Abstract
Equine infectious anemia virus (EIAV) infection of horses is characterized by well-defined waves of viremia associated with the sequential evolution of distinct viral populations displaying extensive envelope gp90 variation; however, a correlation of in vivo envelope evolution with in vitro serum neutralization phenotype remains undefined. Therefore, the goal of the present study was to utilize a previously defined panel of natural variant EIAV envelope isolates from sequential febrile episodes to characterize the effects of envelope variation during persistent infection on viral neutralization phenotypes and to define the determinants of EIAV envelope neutralization specificity. To assess the neutralization phenotypes of the sequential EIAV envelope variants, we determined the sensitivity of five variant envelopes to neutralization by a longitudinal panel of immune serum from the source infected pony. The results indicated that the evolution of the EIAV envelope sequences observed during sequential febrile episodes produced an increasingly neutralization-resistant phenotype. To further define the envelope determinants of EIAV neutralization specificity, we examined the neutralization properties of a panel of chimeric envelope constructs derived from reciprocal envelope domain exchanges between selected neutralization-sensitive and neutralization-resistant envelope variants. These results indicated that the EIAV gp90 V3 and V4 domains individually conferred serum neutralization resistance while other envelope segments in addition to V3 and V4 were evidently required for conferring total serum neutralization sensitivity. These data clearly demonstrate for the first time the influence of sequential gp90 variation during persistent infection in increasing envelope neutralization resistance, identify the gp90 V3 and V4 domains as the principal determinants of antibody neutralization resistance, and indicate distinct complex cooperative envelope domain interactions in defining sensitivity to serum antibody neutralization.
Equine infectious anemia virus (EIAV) is a member of the lentivirus subfamily of retroviruses; it causes a rapid and dynamic disease in persistently infected horses that is in marked contrast to the slowly progressive degenerative diseases typically associated with infections by other human and animal lentiviruses (23). Natural or experimental EIAV infections of horses follow a characteristic progression of disease during the first year from acute, to chronic (recurring disease cycles), to long-term inapparent carrier, as the result of a complex and lengthy development of enduring and broadly controlling virus-specific host immunity that suppresses viral replication to subclinical levels (13, 14, 23). Therefore, the EIAV system provides a useful model lentivirus system for examining the nature and role of antigenic variation during persistent infection, investigating the dynamic interaction with host immune responses, and defining the critical components in the development of immunologic control of lentivirus replication and disease.
Several studies have demonstrated that the control of EIAV replication and disease is directly related to host immune responses and not to the attenuation of the infecting virus (16, 18, 26). For example, immune suppression of inapparent carriers by dexamethasome treatment can cause a recrudescence of disease associated with increased levels of viremia (9, 18, 32), and transfer of whole blood from inapparent carriers to naive horses usually results in infection and disease in the recipients (16, 32). In addition, Perryman et al. have reported that experimental infection of immunodeficient foals with EIAV causes an aggressively progressive fatal disease, emphatically demonstrating the potential virulence of EIAV in the absence of host immune responses (26). Equally important, but probably less well recognized, is the observation that inapparent carriers of EIAV are protected from subsequent exposure to virus by horsefly transmission in the field or by experimental intravenous inoculation in the laboratory (16, 17). Therefore, the ability of the equine immune system to routinely achieve lifelong control of a persistent virulent lentivirus infection and protection from reexposure provides an important natural animal lentivirus model for enduring, broadly protective immunity that can overcome the diverse array of immune escape mechanisms used by lentiviruses.
The development of host immune responses in immunocompetent equids experimentally infected with EIAV has been examined in detail (12, 22, 34). These studies demonstrate that the characteristic progression to an inapparent carrier stage of EIAV infection is associated with an evolution of humoral and cellular immune responses during the first 8 to 10 months postinfection into a mature, steady-state immunity that effectively controls EIAV replication and restricts viral infection predominantly to tissue reservoirs (14). Paralleling the development of enduring broadly protective immunity, neutralizing antibodies to the infecting virus are first detected 2 to 3 months postinfection and their levels continue to increase for up to 10 months before reaching a steady state that is maintained indefinitely (12, 13). In addition to the progressive increases in serum neutralization titer during the first year post infection, Rwambo et al. (28) have reported a steady increase in the breadth of serum antibody neutralization specificity in experimentally infected ponies during the first year postinfection, presumably reflecting an accumulation of antibody responses to variant EIAV envelope quasispecies that evolve during this period. However, the role of neutralizing antibodies in achieving and sustaining control of EIAV replication remains controversial (10, 11, 31).
Extensive studies of EIAV recovered from experimentally infected equids during chronic disease have revealed a unique population of viral envelope quasispecies associated with each disease cycle, apparently reflecting the sequential evolution of antigenic variants that temporarily escape the prevailing host immune responses (20, 21, 25). Interestingly, a similar rate and extent of EIAV envelope evolution have been reported in progressor and nonprogressor ponies experimentally infected with a reference EIAV strain, suggesting ongoing viral variation and immune selection in the absence of clinical symptoms and relatively low levels of systemic virus replication (19). These data indicate that antigenic variants of EIAV are probably produced in tissue reservoirs that are rich in infected macrophages (liver and spleen) as a result of ongoing virus replication and evolution, even in the face of robust host immune responses.
Detailed serologic and genetic characterizations of the evolution of EIAV populations during chronic EIAV have demonstrated a close correlation between changes in viral neutralization specificity and variations in the sequence of the viral SU and TM proteins, respectively designated gp90 and gp45, that are observed in viral populations associated with sequential disease cycles (15, 20, 25, 29, 36). These studies of EIAV envelope variation have led to an identification of conserved and variable domains in the heavily glycosylated gp90 glycoprotein and have suggested that the presence of a hypervariable principal neutralizing domain (PND) in the V3 segment of the gp90 envelope glycoprotein (1, 19, 20). Despite the extensive information available on the nature of EIAV envelope variation during persistent infection and the differences in serum antibody neutralization phenotypes among viral variants, there is to date no definitive information on the neutralization determinants of EIAV envelope proteins or the effects of natural envelope variation in these determinants on viral neutralization sensitivity. A higher-resolution definition of EIAV envelope neutralization determinants could provide important fundamental information to further our understanding of the effects of specific envelope variations on antigenic and immunogenic properties and to aid in design and evaluation of vaccine strategies for this virus.
We report here a series of experiments with the specific aim of defining neutralization determinants of EIAV gp90 envelope protein and of evaluating the influence of natural envelope variation during persistent infection on viral neutralization properties. For these studies, we have used a novel panel of longitudinal immune serum samples and well-defined EIAV envelope variants isolated from sequential febrile episodes in a comprehensively studied experimentally infected pony (12, 13, 19, 20). The results of these studies reveal new basic information about the antigenic architecture of EIAV envelope glycoproteins and the influence of longitudinal natural envelope variation on EIAV neutralization properties.
MATERIALS AND METHODS
Experimental infections, clinical evaluation, and virus and serum isolations.
Four outbred ponies were experimentally inoculated intravenously with 103 50% tissue culture infectious doses (TCID50) of the reference pathogenic biological clone, EIAVPV (20). The clinical progression, viral evolution, and host immune responses of these experimentally infected ponies have been extensively described previously (12, 13, 19, 20). The EIAV envelope variants used in this study were isolated during sequential disease cycles observed in pony 564 (see Fig. 1) and were characterized in detail by cloning and gene sequencing, as described by Leroux et al. (20). A comparison of the deduced amino acid sequences of the gp90 variable domains of the respective variant viral envelope quasispecies selected for this study is summarized in Fig. 2 (see below). Selected immune serum samples taken from pony 564 and the three other experimentally infected ponies (ponies 561, 562, and 567) at monthly intervals and during disease cycles over a 36-month observation period were used in this study to define the neutralization phenotypes of the EIAV envelope variants derived from pony 564.
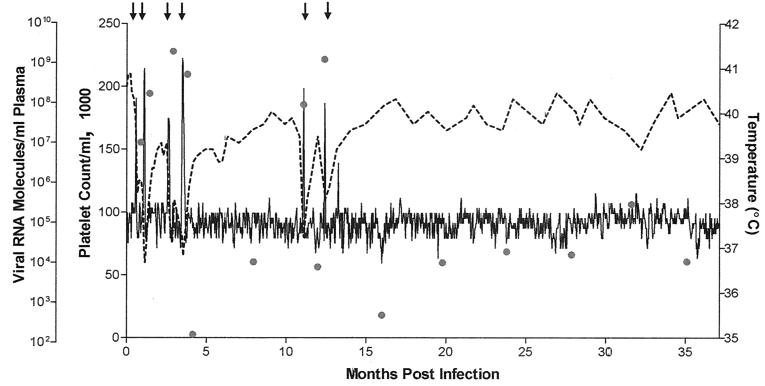
FIG. 1.
Clinical and virological profile of pony 564 experimentally infected with EIAVPV. Pony 564 was experimentally infected with 103 TCID50 of the biological clone EIAVPV. Rectal temperature (solid line, right y axis) and platelet count (dashed line, second left y axis) were monitored regularly for 36 months after infection as standard measures of disease. Febrile episodes (arrows) occurred at 18 (I), 34 (II), 80 (III), 106 (IV), 337 (V), and 378 (VI) days postinfection and were defined by a rectal temperature above 39°C in conjunction with a reduction in the number of platelets below 100,000/μl of whole blood and other characteristic clinical symptoms. Quantification of the virus load (shaded circles, first left y axis) was performed on the viral RNA extracted from plasma during disease cycles and at periodic time points during the observation period. Adapted from reference 13.
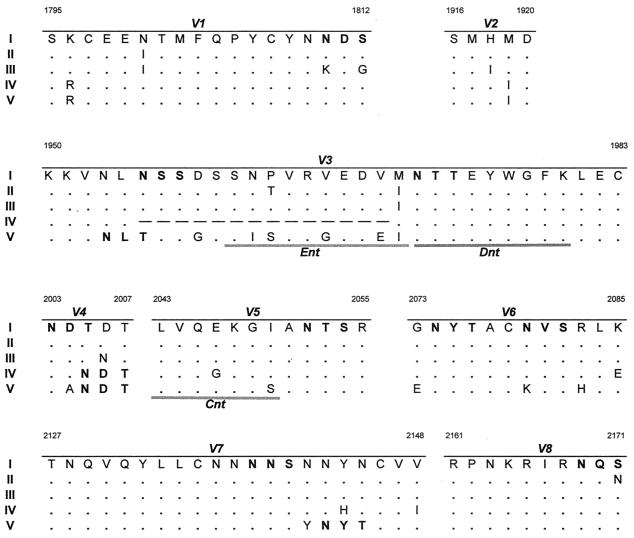
FIG. 2.
Comparison of deduced amino acid gp90 variable region sequences of the predominant quasispecies of EIAV isolates from pony 564 during sequential disease cycles. The region of the SU envelope gene was sequenced from EIAVPV viral stock and plasma viral RNA obtained during disease cycles I to V of EIAVPV-infected pony 564 (20). Based on the sequence data, the predominant quasispecies from each disease cycle were determined and variable regions V1 through V8 were compared. Only the amino acid residues different from EIAVPV are reported. Dots indicate residues identical to the EIAVPV sequence. Dashes indicate amino acid deletions, and boldface type indicates potential N-glycosylation sites (NXS/T). Shaded bars indicate the previously defined neutralizing epitopes ENT, DNT, and CNT (1). Adapted from reference 20.
Construction and titer determination of variant envelope quasispecies in the EIAVUK molecular clone.
To compare the neutralization properties of the variant envelopes, the gp90 segment of the representative predominant envelope clone associated with each of the first five disease cycles observed in pony 564 was substituted into the common reference EIAVUK proviral backbone (8) using the pLG338.30 vector (GenBank accession no. AF016316). The predominant viral envelope from the first febrile episode was the reference EIAVPV (20) and is designated the gp90I variant envelope provirus in this paper. The variant envelope proviral clones containing the predominant gp90 quasispecies from the second, third, and fourth disease cycles in pony 564 were generated by restriction enzyme fragment exchanges using unique NcoI and BstXI restriction sites common to these variant envelope clones. Thus, the respective NcoI-BstXI fragments (containing the V1 to V8 env regions) of clones p564II.21, p564III.35, and p564IV.9 (20) were used to replace the corresponding fragment of pEIAVUK to produce the respective variant envelope proviruses designated gp90II, gp90III, and gp90IV. The envelope variant V provirus was generated by digesting clone p564V.6 with HindIII and then ligating the digested fragment containing the V3 to V8 env segment into the corresponding segment of EIAVUK. The in vitro replication properties of each of the variant envelope proviral DNAs were then assessed by individually transfecting a 4-μg sample of purified DNA from each of the resulting env variant proviral clones into 105 fetal equine kidney (FEK) cells as specified by the manufacturer of the GenePorter Transfection kit (GTS, San Diego, Calif.). Virus production was monitored by weekly measurements of the reverse transcriptase activity in the supernatants of the transfected cells by using a standard micro-RT assay (20). The TCID50s of supernatants from transfected FEK cell cultures were then determined in a standardized infectious-center assay that uses a cell-based enzyme-linked immunosorbent assay detection system to study FEK cells (12).
Construction of chimeric envelopes between neutralization-sensitive and -resistant EIAV envelope variants.
Based on the observed in vitro neutralization phenotypes of the variant envelope proviruses, the gp90II and gp90V proviruses were chosen as reference neutralization-sensitive and -resistant envelopes, respectively. To elucidate the gp90 neutralization determinants, reciprocal chimeric envelopes exchanging defined variable domains were constructed as depicted in Fig. 4 and tested for their neutralization properties against the panel of reference immune serum from pony 564. To generate the desired mutations in the V3 and/or V4 regions, internal primers containing overlapping sequence mutations were used with external primers flanking the BlpI and BstXI restriction enzyme sites. The PCR amplification was performed by using the Expand High Fidelity PCR system (Roche, Indianapolis, Ind.), 0.025 mM each deoxynucleoside triphosphate, 0.1 μM each primer, and 1.5 μl of original template or purified PCR fragments in a final volume of 100 μl. The following conditions were used: 4 min at 95°C, after which 0.4 μl of Expand High Fidelity enzyme was added; 1 min at 95°C, 1.5 min at 50°C, and 1 min at 72°C for 35 cycles; and finally 10 min at 72°C for one cycle. The resulting 1.6-kb env fragments were digested with BlpI and BstXI prior to ligation with T4 ligase (New England Biolabs, Beverly, Mass.) into the vector containing the EIAVUK genome (GeneBank accession no. AF016316) with the corresponding 1.6-kb fragment removed. The ligation products were used to transform competent Escherichia coli DH5α cells (Invitrogen, Carsbad, Calif.). Clones from each of the new chimera constructs were screened by BamHI restriction enzyme digestion for the presence of the insert and sequenced as previously described (20) to confirm the correct envelope sequence. The correct clones for each of the desired variable region substitutions were transfected into FEK cells and monitored by micro-RT assays for virus production, as described above.
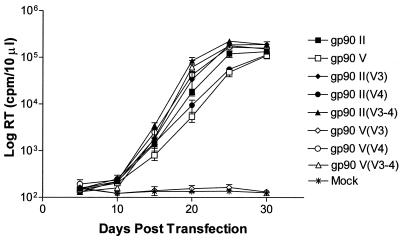
FIG. 4.
Schematic representation of the variable-region exchange chimeric envelope constructs. Neutralization-sensitive envelope from pony 564 febrile episode II (hatched bars) and neutralization-resistant envelope from pony 564 febrile episode V (solid bars) were used as reference envelope species for the variable-region exchanges. The names of the new variable-region exchange chimeric viruses are listed in italics at the left of each construct, starting with the name of the backbone followed by the exchanged variable region in parentheses. The gp90 envelope variable regions V1 to V8 are schematically identified above the bars.
Serum antibody neutralization assays.
The neutralization activity of the panel of reference immune serum from the experimentally infected ponies against the variant and chimeric envelope proviruses was determined as described previously by Hammond et al. (12) using a standard viral infectious-center assay. Briefly, 105 FEK cells were added to each well of a 24-well tissue culture plate and allowed to adhere overnight at 37°C. The desired serum samples were heat inactivated before use in the assay. Twofold serial dilutions of each of the serum samples were incubated in the presence of 100 IU of the selected chimeric virus at 37°C for 1 h. The serum-virus mixture was then added to the cells and again incubated overnight at 37°C. An overlay of 0.8% carboxmethylcellulose was added to the infected cultures, which were then incubated for a further 7 days at 37°C. The cells were then fixed and permeablized. Reference immune serum from an EIAV-infected horse (Lady) was used as a primary antibody, followed by an affinity-purified, horseradish peroxidase-conjugated goat anti-horse immunoglobulin G (Sigma, St. Louis, Mo.). The peroxidase substrate 3-amino-9-ethylcarbazole (Sigma) in a sodium acetate buffer (pH 5.5) supplemented with H2O 2 was used to visualize the EIAV infectious centers. The number of infectious centers was determined, and the 50% reciprocal neutralization titer of each serum sample was determined by linear-regression analysis. Each neutralization assay was repeated at least twice to determine standard error values. Neutralization titers were compared using a paired t-test analysis to determine statistical significance.
RESULTS
Clinical progression and viral envelope evolution in experimentally infected ponies.
To determine the effect of in vivo EIAV envelope variation on neutralization sensitivity in vitro, we utilized a unique panel of viral variants and immune serum samples collected from a group of four ponies experimentally infected with a reference pathogenic biological clone, EIAVPV. The clinical progression, viral evolution, and host immune response maturation in these ponies over a 36-month observation period have been described in detail previously (12, 13, 19, 20). Pony 564 was used as a source of EIAV envelope variants for the current study. The clinical and virologic profiles observed for pony 564 are summarized in Fig. 1. As described by Leroux et al. (20), analyses of the circulating EIAV population associated with each of the sequential waves of viremia and disease cycles observed in pony 564 revealed a distinct population of variant envelope quasispecies, apparently reflecting a dynamic but undefined host immune selection of EIAV envelope species. Thus, to examine the role of EIAV-specific neutralizing antibodies in envelope variation, we selected a representative predominant variant envelope from the various clones isolated during each of the first five disease cycles in pony 564. A comparative summary of the variable domains of the deduced variant envelope gp90 amino acid sequences for the panel of sequential virus envelope quasispecies is presented in Fig. 2. These sequence data indicate a unique envelope for each of the five reference envelope gp90 species, with each quasispecies having distinct signature V3 and V4 domains. For convenience, the individual variant envelope species are designated by the disease cycle from which they were isolated; e.g., gp90II is the representative predominant envelope quasispecies observed during the second disease cycle in pony 564.
Influence of sequential gp90 variation on serum antibody neutralization properties.
To determine the effects of the observed evolution of gp90 sequences on serum antibody neutralization properties, we inserted each of the five gp90 variants depicted in Fig. 2 into the env gene of a common reference provirus, EIAVUK, as described in Materials and Methods. The replication properties of each of the variant envelope proviruses were then examined by parallel transfections of FEK cells with each proviral DNA. All of the variant envelope proviral constructs were replication competent in FEK cells and appeared to replicate with similar kinetics (data not shown), despite the observed differences in gp90 sequences. These observations demonstrate the functional competence of the variant gp90 sequences in the context of the EIAVUK provirus and validate the use of these proviruses in comparative neutralization assays.
We next analyzed the neutralization properties of each of the variant envelope proviruses in our standard in vitro neutralization assay using FEK cell targets and selected longitudinal serum samples collected from the host pony 564 and the other three experimentally infected ponies. The results of these serum neutralization assays are summarized in Fig. 3. As observed previously (12, 13), neutralizing-antibody activity became detectable against the infecting EIAV gp90I between 2 and 3 months postinfection in most of the experimentally infected ponies and its level continued to increase during the next 10 months. Interestingly, serum neutralization titers against EIAV gp90I were sustained at somewhat higher levels in the two nonprogressor ponies that experienced only the single acute disease cycle than in the two progressor ponies that experienced multiple disease cycles. In general, steady-state serum neutralization titers averaged about 1:500 in nonprogressor ponies and about 1:200 in progressors over the 3-year observation period. These data demonstrate a sustained neutralizing-antibody response targeted to the infecting gp90I envelope contained in the EIAVPV inoculum, even though this envelope species is not detectable after the initial acute disease cycle (20).
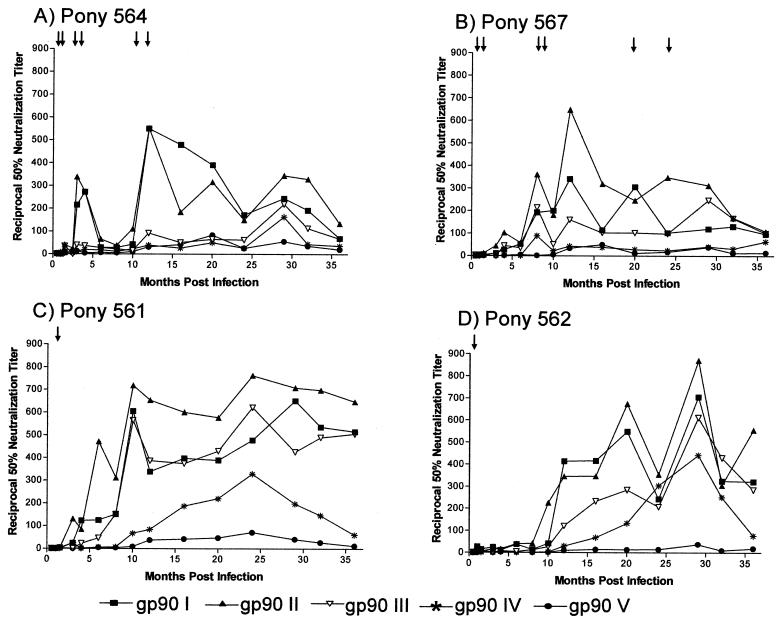
FIG. 3.
Serum neutralization properties of sequential EIAV envelope variants isolated from pony 564. The mean reciprocal 50% neutralizing-antibody titers in the sera of two progressor ponies, 564 (A) and 567 (B), and two nonprogressor ponies, 561 (C) and 562 (D), obtained at regular intervals, were determined against the panel of envelope variants in a common EIAVUK provirus by using an infectious-center assay as detailed in Materials and Methods. Arrows indicate disease cycles. Data presented are representative of two independent experiments run in duplicate.
We next determined the evolution of the neutralization properties of the sequential viral envelope gp90 variants from pony 564 in the context of the EIAVUK provirus, initially using longitudinal serum samples from the source pony 564 (Fig. 3A). The results of these neutralization assays with most of the pony 564 serum samples indicated that the gp90I and gp90II envelopes were substantially more sensitive to neutralization than were the envelope variants gp90III, gp90IV, and gp90V. This differential neutralization sensitivity is exemplified by the 12-month immune serum from pony 564, which displayed a neutralization titer of 1:600 against the gp90I and gp90II envelope proviruses but failed to yield detectable neutralization activity against the other three gp90 variant envelopes. The relative resistance of the gp90III, gp90IV, and gp90V envelope variants to neutralization by the serum antibody responses that evolved during persistent infection of pony 564 is highlighted by the fact that most of the immune serum samples from the 3-year observation period failed to display substantial neutralization titers against these envelope variants; transient serum neutralization was in general observed only with serum samples taken around 30 months postinfection, and this activity was not detectable by 36 months postinfection. Taken together, these data indicate that that the evolution of the EIAV envelope during persistent infection in pony 564 resulted in increased resistance to host serum-neutralizing antibodies.
We next sought to determine if the variant envelope neutralization sensitive and resistant phenotypes defined by the pony 564 immune serum samples would also be evident with longitudinal immune serum samples from the other three experimentally infected ponies. We have previously reported a similar development of broadly neutralizing antibodies in experimentally infected ponies, regardless of the number or severity of disease cycles (28). Figure 3B to D summarizes the neutralization properties of the envelope variants against heterologous longitudinal serum samples from the three other persistently infected ponies. As observed with the homologous pony 564 immune serum samples, the envelope variants display a spectrum of sensitivity to neutralization by the heterologous immune serum samples. In all three ponies, the gp90I and gp90II variants were neutralized by all serum samples taken after about 8 months postinfection, although the level of neutralization differed among the ponies, as expected for an outbred population and the independent EIAV variation observed in individual infected ponies. In marked contrast, the gp90V envelope variant was resistant to neutralization by all of the heterologous test serum samples, regardless of the time postinfection in each pony. These data indicated that the amino acid variations in the gp90V envelope from the parental gp90I envelope (Fig. 2) sequences impart a remarkable resistance to antibody neutralization. The gp90III and gp90IV envelope variants displayed neutralization phenotypes with the heterologous immune serum that were intermediate between the very sensitive gp90I and gp90II and the resistant gp90V envelopes. The gp90III envelope was efficiently neutralized by serum from pony 561 and had intermediate neutralization properties with serum from ponies 562 and 567, usually with neutralizing activities increasing with serum samples obtained later. The gp90IV envelope was somewhat more resistant to serum neutralization by serum from ponies 561 and 562 than was the gp90III envelope and was completely resistant to neutralization by any of the serum samples from pony 567. Thus, these cumulative serum neutralization data from the heterologous serum samples reveal an increasing neutralization sensitivity in the order of gp90II > gp90I > gp90III > gp90IV > gp90V. Conversely, the viral envelopes in general developed increasing neutralization resistance during the persistent infection.
Characterization of gp90 neutralization determinants.
To date, the envelope determinants of EIAV neutralization properties are undefined. The combination of in vivo-derived neutralization-sensitive and neutralization-resistant gp90 variants and immune serum from pony 564 provided a unique set of reagents to define the contribution of defined variable domains to the neutralization phenotype. For these studies, the gp90II variant was selected as a representative neutralization-sensitive envelope and the gp90V variant was selected as the neutralization-resistant envelope. Using these two envelope variants, we constructed a panel of chimeric envelopes that exchanged either V3 domains, V4 domains, or both V3 and V4 domains, as summarized in Fig. 4. Thus, insertion of the gp90V V3 or V4 domain into the gp90II envelope variant provirus were used to test the ability of these envelope domains to confer resistance to neutralization by the reference immune serum. In a reciprocal manner, insertion of the gp90II V3 or V4 domain into the gp90V envelope provirus were used to determine the ability of these variable segments to confer sensitivity to neutralization by the reference immune serum. The V3 domain was chosen for these exchanges, since it has been proposed as a principal neutralizing domain based on its extensive variation during persistent infection and its reactivity with two neutralizing mouse monoclonal antibodies (1). The V4 domain was selected for exchange to specifically examine the effects of variation observed between the gp90II and gp90V sequences that resulted in a shift in a putative N-linked glycosylation site (20). To determine the functional competence of the chimeric envelope proviruses depicted in Fig. 4, the replication properties of each chimeric provirus was assayed by transfection of FEK cells with proviral DNA (Fig. 5). The results of these assays demonstrated that all of the chimeric envelope proviruses replicated to similar levels to that of the parental envelope variants, except for the gp90V(V3) construct, which was replication defective. The observed replication properties of the various chimeric envelope proviruses in transfection experiments were consistent in infection assays of FEK cells used to determine the TCID50 of each viral stock (data not shown). Thus, with the exception of the gp90V(V3) chimeric envelope provirus, the similar replication properties of the various chimeric envelope proviruses confirmed their utility for the comparative serum antibody neutralization assays.
FIG. 5.
Replication kinetics of the variable-region exchange chimeric envelope proviruses. Equal amounts of DNA of each of the chimeric envelope constructs, depicted in Fig. 4, were individually transfected into FEK cells in duplicate. Supernatants from each transfected culture were collected at regular intervals over a 30-day period. The levels of reverse transcriptase (RT) present in the samples at each time point were measured in a microRT assay as described in Materials and Methods.
The neutralization properties of the two parental envelope variants and each chimeric envelope virus were determined using a longitudinal panel of immune serum samples taken from pony 564 at 3, 12, and 29 months postinfection to simulate each of the three phases of infection. Using our standard in vitro neutralization assay, the reciprocal 50% neutralization titer of each envelope construct was determined against each reference immune serum in FEK cells. The neutralization data are summarized in Fig. 6. As observed above, the gp90II envelope variant was consistently neutralization sensitive to the three reference serum samples (average titers of 1:340 to 1:600), while the gp90V envelope variant was consistently more neutralization resistant to the same serum treatments (average titers of 1:10 to 1:60). Substitution of the parental envelope with either of the neutralization-resistant gp90V variable domains V3 and V4 into the neutralization-sensitive gp90II envelope resulted in a marked reproducible decrease in the serum neutralization titer compared to the parental gp90II envelope variant for each reference immune serum. For example, the insertion of the V3 domain of gp90V into the gp90II envelope [gp90II(V3) in Fig. 6] decreased the average neutralization titer observed with the 12-month serum from 1:600 for the gp90II envelope to only 1:125 for the chimeric envelope. Similarly, insertion of the V4 domain of gp90V into the gp90II envelope [gp90II(V4) in Fig. 6] decreased the average neutralization titer with the 12-month serum from 1:600 to 1:75. For reference, it should be noted that the neutralization titer of the 12-month serum against the gp90V envelope variant was about 1:50. Interestingly, insertion of both the V3 and V4 domains of gp90V into the gp90II envelope [gp90II(V3-4) in Fig. 6] decreased the sensitivity to serum neutralization only slightly more than the single-domain substitutions, more closely resembling the gp90V envelope variant resistance levels. For example, the 12-month serum average neutralization titer was 1:25 against the gp90II(V3-4) chimera, compared to the 1:50 average titer observed against the gp90V envelope variant. Thus, these data demonstrated that the gp90 V3 and gp90 V4 domains individually were able to confer a level of serum antibody neutralization resistance similar to that observed with the gp90V envelope.
FIG. 6.
Serum neutralization properties of variable-region chimeric envelope proviral constructs. Three serum samples from pony 564 were selected to test the effect of exchanging specific variable regions on in vitro neutralization phenotypes as described in Materials and Methods. The 50% neutralizing-antibody titers were determined for 3, 12, and 29 months postinfection for each of the replication-competent variable-region exchange chimeric viruses. Each experiment was run in duplicate and repeated twice. Asterisks indicate the neutralizing antibody titers that are significantly different (P ≤ 0.05) from the parental backbone at that specific time point.
In a reciprocal set of experiments, we tested the ability of neutralization-sensitive gp90II domain substitutions into the neutralization-resistant gp90V envelope to confer sensitivity to neutralization by the panel of reference immune serum. As shown in Fig. 6, insertion of the V4 domain of gp90II into the gp90V envelope [gp90V(V4) construct] failed to increase serum neutralization sensitivity compared to that for the gp90V envelope variant. Because of the replication-defective nature of the gp90V(V3) chimeric envelope, it was not possible to assess the effects of a V3 substitution on gp90V envelope neutralization sensitivity. However, analysis of the gp90V(V3-4) chimeric envelope (Fig. 6), in which both the V3 and V4 domains of gp90II were inserted into the gp90V envelope, indicated an increase in serum neutralization sensitivity, as reflected by the observed increase in the average serum neutralization titer of the chimeric envelope compared to the parental gp90V envelope. For example, the 12-month serum neutralization titer for the gp90V(V3-4) chimeric envelope was 1:130, compared to the 1:50 neutralization titer observed for the parental gp90V envelope. However, it is important to note that even the double-domain substitution did not increase serum neutralization sensitivity to the levels observed for the gp90II envelope, for which the 12-month serum displayed a 1:600 average neutralization titer. Thus, these data indicate an unexpected lack of reciprocity in the influence of the gp90 V3 and V4 domains on serum antibody neutralization sensitivity and suggest that envelope sequences outside of these two domains also contribute to the overall envelope neutralization phenotypes.
DISCUSSION
The recurrent nature of EIAV disease cycles coupled with the development of distinct quasispecies provides a unique system to study the dynamic interactions between evolving lentivirus populations and neutralizing-antibody responses. In this study, we investigated how the in vivo evolution of novel envelope quasispecies, specifically within the gp90 variable regions V3 and V4, affect neutralization specificity. The results of these studies provide new insights into the dynamic interplay between envelope evolution during persistent infection and the viral neutralization phenotype and the nature of the envelope determinants of antibody neutralization resistance and sensitivity.
The evolution of novel envelope quasispecies during sequential disease cycles in experimentally infected horses has been well documented (20, 35). During persistent EIAV infection, amino acid variations accumulate predominantly within the antigenically dominant gp90 variable regions, and these variations have been associated with the distinct neutralization phenotypes associated with EIAV isolates. It has been proposed that the modifications seen within the envelope variable regions contribute to viral persistence, in particular by temporarily evading established neutralizing-antibody responses. However, the direct influence of the defined clonal in vivo variation within the gp90 region on in vitro neutralization specificity remains largely undefined. The present study for the first time reveals that the evolution of envelope observed during five sequential disease cycles in an experimentally infected pony over a 12-month period markedly increased the overall neutralization resistance of the gp90 proteins to both homologous and heterologous immune serum. The high level of neutralization resistance observed with the gp90V envelope is a unique observation and suggests that while changes in neutralization specificity may contribute to persistence and the recurring disease cycles during chronic disease, the evolution of predominantly neutralization-resistant viral envelopes may be a major factor in maintaining a long-term persistence in inapparent carriers in the face of robust and broadly neutralizing host serum antibodies. It is important that this model be tested in more detail by characterizing the neutralization phenotypes of other serial EIAV isolates obtained from experimental infections and to extend this type of study to other lentivirus systems.
Based on structural and antigenic similarities to the V3 domain of human immunodeficiency virus type 1IIIB (HIV-1 IIIB) and reactivity, we have previously defined the V3 segment of EIAV gp90 as the PND of the virus. The PND designation was supported further by studies that localized two linear determinants targeted by two highly type-specific neutralizing mouse monoclonal antibodies within the V3 domain (1). However, subsequent studies from our laboratory comparing neutralization determinants of laboratory strains of EIAV indicated the importance of gp90 sequences outside of the PND in defining overall neutralization properties (7). The present studies now extend these observations and conclusively reveal that both the V3 and V4 domains can equally confer antibody neutralization resistance when inserted into a neutralization-sensitive envelope. In fact, the V4 domain substitution actually conferred a greater level of neutralization resistance than did the parallel V3 domain substitution. The ability of the two independent domain substitutions to confer neutralization resistance indicates that gp90 sequences outside the V3 domain can in fact serve as major determinants of envelope neutralization sensitivity. These observations suggest that gp90 neutralization resistance may be determined by a cooperative interaction between the V3 and V4 domains. The proposed interaction of the EIAV gp90 V3 and V4 domains is further supported by the observation that the gp90V(V3) chimera was replication defective, thus indicating a required compatibility between the V3 and V4 domains of gp90. These observations with EIAV are similar to studies with simian immunodeficiency virus and HIV-1, indicating required compatibility between distinct variable domains, suggesting functional interactions (5).
The mechanism(s) by which the V3 and V4 domains individually confer equal levels of neutralization resistance to an otherwise neutralization sensitive envelope is undefined. Our current working model is that the V3 domain is indeed the predominant target for neutralizing serum antibodies but that V3 domain accessibility is determined by a conformational interaction with the V4 domain of gp90. In this regard, it is interesting that the amino acid differences between the respective V4 domains of the neutralization-sensitive (gp90II) and -resistant (gp90V) gp90 envelopes alter the location of single glycosylation site in the V4 domain. Thus, it is conceivable that the location of this single V4 glycosylation site affects the accessibility of V3 neutralization epitopes to serum antibodies. Modification of glycosylation sites by deletion, addition, or relocation is a common result of envelope variations observed in various lentiviruses, including EIAV. The current observations lend support to the concept that the characteristic variations in envelope glycosylation patterns influence neutralization properties by shielding sensitive neutralization epitopes from serum antibodies.
As with the V4 domain, the mechanism by which the V3 domain of the neutralization-resistant gp90V confers resistance to the sensitive gp90II envelope is uncertain. The respective V3 domains differ in amino acid sequences within the previously defined neutralizing epitope Ent and in the position of the upstream potential N-linked glycosylation site. It is conceivable that either or both of these modifications could contribute to the neutralization resistance, even in an envelope containing a sensitive V4 domain.
The present studies defined the V3 and V4 domains as determinants of neutralization resistance, but the reciprocal studies of determinants of neutralization sensitivity were not as clearly defined. An assessment of the ability of the sensitive gp90II V3 domain to confer neutralization sensitivity to the resistant gp90V envelope was not feasible because of the replication-defective nature of the gp90V(V3) chimera. However, the parallel single insertion of the sensitive gp90 envelope V4 into the resistant gp90V envelope failed to increase the sensitivity of the envelope to serum neutralization. The double V3 and V4 insertion into the gp90V envelope did confer partial serum neutralization sensitivity, but only at a level that was about one-fourth of that of the parental gp90II envelope. These observations once again indicate that the resistance is a dominant phenotype, in that the resistant gp90V V3 or V4 domain was the dominant determinant in the presence of sensitive domains from gp90II. The fact that the double V3 and V4 substitution only partially conferred neutralization sensitivity further suggests that gp90 envelope domains outside of these V3 and V4 domains are also contributing to envelope resistance. In this regard, it should be noted that additional sequence variations exist between the neutralization-sensitive gp90II and neutralization-resistant gp90V envelopes. These include one to three amino acid differences in the V2, V5, V6, V7, and V8 domains, some of which alter the respective glycosylation patterns in the respective domains (Fig. 2). Thus, it is likely that one or more of these additional envelope variations contribute to neutralization resistance, reflecting a complex of neutralization determinants at distant but conformationally interactive sites.
A number of studies of EIAV envelope variation have demonstrated a distinct envelope quasispecies population associated with sequential disease cycles in experimentally infected equids (20, 35). The dynamic and diverse nature of EIAV envelope variation in vivo strongly suggests a highly active immune selection, including neutralizing antibodies. The present studies extend our understanding of at least one role of EIAV envelope variation in persistence in that the envelope evolution is driven toward neutralization resistance that can be optimized by multiple, synergistic envelope determinants via changes in neutralization epitopes and in domains that can sequester these sensitive sites. Based on this hypothesis, it may be implied that host neutralizing antibodies can play a dominant role in immune control of EIAV replication during early stages of infection (chronic EIA) while viral envelopes are neutralization sensitive, but that the sustained immune suppression of neutralization-resistant envelope variants present in long-term inapparent carriers is probably due to cellular immune responses. The primary role of the broadly neutralizing antibodies that are associated with mature immunity in EIAV-infected horses is then to protect against natural EIAV exposure by horsefly bites and to eliminate any neutralization-sensitive viral quasispecies that may arise in the tissue reservoirs of infection. In this way, humoral and cellular immune responses act in a timely synergistic manner to gain and then sustain control of EIAV infection and subsequent exposure, a lesson from nature relevant to vaccine development.
The observed evolution in EIAV toward a neutralization-resistant phenotype appears to support the concept that envelope variations observed during persistent lentivirus infection result from immune selections that increase antibody neutralization resistance, providing an important mechanism of persistence in the face of competent host immunity. In this regard, Burns et al. first reported the development of broadly neutralization-resistant envelope variants in monkeys experimentally infected with the reference molecular clone SIVmac239 (3). This seminal paper has been followed by several other published studies demonstrating a temporal relationship between envelope sequence changes and increases in neutralization resistance for viruses that evolve in monkeys during the course of persistent SIV infection (3, 4, 27). Similarly, the evolution from neutralization-sensitive to -resistant envelope phenotypes has been documented in persistent infections of monkeys with SHIV constructs containing the HIV-1 envelope (6, 24, 30, 33). Experiments on immune selection and changes in neutralization phenotypes in HIV-1-infected patients are generally complicated by the lack of knowledge of the infecting viral strain and time of infection and by the low levels of in vitro serum antibody neutralization characteristic of primary HIV-1 neutralization. However, a recent study did characterize the neutralization phenotypes of two HIV-1IIIB variants that were isolated from a laboratory worker accidentally infected with the neutralization-sensitive HIV-1IIIB strain (2). Compared to the original virus in the viral inoculum, the viral isolates displayed an increased resistance to neutralization over time. The emergence of neutralization-resistant virus preceded disease development in this laboratory worker, perhaps indicating the role of neutralizing antibodies in humoral immune control. Thus, these limited observations indicate the need for more detailed studies of the evolution of animal and human lentivirus neutralization properties during persistent infection to provide a more definitive characterization of functional outcome of the dynamic interaction between evolving lentivirus envelopes and host immune response.
Acknowledgments
This research was supported by grant RO1 AI25850 from the National Institutes of Health (R.C.M.) and by funds from the Lucille P. Markey Charitable Trust (C.J.I.) and the Kentucky Agricultural Experiment Station (C.J.I.).
We acknowledge Brian McKeon for excellent technical assistance in these studies, and we thank Kelly Stefano Cole and Frank Jenkins for a critical reading of the manuscript and for helpful suggestions.
REFERENCES
- 1.Ball, J. M., K. E. Rushlow, C. J. Issel, and R. C. Montelaro. 1992. Detailed mapping of the antigenicity of the surface unit glycoprotein of equine infectious anemia virus by using synthetic peptide strategies. J. Virol. 66:732-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaumont, T., A. van Nuenen, S. Broersen, W. A. Blattner, V. V. Lukashov, and H. Schuitemaker. 2001. Reversal of human immunodeficiency virus type 1 IIIB to a neutralization-resistant phenotype in an accidentally infected laboratory worker with a progressive clinical course. J. Virol. 75:2246-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns, D. P., C. Collignon, and R. C. Desrosiers. 1993. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J. Virol. 67:4104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chackerian, B., L. M. Rudensey, and J. Overbaugh. 1997. Specific N-linked and O-linked glycosylation modifications in the envelope VI domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 71:7719-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, M., C. Shi, V. Kalia, S. B. Tencza, R. C. Montelaro, and P. Gupta. 2001. HIV gp120 V(1)/V(2) and C(2)-V(3) domains glycoprotein compatibility is required for viral replication. Virus Res. 79:91-101. [DOI] [PubMed] [Google Scholar]
- 6.Cheng-Mayer, C., A. Brown, J. Harouse, P. A. Luciw, and A. J. Mayer. 1999. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J. Virol. 73:5294-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook, R. F., S. L. Berger, K. E. Rushlow, J. M. McManus, S. J. Cook, S. Harrold, M. L. Raabe, R. C. Montelaro, and C. J. Issel. 1995. Enhanced sensitivity to neutralizing antibodies in a variant of equine infectious anemia virus is linked to amino acid substitutions in the surface unit envelope glycoprotein. J. Virol. 69:1493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook, R. F., C. Leroux, S. J. Cook, S. L. Berger, D. L. Lichtenstein, N. N. Ghabrial, R. C. Montelaro, and C. J. Issel. 1998. Development and characterization of an in vivo pathogenic molecular clone of equine infectious anemia virus. J. Virol. 72:1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craigo, J. K., C. Leroux, L. Howe, J. D. Steckbeck, S. J. Cook, C. J. Issel, and R. C. Montelaro. 2002. Transient immune-suppression of inapparent-carriers infected with a principal neutralizing domain-deficient EIAV induces neutralizing antibodies and lowers steady-state viral replication. J. Gen. Virol. 83:1353-1359. [DOI] [PubMed] [Google Scholar]
- 10.Fujimiya, Y., L. E. Perryman, and T. B. Crawford. 1979. Leukocyte cytotoxicity in a persistent virus infection: presence of direct cytotoxicity but absence of antibody-dependent cellular cytotoxicity in horses infected with equine infectious anemia virus. Infect. Immun. 24:628-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerencer, M., I. Valpotic, B. Jukic, M. Tomaskovic, and I. Basic. 1989. Qualitative analyses of cellular immune functions in equine infectious anemia show homology with AIDS. Arch. Virol. 104:249-257. [DOI] [PubMed] [Google Scholar]
- 12.Hammond, S. A., S. J. Cook, D. L. Lichtenstein, C. J. Issel, and R. C. Montelaro. 1997. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J. Virol. 71:3840-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond, S. A., F. Li, B. M. McKeon, Sr., S. J. Cook, C. J. Issel, and R. C. Montelaro. 2000. Immune responses and viral replication in long-term inapparent carrier ponies inoculated with equine infectious anemia virus. J. Virol. 74:5968-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrold, S. M., S. J. Cook, R. F. Cook, K. E. Rushlow, C. J. Issel, and R. C. Montelaro. 2000. Tissue sites of persistent infection and active replication of equine infectious anemia virus during acute disease and asymptomatic infection in experimentally infected equids. J. Virol. 74:3112-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussain, K. A., C. J. Issel, K. L. Schnorr, P. M. Rwambo, and R. C. Montelaro. 1987. Antigenic analysis of equine infectious anemia virus (EIAV) variants by using monoclonal antibodies: epitopes of glycoprotein gp90 of EIAV stimulate neutralizing antibodies. J. Virol. 61:2956-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issel, C. J., W. V. Adams, Jr., L. Meek, and R. Ochoa. 1982. Transmission of equine infectious anemia virus from horses without clinical signs of disease. J. Am. Vet. Med. Assoc. 180:272-275. [PubMed] [Google Scholar]
- 17.Issel, C. J., and L. D. Foil. 1984. Studies on equine infectious anemia virus transmission by insects. J. Am. Vet. Med. Assoc. 184:293-297. [PubMed] [Google Scholar]
- 18.Kono, Y., K. Hirasawa, Y. Fukunaga, and T. Taniguchi. 1976. Recrudescence of equine infectious anemia by treatment with immunosuppressive drugs. Natl. Inst. Anim. Health Q. (Tokyo) 16:8-15. [PubMed] [Google Scholar]
- 19.Leroux, C., J. K. Craigo, C. J. Issel, and R. C. Montelaro. 2001. Equine infectious anemia virus genomic evolution in progressor and nonprogressor ponies. J. Virol. 75:4570-4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leroux, C., C. J. Issel, and R. C. Montelaro. 1997. Novel and dynamic evolution of equine infectious anemia virus genomic quasispecies associated with sequential disease cycles in an experimentally infected pony. J. Virol. 71:9627-9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtenstein, D. L., C. J. Issel, and R. C. Montelaro. 1996. Genomic quasispecies associated with the initiation of infection and disease in ponies experimentally infected with equine infectious anemia virus. J. Virol. 70:3346-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuire, T. C., D. B. Tumas, K. M. Byrne, M. T. Hines, S. R. Leib, A. L. Brassfield, K. I. O'Rourke, and L. E. Perryman. 1994. Major histocompatibility complex-restricted CD8+ cytotoxic T lymphocytes from horses with equine infectious anemia virus recognize Env and Gag/PR proteins. J. Virol. 68:1459-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montelaro, R., J. M. Ball, and K. Rushlow. 1993. Equine retroviruses, p. 257-360. In J. A. Levy (ed.), The Retroviridae. Plenum Press, New York, N.Y.
- 24.Narayan, S. V., S. Mukherjee, F. Jia, Z. Li, C. Wang, L. Foresman, C. McCormick-Davis, E. B. Stephens, S. V. Joag, and O. Narayan. 1999. Characterization of a neutralization-escape variant of SHIVKU-1, a virus that causes acquired immune deficiency syndrome in pig-tailed macaques. Virology 256:54-63. [DOI] [PubMed] [Google Scholar]
- 25.Payne, S. L., F. D. Fang, C. P. Liu, B. R. Dhruva, P. Rwambo, C. J. Issel, and R. C. Montelaro. 1987. Antigenic variation and lentivirus persistence: variations in envelope gene sequences during EIAV infection resemble changes reported for sequential isolates of HIV. Virology 161:321-331. [DOI] [PubMed] [Google Scholar]
- 26.Perryman, L. E., K. I. O'Rourke, and T. C. McGuire. 1988. Immune responses are required to terminate viremia in equine infectious anemia lentivirus infection. J. Virol. 62:3073-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudensey, L. M., J. T. Kimata, E. M. Long, B. Chackerian, and J. Overbaugh. 1998. Changes in the extracellular envelope glycoprotein of variants that evolve during the course of simian immunodeficiency virus SIVMne infection affect neutralizing antibody recognition, syncytium formation, and macrophage tropism but not replication, cytopathicity, or CCR-5 coreceptor recognition. J. Virol. 72:209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rwambo, P. M., C. J. Issel, W. V. Adams, Jr., K. A. Hussain, M. Miller, and R. C. Montelaro. 1990. Equine infectious anemia virus (EIAV) humoral responses of recipient ponies and antigenic variation during persistent infection. Arch. Virol. 111:199-212. [DOI] [PubMed] [Google Scholar]
- 29.Rwambo, P. M., C. J. Issel, K. A. Hussain, and R. C. Montelaro. 1990. In vitro isolation of a neutralization escape mutant of equine infectious anemia virus (EIAV). Arch. Virol. 111:275-280. [DOI] [PubMed] [Google Scholar]
- 30.Si, Z., M. Cayabyab, and J. Sodroski. 2001. Envelope glycoprotein determinants of neutralization resistance in a simian-human immunodeficiency virus (SHIV-HXBc2P 3.2) derived by passage in monkeys. J. Virol. 75:4208-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tschetter, J. R., K. M. Byrne, L. E. Perryman, and T. C. McGuire. 1997. Control of equine infectious anemia virus is not dependent on ADCC mediating antibodies. Virology 230:275-280. [DOI] [PubMed] [Google Scholar]
- 32.Tumas, D. B., M. T. Hines, L. E. Perryman, W. C. Davis, and T. C. McGuire. 1994. Corticosteroid immunosuppression and monoclonal antibody-mediated CD5+ T lymphocyte depletion in normal and equine infectious anaemia virus-carrier horses. J. Gen. Virol. 75:959-968. [DOI] [PubMed] [Google Scholar]
- 33.Ye, Y., Z. H. Si, J. P. Moore, and J. Sodroski. 2000. Association of structural changes in the V2 and V3 loops of the gp120 envelope glycoprotein with acquisition of neutralization resistance in a simian-human immunodeficiency virus passaged in vivo. J. Virol. 74:11955-11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, W., S. M. Lonning, and T. C. McGuire. 1998. Gag protein epitopes recognized by ELA-A-restricted cytotoxic T lymphocytes from horses with long-term equine infectious anemia virus infection. J. Virol. 72:9612-9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng, Y. H., T. Nakaya, H. Sentsui, M. Kameoka, M. Kishi, K. Hagiwara, H. Takahashi, Y. Kono, and K. Ikuta. 1997. Insertions, duplications and substitutions in restricted gp90 regions of equine infectious anaemia virus during febrile episodes in an experimentally infected horse. J. Gen. Virol. 78:807-820. [DOI] [PubMed] [Google Scholar]
- 36.Zheng, Y. H., H. Sentsui, T. Nakaya, Y. Kono, and K. Ikuta. 1997. In vivo dynamics of equine infectious anemia viruses emerging during febrile episodes: insertions/duplications at the principal neutralizing domain. J. Virol. 71:5031-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]