Abstract
We examined lymph nodes and tonsils from patients with infectious mononucleosis by combined detection of EBV-encoded RNA and a specific marker of natural killer (NK) cells, PEN5. A small number of Epstein-Barr virus (EBV) latently infected nonneoplastic NK cells were detected. Our data demonstrate that NK cells are natural targets of EBV and that infection of these cells is an early event observed during primary EBV infection.
Natural killer (NK) cells and T cells may occasionally be the targets of Epstein-Barr virus (EBV). In nasal T- or NK cell lymphoma (4, 7) and rare chronic lymphoproliferative disorders (8, 9), T and NK cells are latently infected by EBV. The description of NK cell chronic lymphoproliferation associated with EBV (8) and the recent detection by flow cytometry of EBV-positive (EBV+) CD16+ cells in EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection (11) suggest that nonneoplastic cells may also be infected. However, there is no clear understanding of how these cells are infected or when this process occurs during the course of infection.
To address this question, we sought to investigate tonsils and lymph nodes of patients affected with primary EBV infection (i.e., infectious mononucleosis [IM]). We developed a strategy of double labeling that combined EBV-encoded RNA (EBER) in situ hybridization and immunohistochemistry (3) with several antibodies directed against B- and T-cell-associated differentiation antigens and against PEN5, a mucin-like glycoprotein selectively expressed on peripheral blood NK cells (16, 17). Among hematopioetic cells, PEN5 is restricted to the CD56dim subset of NK cells. The latter antigen is expressed on a subset of cytotoxic T cells, while PEN5 is not (16, 17). In a previous report, PEN5+ TiA1+ NK cells were detected in large numbers in different lymphoid and nonlymphoid tissues, suggesting a wide distribution throughout the body (16).
Three samples of lymph nodes and three samples of tonsils from six patients with IM were selected for this study. All patients satisfied the clinical criteria for the diagnosis of IM (clinical symptoms and high levels of serum immunoglobulin M [IgM] antibodies against viral capsid antigen [VCA]). The tissue samples were processed routinely (i.e., formalin fixed and paraffin embedded). A number of monoclonal antibodies that react on paraffin sections were used, including anti-CD20/L26 (Dako), anti-CD3 (two clones, one from our laboratory [1] and one from Dako), anti-CD2 (Dako), anti-CD56 (Dako and Novocastra), anti-CD8 (Dako), anti-Granzyme B (Dako), and anti-PEN5. In addition, we used the anti-EBNA2 antibody PE2 from Dako. In situ hybridization with the Dako EBER PNA kit and double staining were performed as previously described (3). The anti-PEN5 antibody has been previously studied and corresponds to anti-5H10, a murine antibody of the IgM subclass (16). It works well on paraffin sections and detects large numbers of NK cells in comparison with anti-CD56 antibody. Indeed, the density of CD56 expression on the surface of NK cells is too low, making anti-PEN5 antibody the preferred NK cell marker (16). In addition, none of the anti-CD16 or anti-CD56 antibodies stain NK cells reliably on paraffin sections (16). We tested two different clones of anti-CD16 antibody (Dako and Novocastra), both of which failed to stain NK cells. Similarly, for anti-CD56 antibody, two different clones were tested from the same commercial sources as anti-CD16 antibody (Dako and Novocastra), but the observed staining of the putative NK cells was weak and showed significant variability. Moreover, we have tested several samples of reactive lymph nodes and tonsils, and as previously published (16), we found PEN5 to be the most reliable NK marker in paraffin sections (data not shown). As described, anti-PEN5 antibody stains subsets of small cells in interfollicular areas, with some of these cells showing abundant cytoplasm.
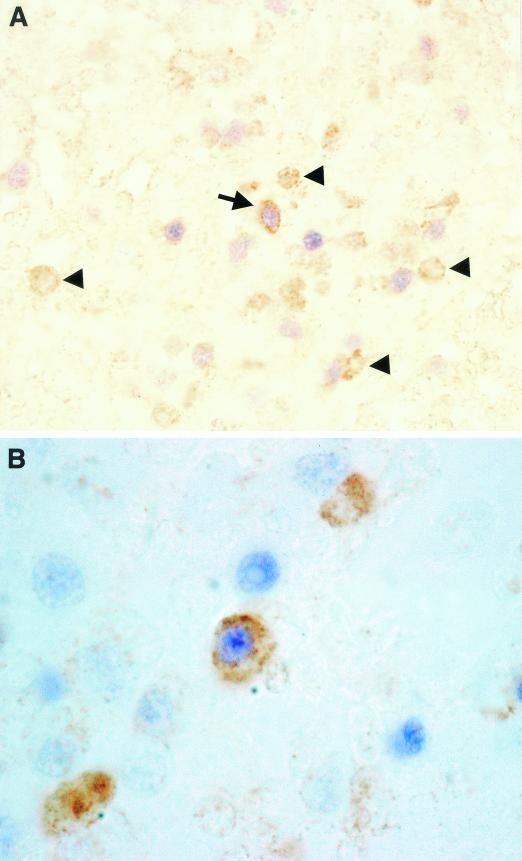
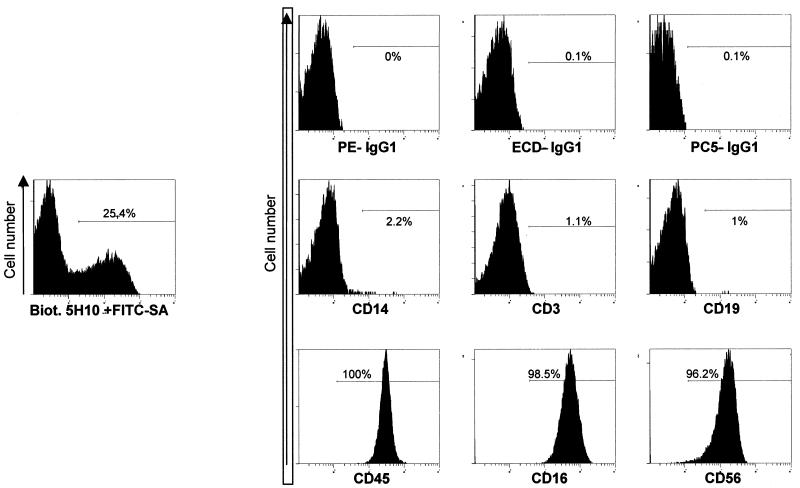
In all samples of IM, we could detect EBER RNA+ PEN5+ cells (Fig. 1A and B). These cells were of small size, but their cytoplasm was slightly more abundant than that of reactive lymphocytes. These cells were rare and corresponded to less than 10−2 to 10−3 EBER RNA+ cells (Fig. 1B). The vast majority of PEN5+ cells were not stained with EBER probes (Fig. 1A, arrowheads). The majority of EBER RNA+ cells were B cells (CD20+), many of which expressed the EBNA2 protein. However, we were unable to detect any EBNA2+ PEN5+ double-positive cells. This may suggest that the rare EBER+ PEN5+ cells display a type I or II latency program. The absence of EBNA2 in EBV+ NK cells is well known and has been documented in both neoplastic (4) and nonneoplastic (9) cells. Finally, the results of fluorescence-activated cell sorter analysis with PEN5 on peripheral blood mononuclear cells (PBMCs) clearly demonstrate that PEN5 is not expressed on T cells, monocytes, or B cells (Fig. 2). In addition, we have tested lymphoblastoid cell lines and EBV+ lymphoblastoid tumors grown in SCID mice (13), both of which failed to demonstrate PEN5 expression (data not shown).
FIG. 1.
Double staining with the EBER probes and anti-PEN5 antibody. (A) Low magnification showing a double-positive PEN5+ EBER+ NK cell (arrow) among other EBV-infected cells (B cells with blue nucleus) and PEN5+ EBER− NK cells (arrowheads). Brown staining with peroxidase-diaminobenzidine indicates PEN5+ membrane and cytoplasm, and blue staining with alkaline phosphatase-nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) indicates EBER probes and nuclear staining. Magnification, ×250. (B) High magnification showing rare cells (in the center) with colocalization of the EBER probes (blue) and the PEN5 molecule (brown). Magnification, ×1,000.
FIG. 2.
Distribution of PEN5+ cells among PBMC subsets (see references 16 and 17). Freshly isolated PBMCs from a healthy volunteer were stained with the monoclonal antibodies PE-IgG1, ECD-IgG1, PC5-IgG1, PE-CD14, ECD-CD3, PE-CD14, PE-CD19, PE-CD16, PC5-CD45, and PC5-CD56 (Beckman Coulter); biotinylated 5H10 (1 μg/ml); and then FITC-neutralite-avidine (FITC-SA; Southern Biotechnology). Samples were run on an XL/MCL cytometer (Beckman Coulter). Acquisition and analysis were performed with EXPO 32 version 1.2 software (Beckman Coulter). For this donor, 5H10+ cells represent 18.9% PBMCs and 25.4% lymphocytes. Anti-CD14 and anti-CD45 stainings were gated on 5H10+ PBMCs; the other histograms were gated on 5H10+ lymphocytes. (Lymphocytes are identified by their forward- and side-scatter features.)
Surprisingly we could not definitely isolate EBV+ T cells by using anti-CD2, anti-CD3, or anti-CD8 labeling. This might be explained by the presence of numerous CD2+ CD3+ and/or CD8+ cells around the EBER RNA+ cells, thus rendering the double-labeling technique difficult to interpret. The latter results are in keeping with the data of Quintanilla-Martinez et al. (14), who failed to detect EBV+ T cells in lesions of IM, but are at variance with those reported by Deamant et al. (5), who reported the presence of CD3+ CD57− EBER RNA+ cells in reactive lymphoid tissues. Similar results have been observed in patients from South America with a history of acute infection (5). However, if these cells are truly EBER+ CD2+ or EBER+ CD3+, one cannot exclude that they represent cells of the NK lineage, since both CD2 and the intracytoplasmic portion of the epsilon chain of the CD3 complex are expressed on NK cells. However, we could not detect isolated CD3+ or CD2+ EBER RNA+ cells, but only observed rare EBER RNA+ cells scattered among the reactive T cells. Unfortunately, the antibodies that recognize the external portion of the CD3 epsilon chain do not work well on paraffin sections, because this portion of the protein is degraded by formalin fixation. EBER RNA in situ hybridization works optimally on a paraffin section in which the small ribonucleoproteins survive the fixation. This technique is unfortunately much less reliable on frozen sections, where no signal is observed after double staining. Indeed, the strongest argument against T-cell infection by EBV might be the absence of EBER RNA+ CD8+ cells. Our failure to detect EBV+ CD8+ T cells may be explained by the absence of an efficient cytotoxic T-cell response during the acute phase of IM.
Our results confirm that EBV can latently infect NK cells and that this appears to occur early during primary infection. Previous studies have shown that normal T cells express both the CD21 receptor and HLA class II molecules (6), thus providing a theoretical means for infection by EBV. HLA class II molecules are also detected on the surface of NK cells (17) and may function as a coreceptor (2). However, NK cells typically lack expression of CD21. Thus, if NK cells were at risk for infection by a virus originating from nearby epithelial cells (2), it would require expression of CD21 or a related receptor. Despite the lack of definitive evidence for an appropriate EBV receptor, recent data have demonstrated that nonneoplastic cells can be the targets of EBV infection (8-10). As described above, the existence of both an NK cell chronic lymphoproliferation associated with EBV infection (8) and nasal T- or NK cell lymphoma as EBV-related clinicopathological entities (7) strongly suggests that these cells can function as a reservoir for the virus. The frequent localization of these lymphomas in the nasopharynx is difficult to explain, since they are not classically observed in the tonsils and in other sites where nonneoplastic EBV-positive NK cells are present (peripheral blood, lymph nodes). However, the nasopharynx is the site of another EBV-associated tumor, nasopharyngeal carcinoma, which in virtually all cases is associated with EBV (12). This site might represent the major localization for active viral replication during primary infection and may serve as a reservoir in patients with chronic shedding of viral particles. Interestingly, large numbers of NK cells are present in this site (personal unpublished data), providing additional evidence that they may participate in an immune response against EBV and/or EBV-infected cells. Nevertheless, other unknown cofactors may favor the development of T- or NK cell lymphoma in this anatomic site. Possible mechanisms for epithelial cell infection have been previously postulated (15); however, little is known regarding the role of T cells and particularly NK cells. It seems reasonable to hypothesize that the infection of NK cells occurs while they are attempting to kill an EBV+ cell target.
In conclusion, we provide for the first time strong evidence that EBV+ nonneoplastic NK cells can be detected in reactive lymphoid tissues. In addition, infection of these cells seems to occur early during the primary infection with EBV.
Acknowledgments
Pierre Brousset is supported by grants from the Institut Universitaire de France.
REFERENCES
- 1.Alibaud, L., R. Llobera, T. Al Saati, M. March, G. Delsol, and B. Rubin. 2000. A new monoclonal anti-CD3ε antibody reactive on paraffin sections. J. Histochem. Cytochem. 48:1609-1616. [DOI] [PubMed] [Google Scholar]
- 2.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. [DOI] [PubMed] [Google Scholar]
- 3.Brousset, P., D. Schlaifer, D. Roda, P. Massip, B. Marchou, and G. Delsol. 1996. Characterization of Epstein-Barr virus-infected cells in benign lymphadenopathy of patients seropositive for human immunodeficiency virus. Hum. Pathol. 27:263-268. [DOI] [PubMed] [Google Scholar]
- 4.Chiang, A. K., Q. Tao, G. Srivastava, and F. C. Ho. 1996. Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin's disease. Int. J. Cancer 68:285-290. [DOI] [PubMed] [Google Scholar]
- 5.Deamant, F. D., P. F. Albujar, Y. Y. Chen, and L. M. Weiss. 1993. Epstein-Barr virus distribution in nonneoplastic lymph nodes. Mod. Pathol. 6:729-732. [PubMed] [Google Scholar]
- 6.Fisher, E., C. Delibrias, and M. D. Kazatchkine. 1991. Expression of the CR2 (the C3dg/EBV receptor, CD21) on normal human peripheral blood T lymphocytes. J. Immunol. 146:865-872. [PubMed] [Google Scholar]
- 7.Kanavaros, P., M. C. Lescs, J. Briere, M. Divine, F. Galateau, I. Joab, J. Bosq, J. P. Farcet, F. Reyes, and P. Gaulard. 1993. Nasal T-cell lymphoma: a clinicopathologic entity associated with peculiar phenotype and with Epstein-Barr virus. Blood 81:2688-2695. [PubMed] [Google Scholar]
- 8.Kanegane, H., T. Wado, K. Nunogami, H. Seki, N. Taniguchi, and G. Tosato. 1996. Chronic persistent Epstein-Barr virus infection of natural killer cells and B cells associated with granular lymphocytes expansion. Br. J. Haematol. 95:116-122. [DOI] [PubMed] [Google Scholar]
- 9.Kanegane, H., F. Wang, and G. Tosato. 1996. Virus-cell interactions in a natural killer-like cell line from a patient with lymphoblastic lymphoma. Blood 88:4667-4675. [PubMed] [Google Scholar]
- 10.Kanegane, H., A. Yachie, T. Miyawaki, and G. Tosato. 1998. EBV-NK cells interactions and lymphoproliferative disorders. Leuk. Lymphoma 29:491-498. [DOI] [PubMed] [Google Scholar]
- 11.Kasahara, Y., A. Yachie, K. Takei, C. Kanegane, K. Okada, K. Ohta, H. Seki, N. Igarashi, K. Maruhashi, K. Katayama, E. Katoh, G. Terao, Y. Sakiyama, and S. Koizumi. 2001. Differential cellular targets of Epstein-Barr virus (EBV) infection between acute EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Blood 98:1882-1888. [DOI] [PubMed] [Google Scholar]
- 12.Klein, G. 1979. The relationship of the virus to nasopharyngeal carcinoma, p. 339-350. In M. A. Epstein and B. G. Achong (ed.),The Epstein-Barr virus. Springer-Verlag, Berlin, Germany.
- 13.Meggetto, F., P. Brousset, J. Selves, G. Delsol, and B. Mariame. 1997. Reed-Sternberg cells and “bystander” lymphocytes in lymph nodes affected by Hodgkin's disease are infected with different strains of Epstein-Barr virus. J. Virol. 71:2547-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quintanilla-Martinez, L., S. Kumar, F. Fend, E. Reyes, J. Teruya-Feldstein, D. W. Kingma, L. Sorbara, M. Raffeld, S. E. Straus, and E. S. Jaffe. 2000. Fulminant EBV+ T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood 96:443-451. [PubMed] [Google Scholar]
- 15.Sixbey, J. W., and Q. Y. Yao. 1992. Immunoglobulin A-induced shift of Epstein-Barr virus tissue tropism. Science 255:1578-1580. [DOI] [PubMed] [Google Scholar]
- 16.Vivier, E., M. Munroe, P. Ariniello, and P. Anderson. 1995. Identification of tissue-infiltrating lymphocytes expressing PEN5, a mucin-like glycoprotein selectively expressed on natural killer cells. Am. J. Pathol. 146:409-418. [PMC free article] [PubMed] [Google Scholar]
- 17.Vivier, E., J. M. Sorrell, M. Ackerly, M. J. Robertson, R. A. Rasmussen, H. Levine, and P. Anderson. 1993. Developmental regulation of a mucinlike glycoprotein selectively expressed on natural killer cells. J. Exp. Med. 178:2023-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]