important molecules after HTNV infection.
The important role which DCs play as professional APCs in antiviral immune responses depends on the fine-tuned and coordinated expression of adhesion, costimulatory, and MHC molecules. By using flow cytometry, we investigated whether HTNV affects the level of these immunologically relevant molecules. To assess which HTNV-induced changes were cell type specific, HUVECs were included in this analysis. Furthermore, TNF-α-treated cells were used as positive controls because TNF-α activates both DCs and HUVECs and is implicated in HTNV-associated pathogenesis (27).
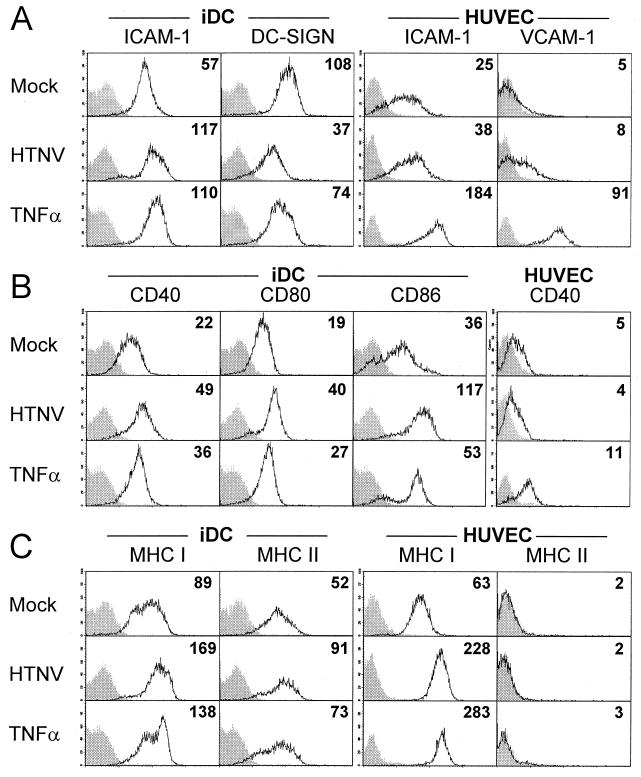
Figure 5A shows that in comparison to mock-infected cells, the expression of ICAM-1 on HTNV-infected immature DCs was enhanced 4 days postinfection whereas DC-SIGN was strongly down-regulated at this time point. Similarly to the situation in immature DCs, the level of ICAM-1 expression was increased on HTNV-infected HUVECs although not as greatly as on TNF-α-treated HUVECs. We could also detect a slight HTNV-induced increase in the density of VCAM-1 molecules on the surface of HUVECs. Figure 5B depicts the density of costimulatory molecules on HTNV-infected immature DCs. These cells upregulated CD40, CD80, and CD86 compared to mock-infected immature DCs. In contrast, HTNV-infected HUVECs did not show increased levels of CD40, although this might be due to the limited ability of HUVECs to express CD40, e.g., after TNF-α treatment. We observed increased expression of MHC class I and II molecules on immature DCs after infection with HTNV. The levels of MHC class I molecules on infected immature DCs were even higher than on TNF-α-treated immature DCs. Intriguingly, MHC class I molecules were also strongly upregulated on HTNV-infected HUVECs whereas expression of MHC class II molecules could not be detected on this cell type (Fig. 5C). HTNV infection of already mature DCs did not significantly alter the pattern of surface molecules (data not shown). Infection with higher viral titers (MOI = 5) enhanced the depicted changes in surface expression only slightly, whereas lowering the MOI to 0.1 diminished (immature DCs) or removed (HUVECs) these changes (data not shown). The expression levels of additional molecules associated with DC maturation were also altered in the course of HTNV infection in comparison to those for the mock-infected immature DCs (data not shown). For example, CD1a was downregulated whereas CD83 and CD44 were upregulated. Immature DCs infected for 2 days with HTNV showed similar although less pronounced phenotypic changes in comparison to immature DCs infected for 4 days, with the exception of CD11c, which was upregulated transiently on day 2 postinfection (data not shown).
FIG. 5.
Expression of immunologically relevant molecules on HTNV-infected immature DCs and HUVECs. Cells were mock infected (infected with UV-inactivated virus), infected with HTNV (MOI = 1), or treated with TNF-α (1,000 U/ml) as indicated. After 3 (HUVECs) or 4 (DCs) days, the cells were analyzed by flow cytometry. (A) Levels of adhesion molecules on immature DCs (ICAM-1 and DC-SIGN) and HUVECs (ICAM-1 and VCAM-1). (B) Expression of costimulatory molecules on immature DCs (CD40, CD80, and CD86) and HUVECs (CD40). (C) Density of MHC class I and II molecules on immature DCs and HUVECs. The unfilled curves represent expression of the marker molecules as indicated, whereas the gray filled-in curves show staining of cells with an irrelevant antibody (isotype control). The x axis shows the fluorescence intensity (log scale, four decades), whereas the y axis depicts the relative cell number. The mean fluorescence intensity is given in the upper right corner of each histogram. The data are representative of three individual experiments.
Taken together, these experiments demonstrated the upregulation of costimulatory, MHC, and adhesion molecules on the cell surface of immature DCs that have been infected with HTNV in vitro. Such an activated DC phenotype indicates that infection with HTNV constitutes a maturation signal for immature DCs.
DCs functionally mature and release proinflammatory cytokines after HTNV infection.
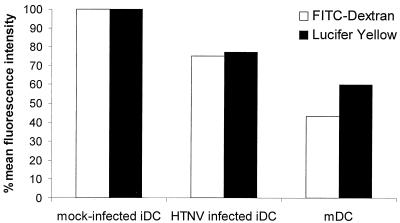
Immature DCs efficiently capture and process antigen (65). This DC function decreases with maturation. Therefore, we wanted to study the effect of HTNV on the uptake of exogenous antigen by analysis of macropinocytosis and receptor-mediated endocytosis. For this type of experiment, immature DCs were infected with HTNV for 4 days. Control cells were treated with TNF-α to generate mature DCs. For measuring macropinocytosis, the uptake of Lucifer Yellow was quantified. Receptor-mediated endocytosis was studied by assessing the uptake of FITC-conjugated dextran. Figure 6 shows that macropinocytosis and receptor-mediated endocytosis of HTNV-infected immature DCs were higher than that observed with mature DCs. However, immature DCs had a reduced capacity for antigen uptake 4 days postinfection compared to the maximal possible antigen uptake as shown by mock-infected immature DCs. Thus, HTNV reduces antigen capture by DCs, illustrating its role as maturation signal for these important APCs.
FIG. 6.
Antigen uptake by immature DCs after infection with HTNV. After 4 days of infection with HTNV, the capacity of immature DC (iDC) for antigen uptake by receptor-mediated phagocytosis (FITC-conjugated dextran) and by macropinocytosis (Lucifer Yellow) was analyzed. For comparison, DCs after maturation with TNF-α (1,000 U/ml) were included in the experiments (mDC). The results are given as the percentage of the maximal possible antigen uptake as shown by mock-infected (infected with UV-inactivated virus) immature DCs. Similar results were obtained in two repeat experiments.
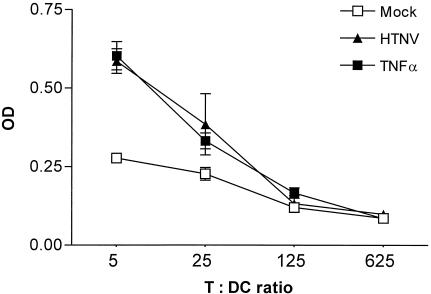
Another characteristic feature of mature DCs is their capacity for T-cell priming. Therefore, we analyzed the ability of HTNV-infected immature DCs to stimulate the proliferation of naive allogeneic T cells (Fig. 7). Mock-infected immature DCs did not efficiently induce the proliferation of allogeneic T lymphocytes. In contrast, stimulation of allogeneic T cells with immature DCs infected for 4 days with HTNV resulted in a proliferative response equivalent to that observed after stimulation with mature DCs.
FIG. 7.
T-cell stimulatory capacity of HTNV-infected DCs. Mock-infected (infected with UV-inactivated virus), HTNV-infected (4 days postinfection), and TNF-α (1,000 U/ml)-treated immature DCs were added to purified allogeneic T cells at different ratios (the T/DC ratio is shown on the x axis). After incubation for 5 days, BrdU was added to the culture for 18 h. Thereafter, the cells were fixed and BrdU incorporation was determined by measuring the optical density (OD, y axis). Data shown are representative of three individual experiments.
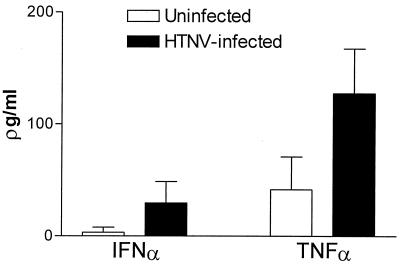
In further experiments, we explored whether HTNV could induce the production of proinflammatory cytokines by DCs. For this purpose, supernatants from uninfected or HTNV-infected DCs were collected at 4 days after infection and analyzed for their concentration of TNF-α, IFN-α, and IL-12. Figure 8 shows that HTNV-infected DCs produced higher levels of IFN-α and TNF-α. In contrast, HTNV did not induce the production of IL-12 (data not shown). In conclusion, these experiments verified that in vitro HTNV induces DC maturation not only in phenotypic but also in functional terms. Moreover, HTNV enhances the production of important proinflammatory cytokines.
FIG. 8.
Cytokine production in immature DCs after infection with HTNV. The supernatant was collected 4 days after infection of immature DCs with HTNV (MOI = 1), and enzyme-linked immunosorbent assays were used to determine the concentrations of cytokines as indicated. The mean values of supernatants derived from three separate experiments are depicted.
DISCUSSION
In this study we demonstrated that DCs derived from different sources can be productively infected with HTNV in vitro. HTNV neither changed DC morphology nor induced cell death by “inside-in” apoptotic signals mediated by viral replication. Moreover, the virus was unable to block “outside-in” apoptotic signals mediated by MHC class II molecules on the cell surface. However, HTNV delivered a strong maturation stimulus to immature DCs as shown by phenotypic and functional changes and induced the release of proinflammatory cytokines.
We found that monocyte-derived DCs, peripheral blood DCs, and CD34+ progenitor cell-derived DCs are susceptible to infection with HTNV. The kinetics of virus production by HTNV-infected DCs resembled that of hantavirus-infected HUVECs (59, 78). During the first 4 days of infection, the number of virions released by HTNV-infected DCs increased. Later, however, virus yields decreased sharply. Whether this drop was due to adsorption of viral particles onto cellular debris, instability of free virus at 37°C, control of viral replication by antiviral defense mechanisms (e.g., IFN-β and MxA) (9, 13), or a combination of these potential mechanisms remains to be investigated.
The changes of DC morphology induced by HTNV were very subtle. There was only a slight increase in size as judged by flow cytometry, and cytopathic effects were not observed (data not shown). This is in accordance with studies demonstrating that hantaviruses replicate in endothelial cells and monocytes/macrophages without causing cytopathic effects (59, 75, 79). Vero E6 cells, a cloned cell line derived from green monkeys, produce high peak viral titers 7 days after HTNV infection and undergo replication-dependent apoptosis (29). In contrast, the peak titer of HTNV replication in human DCs was lower (5 × 10 4 FFU per ml), had already occurred at 4 days postinfection, and was not associated with cell death. Other DC-tropic RNA viruses have also been analyzed with regard to induction of apoptosis in DCs. For example, dengue viruses, mosquito-borne flaviviruses that cause dengue hemorrhagic fever and dengue hemorrhagic shock syndrome, do not induce significant levels of apoptosis in immature DCs (21). In contrast, influenza A virus encodes several strong inducers of apoptosis causing cell death in various cell types (11, 69) including immature DCs (9, 39, 55).
The absence of apoptosis in DCs infected with HTNV and dengue virus may be explained by virus-triggered release of IFN-α, which induces the production of MxA. This cytoplasmic protein interferes with the replication of a variety of RNA viruses (9, 13, 34, 57). Alternatively, virus-induced NF-κB could protect immature DCs from cell death by inducing the expression of several antiapoptotic proteins including cellular inhibitors of apoptosis (c-IAPs) and cellular FLICE inhibitory protein (c-FLIP) (30). Moreover, NF-κB molecules are essential for the survival pathways in DCs that are induced by TNF-related ligands (56). However, despite the lack of apoptosis triggered by viral replication (inside-in death signals), HTNV-infected DCs were susceptible to outside-in death signals mediated by cross-linking of surface MHC class II molecules. This death-inducing mechanism is thought to be important for the homeostatic control of mature DCs which disappear from the lymph nodes after activating T lymphocytes (5, 24).
The HTNV-induced rapid maturation of immature DCs was accompanied by phenotypic changes including upregulation of MHC class I and II molecules and costimulatory and adhesion molecules. In addition, we found that antigen uptake by HTNV-infected DCs decreased, a characteristic feature of maturation (63). In line with this observation, HTNV-infected DCs downregulate DC-SIGN, another molecule that is involved in internalization of antigen by DCs (12). Other DC-tropic viruses, like influenza A virus and dengue virus, also induce DC maturation (9, 21, 39, 40). Double-stranded RNA intermediates, which are produced during the viral replication cycle, could therefore trigger DC maturation. These molecules activate PKR, a serine/threonine kinase (46). It is known that PKR phosphorylates IκB, thereby activating NF-κB (37). The latter could transactivate genes involved in DC maturation. In addition, the release of IFN-α and TNF-α during productive viral infection could drive the maturation of immature DCs as described for dengue virus-infected DCs (21, 40). Alternatively, double-stranded RNA could bind to Toll-like receptors that recognize molecular patterns associated with microbes and initiate the innate immune response (2). Thus, several alternative pathways may exist that mediate hantavirus-induced DC maturation.
We have observed that HTNV upregulates the level of MHC class I molecules not only on the surface of DCs but also on endothelial cells. Enhanced expression of MHC class I on different cell types has also been found after infection with other RNA viruses, e.g., paramyxoviruses (15, 16, 45), coronaviruses (73), and flaviviruses (32, 48). Flaviviruses, the best-studied example so far, increase the biosynthesis of MHC class I molecules by transactivating MHC I gene transcription (31). These pathogens additionally increase the transporter associated with antigen processing (TAP)-mediated peptide import into the endoplasmic reticulum. This results in the intracellular assembly of more stable MHC class I complexes and increases the density of MHC class I molecules on the cell surface (47). HTNV-infected endothelial cells could become more visible for attacking antiviral T cells in a similar way. However, the mechanisms by which HTNV enhances MHC class I surface expression remain to be analyzed in future studies.
DCs infected with immunosuppressive viruses like measles virus, MCMV, and HCMV fail to properly induce either mitogenic or allogeneic T-cell responses (3, 14, 62, 68, 70). DCs after HTNV-induced maturation were as efficient as DCs after TNF-α-mediated maturation in stimulating the proliferation of T lymphocytes. In a similar way, DCs activated by influenza A virus show an increased T-cell-stimulatory capacity (7, 9). Remarkably, during infection with the cytopathic influenza A virus, uninfected DCs take up debris derived from infected bystander cells undergoing apoptosis. From this exogenous source of viral antigens, peptides are processed and presented on MHC class molecules to stimulate antiviral T cells (1). This cross-presentation via an apoptosis-dependent pathway appears to be unlikely to occur in the course of infection with hantaviruses, given the lack of virus-associated cytopathic effects. Rather, direct infection of DCs may be the most important antigen presentation pathway for inducing a primary T-cell response against these pathogens.
Infection with HTNV induced the production of IFN-α and TNF-α. In contrast, the release of IL-12 was not enhanced by HTNV (data not shown). Increased production of TNF-α by HTNV-infected DCs could contribute to the elevated level of this cytokine found in HFRS patients (41, 74). A pathogenic role for TNF-α is also supported by the relatively frequent occurrence of a genetically fixed high-producer TNF-α phenotype in patients hospitalized with severe PUUV infection (28). In addition, this proinflammatory cytokine is involved in the pathogenesis of hemorrhagic fever caused by other viruses, e.g., filoviruses and dengue virus (18, 67). It is intriguing that HTNV-infected and dengue virus-infected DCs show similar phenotypic and functional changes (21, 40, 77) since immunopathology is thought to play a pivotal role in both hantavirus- and dengue virus-associated hemorrhagic fever (60).
Based on our findings, we propose the following model of hantavirus pathogenesis in which DCs are of central importance. In the airways and the alveoli, a network of immature DCs located in the vicinity of epithelial cells take up pathogens which are inhaled by humans (52, 58). Hantaviruses can productively infect these important immune cells without causing significant cell death. As a consequence of hantavirus infection, DCs start to mature, migrating from the lungs to the lymph nodes. Thus, hantavirus-infected DCs could contribute to virus-associated pathogenesis in several ways. First, infection of immature DCs may be important for the transmission and dissemination of the virus throughout the body. Second, in the secondary lymphoid organs, hantavirus-matured DCs could efficiently stimulate T cells which reach the infected organs via the bloodstream. In the process of transmigration, CD8+ effector T cells could firmly bind to and then damage hantavirus-infected endothelial cells that upregulate ICAM-1 and MHC class I molecules. In addition, the proinflammatory cytokines TNF-α and IFN-α released by infected DCs could enhance the hantavirus-induced endothelial cell leakage.
Hantaviruses do not cause disease in chronically infected rodent hosts. Therefore, it will be of great interest whether DCs isolated from the animal hosts can also be infected by hantaviruses and whether these cells show the same phenotype and function as human DCs after viral infection. Finally, the recently described Syrian hamster model may provide a means of testing the in vivo relevance of hantavirus infection of DC (22). This knowledge will help us to understand the pathological conditions underlying virus-associated disease and will contribute to the development of preventive and therapeutic strategies.
Acknowledgments
We thank T. Kaiser from the FACS facility of the Deutsche Rheumaforschungszentrum, Berlin, for assistance in flow cytometry, U. Noack for excellent technical assistance, Å Lundkvist for providing Vero E6 cells and monoclonal antibody 1C12, and N. Suttorp and S. Hippenstiel for critical discussion.
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (Scho 592/3-1) and the European Commission (QLK2-CT-1999-01119) and by the Charité Medical School, Humboldt University.
REFERENCES
- 1.Albert, M. A., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Bertho, N., B. Drenou, B. Laupeze, C. L. Berre, L. Amiot, J. M. Grosset, O. Fardel, D. Charron, N. Mooney, and R. Fauchet. 2000. HLA-DR-mediated apoptosis susceptibility discriminates differentiation stages of dendritic/monocytic APC. J. Immunol. 164:2379-2385. [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj, N. 1997. Interactions of viruses with dendritic cells: a double-edged sword. J. Exp. Med. 186:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhardwaj, N., A. Bender, N. Gonzalez, L. K. Bui, M. C. Garrett, and R. M. Steinman. 1994. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J. Clin. Investig. 94:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blauvelt, A., M. Clerici, D. R. Lucey, S. M. Steinberg, R. Yarchoan, R. Walker, G. M. Shearer, and S. I. Katz. 1995. Functional studies of epidermal Langerhans cells and blood monocytes in HIV-infected persons. J. Immunol. 154:3506-3515. [PubMed] [Google Scholar]
- 9.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, L. B., and W. S. Yang. 1990. Abnormalities of T cell immunoregulation in hemorrhagic fever with renal syndrome. J. Infect. Dis. 161:1016-1019. [DOI] [PubMed] [Google Scholar]
- 11.Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7:1306-1312. [DOI] [PubMed] [Google Scholar]
- 12.Engering, A., T. B. Geijtenbeek, S. J. van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C. G. Figdor, V. Piguet, and Y. van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118-2126. [DOI] [PubMed] [Google Scholar]
- 13.Frese, M., G. Kochs, H. Feldmann, C. Hertkorn, and O. Haller. 1996. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J. Virol. 70:915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M.-C. Rissoan, Y.-J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and function of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao, J., B. P. De, and A. K. Banerjee. 1999. Human parainfluenza virus type 3 up-regulates major histocompatibility complex class I and II expression on respiratory epithelial cells: involvement of a STAT1- and CIITA-independent pathway. J. Virol. 73:1411-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garofalo, R., F. Mei, R. Espejo, G. Ye, H. Haeberle, S. Baron, P. L. Ogra, and V. E. Reyes. 1996. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression through the induction of IFN-beta and IL-1 alpha. J. Immunol. 157:2506-2513. [PubMed] [Google Scholar]
- 17.Gavrilovskaya, I. N., E. J. Brown, M. H. Ginsberg, and E. R. Mackow. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J. Virol. 73:3951-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green, S., D. W. Vaughn, S. Kalayanarooj, S. Nimmannitya, S. Suntayakorn, A. Nisalak, R. Lew, B. L. Innis, I. Kurane, A. L. Rothman, and F. A. Ennis. 1999. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J. Infect. Dis. 179:755-762. [DOI] [PubMed] [Google Scholar]
- 19.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heider, H., B. Ziaja, C. Priemer, A. Lundkvist, J. Neyts, D. H. Kruger, and R. Ulrich. 2001. A chemiluminescence detection method of hantaviral antigens in neutralisation assays and inhibitor studies. J. Virol. Methods 96:17-23. [DOI] [PubMed] [Google Scholar]
- 21.Ho, L. J., J. J. Wang, M. F. Shaio, C. L. Kao, D. M. Chang, S. W. Han, and J. H. Lai. 2001. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 166:1499-1506. [DOI] [PubMed] [Google Scholar]
- 22.Hooper, J. W., T. Larsen, D. M. Custer, and C. S. Schmaljohn. 2001. A lethal disease model for hantavirus pulmonary syndrome. Virology 289:6-14. [DOI] [PubMed] [Google Scholar]
- 23.Huang, C., B. Jin, M. Wang, E. Li, and C. Sun. 1994. Hemorrhagic fever with renal syndrome: relationship between pathogenesis and cellular immunity. J. Infect. Dis. 169:868-870. [DOI] [PubMed] [Google Scholar]
- 24.Ingulli, E., A. Mondino, A. Khoruts, and M. K. Jenkins. 1997. In vivo detection of dendritic cell antigen presentation to CD4 + T cells. J. Exp. Med. 185:2133-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsson, C. B., and C. S. Schmaljohn. 2001. Replication of hantaviruses. Curr. Top. Microbiol. Immunol. 256:15-32. [DOI] [PubMed] [Google Scholar]
- 27.Kanerva, M., J. Mustonen, and A. Vaheri. 1998. Pathogenesis of puumala and other hantavirus infections. Rev. Med. Virol. 8:67-86. [DOI] [PubMed] [Google Scholar]
- 28.Kanerva, M., A. Vaheri, J. Mustonen, and J. Partanen. 1998. High-producer allele of tumour necrosis factor-alpha is part of the susceptibility MHC haplotype in severe puumala virus-induced nephropathia epidemica. Scand. J. Infect. Dis. 30:532-534. [DOI] [PubMed] [Google Scholar]
- 29.Kang, J. I., S. H. Park, P. W. Lee, and B. Y. Ahn. 1999. Apoptosis is induced by hantaviruses in cultured cells. Virology 264:99-105. [DOI] [PubMed] [Google Scholar]
- 30.Karin, M., and A. Lin. 2002. NF-kappaB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 31.Kesson, A. M., and N. J. King. 2001. Transcriptional regulation of major histocompatibility complex class I by flavivirus West Nile is dependent on NF-κB activation. J. Infect. Dis. 184:947-954. [DOI] [PubMed] [Google Scholar]
- 32.King, N. J., and A. M. Kesson. 1988. Interferon-independent increases in class I major histocompatibility complex antigen expression follow flavivirus infection. J. Gen. Virol. 69:2535-2543. [DOI] [PubMed] [Google Scholar]
- 33.Klagge, I. M., and S. Schneider-Schaulies. 1999. Virus interactions with dendritic cells. J. Gen. Virol. 80:823-833. [DOI] [PubMed] [Google Scholar]
- 34.Kochs, G., C. Janzen, H. Hohenberg, and O. Haller. 2002. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc. Natl. Acad. Sci. USA 99:3153-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruger, D. H., R. Ulrich, and A. Lundkvist. 2001. Hantavirus infections and their prevention. Microbes Infect. 3:1129-1244. [DOI] [PubMed] [Google Scholar]
- 36.Kruse, M., O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer. 2000. Mature dendritic cells infected with herpes simplex virus type 1 exhibited inhibited T-cell stimulatory capacity. J. Virol. 74:7127-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar, A., J. Haque, J. Lacoste, J. Hiscott, and B. R. Williams. 1994. Double-stranded RNA-dependent protein kinase activates transcription factor NF-kappa B by phosphorylating I kappa B. Proc. Natl. Acad. Sci. USA 91:6288-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambrecht, B. N., J. B. Prins, and H. C. Hoogsteden. 2001. Lung dendritic cells and host immunity to infection. Eur. Respir. J. 18:692-704. [PubMed] [Google Scholar]
- 39.Larsson, M., D. Messmer, S. Somersan, J. F. Fonteneau, S. M. Donahoe, M. Lee, P. R. Dunbar, V. Cerundolo, I. Julkunen, D. F. Nixon, and N. Bhardwaj. 2000. Requirement of mature dendritic cells for efficient activation of influenza A-specific memory CD8+ T cells. J. Immunol. 165:1182-1190. [DOI] [PubMed] [Google Scholar]
- 40.Libraty, D. H., S. Pichyangkul, C. Ajariyakhajorn, T. P. Endy, and F. A. Ennis. 2001. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J. Virol. 75:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linderholm, M., C. Ahlm, B. Settergren, A. Waage, and A. Tarnvik. 1996. Elevated plasma levels of tumor necrosis factor (TNF)-alpha, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 173:38-43. [DOI] [PubMed] [Google Scholar]
- 42.Linderholm, M., and F. Elgh. 2001. Clinical characteristics of hantavirus infections on the Eurasian continent. Curr. Top. Microbiol. Immunol. 256:135-151. [DOI] [PubMed] [Google Scholar]
- 43.Liu, Y. J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106:259-262. [DOI] [PubMed] [Google Scholar]
- 44.Macatonia, S. E., M. Gompels, A. J. Pinching, S. Patterson, and S. C. Knight. 1992. Antigen-presentation by macrophages but not by dendritic cells in human immunodeficiency virus (HIV) infection. Immunology 75:576-581. [PMC free article] [PubMed] [Google Scholar]
- 45.Massa, P. T., A. Schimpl, E. Wecker, and V. ter Meulen. 1987. Tumor necrosis factor amplifies measles virus-mediated Ia induction on astrocytes. Proc. Natl. Acad. Sci. USA 84:7242-7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meurs, E., K. Chong, J. Galabru, N. S. Thomas, I. M. Kerr, B. R. Williams, and A. G. Hovanessian. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62:379-390. [DOI] [PubMed] [Google Scholar]
- 47.Momburg, F., A. Mullbacher, and M. Lobigs. 2001. Modulation of transporter associated with antigen processing (TAP)-mediated peptide import into the endoplasmic reticulum by flavivirus infection. J. Virol. 75:5663-5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullbacher, A., and M. Lobigs. 1995. Up-regulation of MHC class I by flavivirus-induced peptide translocation into the endoplasmic reticulum. Immunity 3:207-214. [DOI] [PubMed] [Google Scholar]
- 49.Mustonen, J., H. Helin, K. Pietila, M. Brummer-Korvenkontio, K. Hedman, A. Vaheri, and A. Pasternack. 1994. Renal biopsy findings and clinicopathologic correlations in nephropathia epidemica. Clin. Nephrol. 41:121-126. [PubMed] [Google Scholar]
- 50.Mustonen, J., J. Partanen, M. Kanerva, K. Pietila, O. Vapalahti, A. Pasternack, and A. Vaheri. 1996. Genetic susceptibility to severe course of nephropathia epidemica caused by Puumala hantavirus. Kidney Int. 49:217-221. [DOI] [PubMed] [Google Scholar]
- 51.Nichol, S. T., C. F. Spiropoulou, S. Morzunov, P. E. Rollin, T. G. Ksiazek, H. Feldmann, A. Sanchez, J. Childs, S. Zaki, and C. J. Peters. 1993. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 262:914-917. [DOI] [PubMed] [Google Scholar]
- 52.Nicod, L. P., and L. Cochand. 2001. Dendritic cells in the respiratory tract, p. 315-323. In M. T. Lotze and A. W. Thomson (ed.), Dendritic cells. Academic Press, Inc., San Diego, Calif.
- 53.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 54.Nolte, K. B., R. M. Feddersen, K. Foucar, S. R. Zaki, F. T. Koster, D. Madar, T. L. Merlin, P. J. McFeeley, E. T. Umland, and R. E. Zumwalt. 1995. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum. Pathol. 26:110-120. [DOI] [PubMed] [Google Scholar]
- 55.Oh, S., J. M. McCaffery, and M. C. Eichelberger. 2000. Dose-dependent changes in influenza virus-infected dendritic cells result in increased allogeneic T-cell proliferation at low, but not high, doses of virus. J. Virol. 74:5460-5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ouaaz, F., J. Arron, Y. Zheng, Y. Choi, and A. A. Beg. 2002. Dendritic cell development and survival require distinct NF-κB subunits. Immunity 16:257-270. [DOI] [PubMed] [Google Scholar]
- 57.Pavlovic, J., H. A. Arzet, H. P. Hefti, M. Frese, D. Rost, B. Ernst, E. Kolb, P. Staeheli, and O. Haller. 1995. Enhanced virus resistance of transgenic mice expressing the human MxA protein. J. Virol. 69:4506-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peebles, R. S., Jr., and B. S. Graham. 2001. Viruses, dendritic cells and the lung. Respir. Res. 2:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pensiero, M. N., J. B. Sharefkin, C. W. Dieffenbach, and J. Hay. 1992. Hantaan virus infection of human endothelial cells. J. Virol. 66:5929-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters, C. J., and S. R. Zaki. 2002. Role of the endothelium in viral hemorrhagic fevers. Crit. Care Med. 30:S268-S273. [DOI] [PubMed] [Google Scholar]
- 61.Porstmann, T., T. Ternynck, and S. Avrameas. 1985. Quantitation of 5-bromo-2-deoxyuridine incorporation into DNA: an enzyme immunoassay for the assessment of the lymphoid cell proliferative response. J. Immunol. Methods 82:169-179. [DOI] [PubMed] [Google Scholar]
- 62.Raftery, M. J., M. Schwab, S. Eibert, Y. Samstag, H. Walczak, and G. Schönrich. 2001. Targeting of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997-1009. [DOI] [PubMed] [Google Scholar]
- 63.Romani, N., S. Koide, M. Crowley, M. Witmer-Pack, A. M. Livingstone, C. G. Fathman, K. Inaba, and R. M. Steinman. 1989. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J. Exp. Med. 169:1169-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 65.Salter, R. D., and X. Dong. 2001. Regulation of antigen capture, MHC biosynthesis, and degradation by dendritic cells, p. 151-163. In T. M. Lotze and A. W. Thomson (ed.), Dendritic cells. Academic Press, Inc., San Diego, Calif.
- 66.Schmaljohn, C. S. 1996. Bunyaviridae: the viruses and their replication, p. 1447-1471. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippencott-Raven Press, Philadelphia, Pa.
- 67.Schnittler, H. J., and H. Feldmann. 1999. Molecular pathogenesis of filovirus infections: role of macrophages and endothelial cells. Curr. Top. Microbiol. Immunol. 235:175-204. [DOI] [PubMed] [Google Scholar]
- 68.Schnorr, J.-J., S. Xanthakos, P. Keikavoussi, E. Kämpgen, V. ter Meulen, and S. Schneider-Schaulies. 1997. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc. Natl. Acad. Sci. USA 94:5326-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schultz-Cherry, S., N. Dybdahl-Sissoko, G. Neumann, Y. Kawaoka, and V. S. Hinshaw. 2001. Influenza virus NS1 protein induces apoptosis in cultured cells. J. Virol. 75:7875-7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steineur, M. P., I. Grosjean, C. Bella, and D. Kaiserlian. 1998. Langerhans cells are susceptible to measles virus infection and actively suppress T cell proliferation. Eur. J. Dermatol. 8:413-420. [PubMed] [Google Scholar]
- 71.Steinman, R. M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271-296. [DOI] [PubMed] [Google Scholar]
- 72.Sundstrom, J. B., L. K. McMullan, C. F. Spiropoulou, W. C. Hooper, A. A. Ansari, C. J. Peters, and P. E. Rollin. 2001. Hantavirus infection induces the expression of RANTES and IP-10 without causing increased permeability in human lung microvascular endothelial cells. J. Virol. 75:6070-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzumura, A., E. Lavi, S. R. Weiss, and D. H. Silberberg. 1986. Coronavirus infection induces H-2 antigen expression on oligodendrocytes and astrocytes. Science 232:991-993. [DOI] [PubMed] [Google Scholar]
- 74.Temonen, M., J. Mustonen, H. Helin, A. Pasternack, A. Vaheri, and H. Holthofer. 1996. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: an immunohistochemical study. Clin. Immunol. Immunopathol. 78:47-55. [DOI] [PubMed] [Google Scholar]
- 75.Temonen, M., O. Vapalahti, H. Holthofer, M. Brummer-Korvenkontio, A. Vaheri, and H. Lankinen. 1993. Susceptibility of human cells to Puumala virus infection. J. Gen. Virol. 74:515-518. [DOI] [PubMed] [Google Scholar]
- 76.von Herrath, M. G., and M. B. Oldstone. 1996. Virus-induced autoimmune disease. Curr. Opin. Immunol. 8:878-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]
- 78.Yanagihara, R., and D. J. Silverman. 1990. Experimental infection of human vascular endothelial cells by pathogenic and nonpathogenic hantaviruses. Arch. Virol. 111:281-286. [DOI] [PubMed] [Google Scholar]
- 79.Zaki, S. R., P. W. Greer, L. M. Coffield, C. S. Goldsmith, K. B. Nolte, K. Foucar, R. M. Feddersen, R. E. Zumwalt, G. L. Miller, A. S. Khan, et al. 1995. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552-579. [PMC free article] [PubMed] [Google Scholar]