Abstract
Three defense functions of immunoglobulin A (IgA), immune exclusion, intracellular neutralization, and virus excretion, were assessed in a measles virus model using polarized epithelial cells expressing the polymeric immunoglobulin receptor and monoclonal antibodies against the viral H and F envelope proteins and the internal N protein. Anti-H IgA was the most effective antibody at preventing infection via the apical surface, i.e., immune exclusion. This IgA was also the most effective at intraepithelial cell neutralization after infection at the apical surface and endocytosis of IgA at the basolateral surface, although an antibody against the internal N protein was also effective. In the intracellular neutralization experiments, confocal immunofluorescence microscopy showed prominent colocalization of anti-H IgA and H protein inside virus-infected cells, whereas colocalization of anti-F and F protein and of anti-N and N protein was much less, in agreement with the neutralization results. Combinations of IgA anti-H, anti-F, and anti-N showed no synergistic effects in intracellular neutralization. In the immune excretion experiments, virus immune complexes with either anti-H or anti-F IgA placed beneath polarized epithelial cells could be transported to the apical supernatant. Anti-F IgA, which was relatively poor at immune exclusion and intracellular neutralization, was the most robust at virus excretion. Thus, the studies collectively demonstrated three different antiviral functions of IgA in relation to epithelium and also suggested that the particular viral component with which a given IgA antibody reacts is an important determinant of the magnitude of the antiviral effect.
The mucosal immune system provides the initial immunological barrier against most pathogens (8, 15). In particular, immunoglobulin A (IgA), the predominant mucosal antibody, is thought to mediate defense functions at different anatomic levels in relation to mucosal epithelium (15, 19). For example, after secretion, IgA can bind to microbes and prevent them from attaching to or penetrating the epithelial lining. Exactly how this is accomplished in the case of viruses is not fully understood. Studies on influenza virus suggest that the mechanism of neutralization by IgA may vary according to the number of antibody molecules per virion (1). In addition to the immune exclusion function displayed by secreted antibody, IgA has two other potential modes and sites of action in mucous membranes (14, 18). First, during transport through the lining epithelial cells after polymeric immunoglobulin receptor (pIgR)-mediated endocytosis, IgA is thought to be able to interact with intracellular pathogens such as viruses, blocking replication, assembly, and/or budding. Such intra-epithelial cell neutralization has been demonstrated by IgA monoclonal antibodies (MAbs) against Sendai virus, influenza virus, and rotavirus (7, 17, 18). In mice, an IgA MAb against a rotavirus internal protein was able to prevent infection and cure persistent infection (7). Second, IgA in the lamina propria beneath mucosal epithelium may form a complex with antigens and transport them, via the pIgR, across the epithelial cells and into the secretions (14, 26). With respect to viruses, Epstein-Barr virus-IgA immune complexes were transcytosed in this manner across polarized epithelial cells from the basolateral to the apical surface (9). In a variant of this excretory immune function, when IgA antibodies met free human immunodeficiency virus (HIV) within epithelial cells, the antibodies blocked their apical- to basal-surface transcytosis and transported the viral particles to the apical supernatant (4).
The present study was designed to demonstrate and investigate the three proposed host defense functions of IgA in relation to viruses and epithelium within a single model system. For several reasons, measles virus was chosen. First, measles is historically a major pathogen and continues to be a significant killer of children in the Third World (22). Second, during infection, the virus replicates initially in the epithelial cells lining the oropharynx and upper respiratory tract. The virus spreads to the regional lymph nodes and blood, and then to a number of other sites, including the skin, the kidney, the lower respiratory, intestinal, and genital tracts, the liver, and sometimes the brain. Third, a group of well-characterized IgG MAbs against multiple measles virus proteins, both envelope and internal, is available (28). This enables generation and comparison of pairs of IgG and IgA switch variant derivatives recognizing the same viral epitope. Fourth, measles virus enters polarized Vero C1008 epithelial cells at the apical surface, where the natural receptor for measles virus, CD46, is expressed preferentially (2). In contrast, the receptor for IgA, the pIgR, is expressed on the basolateral surface. This provides an advantageous situation for studies of (i) immune exclusion, because virus-IgA antibody complexes will be taken up only via the virus receptor, (ii) intracellular neutralization, because infection and endocytosis of IgA can occur at opposite poles of the cell, and (iii) antibody-mediated virus excretion, because virus-IgA antibody complexes placed below the cell monolayer will be endocytosed almost exclusively via the pIgR.
MATERIALS AND METHODS
Cell culture and viruses.
Madin-Darby canine kidney cells (MDCK) were stably transfected with cDNA encoding human pIgR by Tamer et al. (29). This receptor transports oligomeric but not monomeric IgA. African green monkey kidney cells, Vero C1008 (ATCC CRL 1587), were obtained from the American Type Culture Collection (Manassas, Va.); these cells were also transfected to express human pIgR (12), and stably expressing cells were selected. Cells were cultured in Eagle's minimal essential medium (Gibco BRL, Gaithersburg, Md.) supplemented with antibiotics and 10% fetal bovine serum at 37°C under a 5% CO2 atmosphere. Cells were cultured on tissue culture-treated 0.4-μm-pore-size Transwell polyester membranes (Costar Corp, Cambridge, Mass.). Polarization of cell monolayers was tested by monitoring electrical resistance between the upper and lower chambers with a Millicell resistance system (Millipore Corp, Bedford, Mass.). Polarized Vero C1008 and MDCK cells had resistances of 50 to 75 Ω per cm2 and 100 to 200 Ω per cm2, respectively. The Edmonston strain of measles virus obtained from the American Type Culture Collection was propagated in Vero C1008 cells, and the virus titer was determined by a plaque assay in Vero C1008 cells.
Production and purification of IgA MAbs.
Hybridomas secreting the anti-measles virus IgG antibodies 16CD11-G (anti-H), 16DC9-G (anti-F), and 16CF7-G (anti-N) were generated in the laboratory of Erling Norrby (28). H and F are envelope proteins, and N is the major internal virus protein. IgA MAbs were obtained after repetitive cycles of limiting dilution and selection of IgG-producing hybridoma cells and isolation of naturally occurring isotype switch variants (5). MAbs were produced by culturing hybridomas in serum-free medium in roller bottles. IgG antibodies were purified from serum-free culture medium on a recombinant protein G-agarose column (Gibco BRL) according to the manufacturer's protocol. IgA antibodies were purified on a Kaptiv-AE column (Tecnogen, Piana di Monte Verna, Italy) (23). Concentrations of purified antibodies were measured by optical density at 280 nm with an ɛ of 1.34 for IgA (1 mg/ml) and 1.50 for IgG and by an enzyme-linked immunosorbent assay (ELISA) with isotype-matched standards of known concentrations. Antibody purity was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. An IgA MAb against HIV gp41 was used as an irrelevant control.
Transport of IgA through polarized cell monolayers.
Transcytosis of antibodies from the basolateral to the apical surfaces of epithelial cells was assessed by addition of 10 μg of purified IgA or IgG in 100 μl of medium to the chamber below polarized Vero C1008 cell monolayers expressing pIgR and incubation at 37°C. Apical supernatants were collected at intervals and analyzed by ELISA for Ig content.
Traditional virus neutralization.
Measles virus at about 100 PFU in 150 μl was mixed with 150 μl of a MAb (anti-H IgA, anti-H IgG, anti-F IgA, anti-F IgG, anti-N IgA, or anti-N IgG) containing 18, 9, or 4.5 μg or medium alone (control) and was incubated at room temperature for 2 h. The virus-antibody mixture was used to infect unpolarized Vero C1008 cell monolayers, and virus titers were analyzed by a plaque assay.
Blocking infection of polarized epithelial cells at the apical surface.
MAbs (100 μl at 400 μg/ml) were added at the apical surfaces of polarized pIgR+ Vero C1008 cells grown on polyester membranes for 30 min at 37°C, followed by measles virus in 100 μl at a multiplicity of infection (MOI) of 0.1. After 2 h at 37°C, the inoculum was removed and the monolayer was washed with medium three times. Fresh medium was added to both apical and basolateral chambers, and the cells were incubated at 37°C for another 26 h. Apical supernatants and cell lysates were collected, and virus titers were assessed by plaque assay. Cell lysates were prepared by scraping the cells in medium, freeze-thawing three times, and centrifuging for 10 min at 2,300 × g to remove cellular debris.
Intracellular colocalization of IgA antibody and measles virus protein.
Polarized pIgR+ Vero C1008 cell monolayers grown on polyester membranes were either infected with measles virus at an MOI of 1 or mock infected via the apical surface for 2 h at 37°C. IgA MAbs specific for H, F, or N protein or an irrelevant IgA MAb was added to the basal surface. After 30 h, the membrane-attached cells were fixed without disruption in 2% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4), permeabilized with 0.1% Triton X-100 in PBS, and washed with PBS containing 1% bovine serum albumin. Two-color immunofluorescence (in the Case Western Reserve University/Ireland Comprehensive Cancer Center confocal microscopy facility) was used to detect H, F, or N protein and IgA simultaneously. IgA was localized with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgA (Southern Biotechnology Inc., Birmingham, Ala.). H, F, or N protein was localized with mouse IgG MAbs (recognizing different epitopes from the IgA antibody that had been added to the basolateral chamber) followed by rhodamine-goat anti-mouse IgG (Pierce, Rockford, Ill.). A Zeiss model 510 laser scanning confocal microscope with a 63× (numerical aperture, 1.4) Planapochromat oil immersion objective lens (Zeiss, Thornwood, N.Y.) was used for all experiments. Confocal images of FITC fluorescence were collected with a 488-nm excitation light from an argon laser, a 488-nm dichroic mirror, and a 500- to 550-nm band-pass barrier filter. Images of rhodamine fluorescence were collected with 543-nm excitation from an He/Ne laser, a 543-nm dichroic mirror, and a 560-nm long pass filter.
Intracellular neutralization.
Polarized pIgR+ Vero C1008 cell monolayers on polyester membranes were infected with measles virus at an MOI of 1 via the apical surface for 2 h at 37°C. Unadsorbed virus was removed by six washes followed by addition of fresh medium. A MAb (10 μg in 100 μl) or medium alone (control) was added to the basolateral chamber. After 4 h at 37°C, residual antibody was removed by washing the basal surface three times. Fresh medium was added, and cell monolayers were incubated for another 24 h at 37°C in a 5% CO2 incubator. Virus titers were determined by plaque assay of both apical supernatants and cell lysates.
Excretion of virus-antibody complexes through polarized epithelial cells.
Measles virus was mixed either with 15 μg of an IgA or IgG MAb or with medium alone (total volume, 150 μl) and incubated at 4°C for 6 h to allow formation of immune complexes, which were then added to the basolateral chamber below polarized, pIgR-expressing MDCK cells. The cells were incubated at 37°C, and samples of apical medium were collected at different times. RNA was extracted immediately from 140-μl aliquots (QIAamp viral RNA mini kit; Qiagen, Valencia, Calif.) and analyzed by reverse transcription-PCR (RT-PCR) with specific primers for the N gene (24) and gel electrophoresis (visualized by ethidium bromide staining).
Statistical analysis.
Statistical analysis was performed with a two-tailed t test by using the computer-fitting program Prism (GraphPAD Software Inc., San Diego, Calif.). The 5% confidence limit was adopted as the criterion for statistical significance.
RESULTS
MAbs.
By limiting dilution and spontanous isotype switching of IgG hybridomas, we obtained three pairs, i.e., sharing V regions, of IgA- and IgG-secreting hybridomas (Table 1). The purity of all the MAbs was greater than 90% as analyzed by reducing and nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
TABLE 1.
Measles virus MAbsa
| Hybridoma | Antigen specificity | Isotype | % Oligomeric IgA |
|---|---|---|---|
| 16CD11-G | H | IgG1 | 0 |
| 16DC9-G | F | IgG1 | 0 |
| 16CF7-G | N | IgG1, IgG2a | 0 |
| 16CD11-A | H | IgA | 73 |
| 16DC9-A | F | IgA | 62 |
| 16CF7-A | N | IgA | 70 |
The hybridomas were established by Sheshberadaran et al. (28). The specificities originally determined were confirmed in the present work by Western blotting after the switch to IgA. Antigens H and F are on the surface of the virus, and N is internal. IgA and IgG isotypes, including IgG subclasses, were determined by ELISA. Hybridoma 16CF7-G secreted both IgG1 and IgG2a. The percentage of the total IgA that was oligomeric was assessed by fast protein liquid chromatography.
Transport of IgA across a polarized epithelial-cell monolayer.
To assess the transcytosis of MAbs across a polarized epithelial-cell monolayer, 10 μg of purified IgA or IgG (anti-H, anti-F, or anti-N) in 100 μl was added to the basolateral chamber of pIgR+ Vero C1008 cells and incubated at 37°C. Apical supernatants were sampled at 0, 2, 4, 8, 12, and 24 h and analyzed by ELISA for antibody content. All three IgA MAbs were transported across the cell monolayer. Transport was mediated by the pIgR, as evidenced by the fact that pIgR− cells did not transport IgA (data not shown). Amounts of IgA transported to the apical chamber at 4, 8, and 12 h were 81 ± 6, 195 ± 21, and 400 ± 14 ng, respectively, for anti-H; 88 ± 3, 205 ± 21, and 360 ± 14 ng, respectively, for anti-F; and 79 ± 1, 260 ± 14, and 420 ± 14 ng, respectively, for anti-N. In contrast, at 12 h, ≤31 ± 4 ng of the corresponding IgG reached the apical chamber; i.e., 31 ± 4 ng was the highest nonspecific transport measured among the three IgG MAbs.
Traditional virus neutralization.
To examine whether the MAbs could reduce the infectivity of measles virus, we performed standard neutralization assays in Vero C1008 cells. Anti-H IgA at 15 μg/ml had significant neutralization activity (70% plaque reduction), whereas anti-H IgG even at 60 μg/ml had only moderate neutralization activity (40% plaque reduction) (data not shown). The anti-F and anti-N MAbs, both IgA and IgG, showed no neutralization activity at 60 μg/ml.
Immune exclusion (blocking apical virus infection of polarized epithelial cells).
To simulate the ability of an antiviral antibody at the mucosal surface to inhibit infection of epithelial cells, the apical surfaces of polarized Vero C1008 cells were exposed to a measles-specific IgA or IgG MAb or to an irrelevant IgA MAb. Measles virus was then added. After 2 h, the apical supernatant was removed and the cell monolayer was washed. Virus titers were evaluated 26 h after initial exposure to virus in both apical supernatants and cell lysates. Anti-H IgA was highly effective and more potent than anti-H IgG in blocking infection, according to the virus titers in both apical supernatants and cell lysates (P ≤ 0.0005 in both cases) (Table 2). Anti-F IgA and IgG were both modestly effective in reducing virus titers in the apical supernatants. Interestingly, anti-N IgA, directed against an internal viral antigen, also showed slight inhibition of virus infection when the apical supernatants were analyzed.
TABLE 2.
Blocking by antibodies of apical measles virus infection of polarized epithelial cellsa
| MAb (400 μg/ml) | Apical supernatant
|
Cell lysate
|
||
|---|---|---|---|---|
| Virus titer (10−2 PFU/ml) | % Reduction of virusb | Virus titer (10−4 PFU/ml) | % Reduction of virusb | |
| Anti-H IgA | 0c | 100 | 0.68 ± 0.07c | 82 |
| Anti-H IgG | 4.55 ± 0.70c | 41 | 3.20 ± 0.42d | 15 |
| Anti-F IgA | 5.00 ± 0.28c | 35 | 2.78 ± 0.81d | 26 |
| Anti-F IgG | 4.40 ± 0.57c | 43 | 3.20 ± 0.28d | 15 |
| Anti-N IgA | 5.75 ± 0.35c | 26 | 3.55 ± 0.35d | 6 |
| Anti-N IgG | 6.90 ± 0.57d | 11 | 3.57 ± 0.11d | 6 |
| Irrelevant IgA | 8.10 ± 0.07 | 0 | 4.40 ± 0.14 | 0 |
| None | 7.75 ± 0.35 | 0 | 3.78 ± 0.60 | 0 |
Experiments were repeated on at least three occasions with similar results. Results of a representative experiment are shown. Data are means ± standard deviations (n = 3).
Compared to no-antibody control.
Mean virus titers were significantly lower (P ≤ 0.002) than those from cells exposed to no antibody.
Mean virus titers were not significantly lower (P ≥ 0.05) than those from cells exposed to no antibody.
Intracellular colocalization of IgA antibodies and viral antigens.
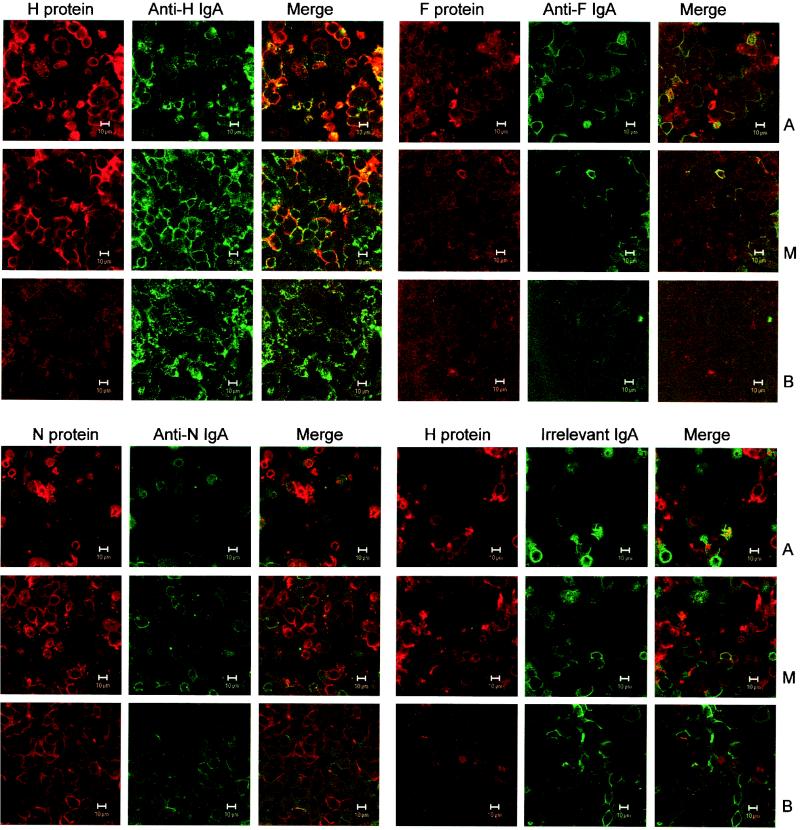
To demonstrate that transcytosing IgA can interact with newly synthesized viral protein inside infected epithelial cells, two-color immunofluorescence microscopy with a confocal laser scanning microscope was used (Fig. 1). H protein (red) stained intensely and was located mainly in the apical and middle portions of the cells, and anti-H IgA (green) was distributed through all three horizontal sections (apical, middle, and basal) (Fig. 1, upper-left quadrant). As shown in the merge image, H protein and anti-H IgA colocalized prominently in the apical and middle sections (orange to yellow according to the relative intensities of the merged red and green signals). The extent of colocalization was greatest in the apical portions of the cells, suggesting that this was the principal site of interaction between the IgA antibody and H protein. The relatively weak F protein immunofluorescence was most visible in the apical portions of the cells (Fig. 1, upper-right quadrant). The slight colocalization with anti-F IgA antibody was mainly apical and much less prominent than that of H with anti-H IgA. N protein (Fig. 1, lower-left quadrant), even though it is quantitatively the major viral protein, stained less prominently than H protein. N protein was distributed throughout the cells, but only moderate amounts of anti-N IgA, less than the amounts of anti-H IgA, were detected. As with F protein and anti-F, only slight colocalization of N and anti-N IgA was observed. In contrast to the marked colocalization of H and anti-H IgA, irrelevant IgA (control) and H protein showed essentially no colocalization (Fig. 1, lower-right quadrant) and stained less prominently than H protein and anti-H IgA in the upper-left quadrant. Collectively, these immunofluorescence results demonstrate strong colocalization and suggest trapping of viral H protein by the anti-H IgA antibody.
FIG. 1.
Intracellular colocalization of IgA MAbs and measles virus antigens in polarized, apically infected pIgR+ Vero cells observed by confocal immunofluorescence microscopy. Apical (A), middle (M), and basal (B) horizontal sections through the cell monolayers are shown. Each section is presented as the red channel (viral protein), green channel (IgA antibody), and merged red and green channels. Where the red and green signals colocalize, the resulting color varies from orange to yellow according to the relative intensities of the red and green signals. (Upper-left quadrant) Colocalization of viral H protein and anti-H IgA. H proteins were located mainly in the apical and middle parts of the cells, whereas anti-H IgA was distributed throughout. Colocalization of H protein and anti-H IgA antibody is especially prominent in the apical and middle sections (merge column). (Upper-right quadrant) Distribution of viral F protein and anti-F IgA. Although both F protein and anti-F IgA could be detected, colocalization was minimal. (Lower-left quadrant) Distribution of viral N protein and anti-N IgA. N, the major viral protein, stained clearly, but colocalization was rare. (Lower-right quadrant) Distribution of viral H protein and irrelevant IgA. H protein and irrelevant IgA overwhelmingly displayed different locations. Bar, 10 μm.
Intracellular virus neutralization.
Polarized Vero C1008 cells grown on the polyester membranes were infected via the apical surface with measles virus at an MOI of 1 per cell. After 2 h, residual virus was removed, the cells were washed, and fresh medium was added. Either anti-H IgA or IgG or medium alone (control) was then added to the basolateral chamber for 4 h at 37°C. Fluid was then removed from the basolateral chamber, the cells were washed, and fresh medium was added. After another 24 h of incubation, the apical supernatant was collected, cells were scraped, and a cell lysate was prepared. Virus was quantified in the apical supernatant and cell lysate by a plaque assay (Table 3). Virus titers were reduced by 97% in the apical supernatants and by 91% in the cell lysates of pIgR+ cells exposed to anti-H IgA MAb; these were significantly greater reductions (P < 0.0001) than those in the no-antibody control cells. However, pIgR+ cells exposed to an anti-H IgG MAb produced virus titers comparable to those of control cells not exposed to an antibody. Furthermore, no significant reduction was observed in pIgR− cells, incapable of endocytosing and transcytosing IgA, that were treated with the same anti-H IgA MAb.
TABLE 3.
Intracellular virus neutralization by anti-H IgAa
| Cells and antibody (100 μg/ml) | Apical supernatant
|
Cell lysate
|
||
|---|---|---|---|---|
| Virus titer (10−4 PFU/ ml) | % Reduction of virusb | Virus titer (10−5 PFU/ ml) | % Reduction of virusb | |
| pIgR+ | ||||
| Anti-H IgA | 0.09 ± 0.02d | 97 | 0.49 ± 0.05d | 91 |
| Anti-H IgG | 3.09 ± 0.32e | 11 | 5.82 ± 0.36e | 0 |
| CMc | 1.72 ± 0.21d | 51 | 5.71 ± 0.19e | 0 |
| None | 3.48 ± 0.34 | 0 | 5.68 ± 0.39 | 0 |
| pIgR− | ||||
| Anti-H IgA | 2.85 ± 0.13d | 16 | 5.63 ± 0.24e | 9 |
| None | 3.41 ± 0.18 | 0 | 6.16 ± 0.25 | 0 |
Experiments were repeated on at least three occasions with similar results. Results of a representative experiment are shown. Data are means ± standard deviations (n = 3).
Compared to no-antibody control.
Conditioned medium (see the text).
Mean virus titers were significantly lower (P < 0.001) than those from cells exposed to no antibody.
Mean virus titers were not significantly lower (P ≥ 0.05) than those from cells exposed to no antibody.
To confirm that significant neutralization was mediated by anti-H IgA MAb intracellularly during transcytosis rather than by an antibody that had already been transported to the apical medium, conditioned medium was prepared by aspirating apical supernatants from uninfected pIgR+ Vero C1008 cell monolayers that had transcytosed anti-H IgA for 4 h, by which time most of the IgA has been transcytosed. After apical virus infection of pIgR+ cells not exposed to a basolateral antibody, conditioned medium was added above the monolayer, and the cells were incubated for 28 h. The decrease in the virus titer of the apical supernatant was 51%, versus 97% for transcytosing IgA (P = 0.0002), but importantly, the conditioned medium did not diminish virus titers in cell lysates (0% versus 91% for transcytosing IgA) (Table 3). Given that anti-H IgA was able to neutralize virus in a traditional neutralization assay (see results above), anti-H IgA which had been transported into the apical chamber could have contributed to the reduction of virus titers in the apical supernatants. The decreased virus titers in the cell lysates, however, must have been due entirely to intracellular neutralization.
Anti-F and anti-N IgA MAbs were also tested for intracellular neutralization at the same concentration (100 μg/ml) as anti-H IgA. Anti-F IgA reduced virus titers by 26% (P = 0.1, not significant) in the apical supernatant and by 57% (P = 0.0001) in cell lysates, and anti-N IgA reduced virus titers by 34% (P = 0.056, not significant) in the apical supernatant and by 62% (P < 0.0001) in cell lysates (Table 4). In comparison, anti-H IgA produced reductions of 98% (P = 0.001) and 84% (P < 0.0001) in the apical supernatant and cell lysates, respectively, in the experiments for which results are shown in Table 4. These results suggest that anti-H IgA is the most potent of the three IgA antibodies at intracellular neutralization, which is consistent with the observation that anti-H IgA and H protein colocalized intracellularly to the greatest extent (Fig. 1). Nevertheless, both anti-F and anti-N IgA are capable of some intracellular neutralization.
TABLE 4.
Intracellular virus neutralization by IgA antibodies to F, N, and H and by antibody combinationsa
| IgA MAb (μg/ml) | Apical supernatant
|
Cell lysate
|
||
|---|---|---|---|---|
| Virus titer (10−3 PFU/ ml) | % Reduction of virusb | Virus titer (10−5 PFU/ ml) | % Reduction of virusb | |
| Anti-H (100) | 0.48 ± 0.04c | 98 | 0.93 ± 0.16c | 84 |
| Anti-F (100) | 18.9 ± 1.80d | 26 | 2.52 ± 0.28c | 57e |
| Anti-N (100) | 16.8 ± 2.40d | 34 | 2.25 ± 0.26c | 62e |
| Anti-H (50) | 6.12 ± 0.69c | 76 | 1.70 ± 0.39c | 71 |
| Anti-H (33) | 8.06 ± 0.52c | 68 | 1.96 ± 0.46c | 66 |
| Anti-H (50) + anti-F (50) | 2.05 ± 0.79c | 92 | 2.13 ± 0.51c | 64e |
| Anti-H (50) + anti-N (50) | 1.67 ± 0.53c | 93 | 2.28 ± 0.76c | 61e |
| Anti-F (50) + anti-N (50) | 25.2 ± 2.40d | 1 | 3.91 ± 0.65c | 33e |
| Anti-H (33) + anti-F (33) + anti-N (33) | 3.29 ± 0.77c | 87 | 2.33 ± 0.54c | 60e |
| Irrelevant (100) | 25.5 ± 4.90 | 0 | 5.81 ± 1.02 | 1 |
| None | 25.5 ± 5.10 | 0 | 5.85 ± 0.28 | 0 |
Experiments were repeated on at least three occasions with similar results. Results of a representative experiment are shown. Data are mean ± standard deviations (n = 3).
Compared to no-antibody control.
Mean virus titers were significantly lower (P < 0.01) than those from cells exposed to no antibody.
Mean virus titers were not significantly lower (P > 0.05) than those from cells exposed to no antibody.
A significantly smaller percent reduction (P < 0.04) than that with anti-H alone at 100 μg/ml.
In order to determine whether the anti-H, anti-F, and anti-N IgA antibodies could interact synergistically in intracellular virus neutralization, different combinations were tested (Table 4). The combinations of anti-H-anti-F and anti-H-anti-N (50 μg/ml each) reduced virus titers by 92 and 93%, respectively, in the apical supernatant, a little more than the reduction with anti-H alone at 50 μg/ml (76%) and comparable to that with anti-H alone at 100 μg/ml (98%). The combinations reduced virus titers by 64 and 61%, respectively, in cell lysates, essentially the same as anti-H alone at 33 and 50 μg/ml (66 and 71%, respectively) but significantly less (P < 0.04 for both combinations) than anti-H alone at 100 μg/ml (84%). The combination of anti-F-anti-N (50 μg/ml each) did not reduce virus titers in the apical supernatant but did produce a 33% reduction (P < 0.01 versus the no-antibody control) in cell lysates. This reduction, however, was lower than that with either anti-F or anti-N alone at 100 μg/ml (57 and 62%, respectively) (P < 0.03) and lower than that with anti-H alone at 100 μg/ml (84%) (P < 0.002). The combination of all three IgA antibodies (anti-H, anti-F, and anti-N at 33 μg/ml each) reduced virus titers by 87% (P = 0.0017 versus the no-antibody control) in the apical supernatant and 60% (P = 0.0006) in cell lysates. The latter reduction was significantly lower (P < 0.02) than that with anti-H alone at 100 μg/ml (84%). Thus, overall anti-H IgA appeared to be the main contributor to virus neutralization, and no synergies were found.
Virus excretion.
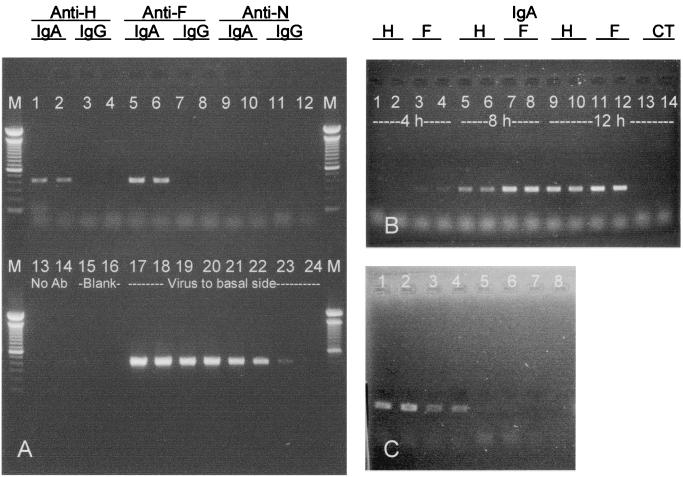
To investigate whether anti-measles virus IgA can transport virus into and through polarized epithelial cells, MAbs were mixed with virus at 4°C for 6 h, and the immune complexes were applied to the basolateral surfaces of polarized pIgR+ MDCK cells, which proved to be better for studying excretion than Vero C1008 cells. Apical medium was sampled after 4, 8, and 12 h at 37°C, and viral RNA was analyzed by RT-PCR and gel electrophoresis. The results (Fig. 2A) show that anti-H and anti-F IgA antibodies against the envelope proteins could transport virus across the cells. In contrast, an IgA antibody to an internal viral protein (N) was unable to excrete virus. Results with the corresponding IgG MAbs and the no-antibody control show that neither virus in a complex with IgG nor virus alone traversed the cells. The quantity of virions excreted by anti-envelope IgA increased as a function of time (Fig. 2B). The RNA of excreted virions could be detected as early as 4 h with anti-F IgA and 8 h with anti-H IgA. Also, the quantity of virions transcytosed to the apical surface increased with increasing amounts of anti-F IgA (Fig. 2C).
FIG. 2.
Excretion of measles virus through polarized pIgR+ MDCK cells by MAbs. (A) Lanes 1 to 14, RT-PCR products from apical supernatants after 12 h of virus excretion by different antibodies and a no-antibody control; lanes 17 to 24, 5−3 to 5−10 serial dilutions of original virus applied to the basal side; M, 100-bp DNA ladders (molecular markers). (B) RT-PCR products from apical supernatants after excretion of virus by anti-H IgA and anti-F IgA at different time points. Lanes 13 and 14, no-antibody control. (C) RT-PCR products from apical supernatants at 12 h after excretion of virus by different concentrations of anti-F IgA: 100 (lanes 1 and 2), 20 (lanes 3 and 4), 4 (lanes 5 and 6), and 0 (lanes 7 and 8) μg/ml.
DISCUSSION
In keeping with the large area of the body's mucosal surfaces and the high local tissue density of mucosal IgA-secreting plasma cells, the quantity of IgA synthesized in the body exceeds that of all the other immunoglobulin classes combined (21). Furthermore, because the IgA made by mucosal plasma cells is secreted in proximity to the lining epithelial cells, which express basolateral pIgR that is capable of binding and transcytosing oligomeric IgA, most of the mucosally produced IgA enters the external secretions. Here secretory IgA plays a critical role in the defense of mucous membranes against microbial pathogens and other antigens (8, 15). In fact, IgA has long been known to be able to act as a mucosal barrier to infection by preventing attachment of viruses to epithelial cells, and experimental studies in vivo have demonstrated that virus-specific IgA antibodies can protect the host from infection and resolve chronic infection (7, 20, 25, 27, 31). Nevertheless, in spite of a considerable literature, the different protective functions of IgA have not been explored in great depth. In the present work, within a single virus system (measles virus), a number of proposed antiviral IgA antibody functions were modeled. We are not aware of any data on the physiological concentrations of IgA immediately adjacent to the apical surfaces of mucosal epithelia or in mucosal lamina propria. Based, however, on the IgA content of human external secretions (13) and dog mesenteric lymph (30), we believe the concentrations of IgA we used are reasonable approximations. We have been able to demonstrate that specific IgA antibodies can exert antiviral functions above, within, and beneath a layer of epithelial cells that express the pIgR. Respectively, these functions include prevention of virus entry (immune exclusion), interruption of virus replication (intracellular neutralization), and transport of virus across the epithelium (immune excretion).
In studying virus immune exclusion in the present work, we used IgA (and paired IgG) MAbs against both envelope proteins, H and F, and the major internal protein, N, of measles virus. The antibody was placed above polarized epithelial cells, which were then challenged with virus apically. The results, especially virus titers in cell lysates, showed that anti-H IgA was the most potent extracellular blocker of virus infection. Anti-F, also directed against an envelope protein, was less effective, and anti-F IgA and IgG were comparable (Table 2). The greater effectiveness of anti-H IgA is not due to a greater proportion of oligomers; the percentages of oligomers in the various IgA MAbs were similar (Table 1). Surprisingly, the antibody against the internal N protein was slightly inhibitory toward virus production (Table 2). Perhaps some of the IgA that had been added to the apical surface was able to bind to apical pIgR prior to the usual proteolysis that leads to the release of free secretory component. In this manner, some of the anti-N IgA could have been apically endocytosed by recycling pIgR (6, 16) and thus could have been able to inhibit virus production to a degree. Inhibition did not occur with an irrelevant IgA antibody, demonstrating the specificity of the inhibition. Taken together, the data indicated that a virion-specific IgA antibody secreted onto a mucosal surface is potentially capable of preventing virus from invading mucosal epithelium, whether the antibody is directed against an envelope protein or an internal protein.
In the original demonstrations of intra-epithelial cell neutralization of viruses by IgA, antibodies to only a single envelope protein per virus (HN for Sendai virus and HA for influenza A virus) were studied (17, 18). In the present work, IgA antibodies against the two envelope proteins of measles virus, H and F, plus the major internal protein, N, were investigated. The intracellular neutralization experiments showed that anti-H IgA was the most effective but that anti-F and anti-N were also able to mediate intracellular neutralization (Table 4). These results were entirely consistent with the morphological data (Fig. 1), which show that anti-H IgA binds better than anti-F or anti-N to the corresponding viral protein intracellularly.
The extent of inhibition of measles virus by anti-H IgA was similar to that of influenza A virus by anti-HA IgA (17) but less than that of Sendai virus by anti-HN IgA (18). Measles virus H and F proteins, like viral envelope proteins generally, are synthesized on membrane-bound ribosomes, mature through the endoplasmic reticulum and the Golgi apparatus, and become integral plasma membrane proteins (11). Furthermore, the F protein is synthesized as an inactive precursor (F0) that is cleaved in the trans-Golgi network to form the biologically active protein containing the disufide-linked subunits F1 and F2 (3). N proteins, in contrast, are synthesized on free cytoplasmic ribosomes (10). These different modes of viral protein synthesis, maturation, and packaging may account for some of the differences we observed in the abilities of specific IgA antibodies to interrupt viral replication, namely, that anti-H was the most potent whereas anti-F and anti-N were similar and only modestly effective. In a study of rotavirus infection in vivo, Burns et al. (7) observed that an IgA antibody to vp6, an internal virus protein, could prevent infection and resolve chronic infection. It is not known whether anti-N IgA, which showed some ability to neutralize measles virus intracellularly in vitro, could be similarly active in vivo.
We tested whether mixtures of two or three antibodies of different specificities might show synergistic effects in intracellular neutralization. No evidence for synergy was found, however. On the contrary, the anti-F-anti-N mixture showed less intracellular neutralization than either antibody alone, and the binary and ternary mixtures containing anti-H were less effective than anti-H alone.
Anti-F IgA proved to be the most effective of the three specific IgA antibodies at complexing with measles virus particles and transporting them from the basolateral chamber to the apical chamber (immune excretion) (Fig. 2). The lower excretory ability of anti-H compared to anti-F IgA cannot be attributed to the more robust neutralization activity of anti-H, because when the same amount of transcytosed but free anti-H IgA was mixed with different quantities of virus particles, it did not hinder RT-PCR amplification and gel electrophoresis detection of viral RNA (data not shown). The results suggest that in vivo, an IgA antibody directed against envelope protein is capable of binding extracellular virus particles that may be present in the mucosal lamina propria and transporting them to the luminal side via the pIgR.
In conclusion, working with IgA antibodies and measles virus, we have illustrated three ways in relation to epithelium in which IgA can protect mucous membranes. These include blocking infection at the apical surface, neutralizing virus intracellularly, and excreting virus across epithelial cells. In each of these functions the relative effectiveness of a given antibody varied according to its specificity for a particular viral protein. Overall, the IgA antibody to the H envelope antigen was the most potent, but in the particular case of immune excretion, the IgA antibody against the F envelope antigen was most effective. Although anti-N IgA, directed against an internal viral protein, was the least effective antibody at immune exclusion and immune excretion, it was able to neutralize virus intracellularly to some extent. Under natural conditions, of course, a host would utilize the advantages of a polyclonal antibody response to the multiple antigens of a given microbe. Finally, all the host defense functions investigated here reflect the ability of oligomeric IgA antibodies to bind to the epithelial-cell receptor pIgR, which accounts for the superiority of IgA over the systemically more abundant IgG with regard to protection of mucous membranes.
Acknowledgments
This research was supported by grants AI-26449, AI-36359, and CA-43703 from the NIH and K2000-06P-13420-01A from The Swedish Medical Research Council.
REFERENCES
- 1.Armstrong, S. J., and N. J. Dimmock. 1992. Neutralization of influenza virus by low concentrations of hemagglutinin-specific polymeric immunoglobulin A inhibits viral fusion activity, but activation of the ribonucleoprotein is also inhibited. J. Virol. 66:3823-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blau, D. M., and R. W. Compans. 1995. Entry and release of measles virus are polarized in epithelial cells. Virology 210:91-99. [DOI] [PubMed] [Google Scholar]
- 3.Bolt, G., and I. R. Pedersen. 1998. The role of subtilisin-like proprotein convertases for cleavage of the measles virus fusion glycoprotein in different cell types. Virology 252:387-398. [DOI] [PubMed] [Google Scholar]
- 4.Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277-287. [DOI] [PubMed] [Google Scholar]
- 5.Boot, J. H., M. E. Geerts, E. R. De Groot, and L. A. Aarden. 1988. Murine monoclonal isotype switch variants. Detection with rat monoclonal antibodies in ELISA and isolation by sequential sublining. J. Immunol. Methods 106:195-202. [DOI] [PubMed] [Google Scholar]
- 6.Breitfeld, P. P., J. M. Harris, and K. E. Mostov. 1989. Postendocytotic sorting of the ligand for the polymeric immunoglobulin receptor in Madin-Darby canine kidney cells. J. Cell Biol. 109:475-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, J. W., M. Siadat-Pajouh, A. A. Krishnaney, and H. B. Greenberg. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272:104-107. [DOI] [PubMed] [Google Scholar]
- 8.Corthésy, B., and J. P. Kraehenbuhl. 1999. Antibody-mediated protection of mucosal surfaces. Curr. Top. Microbiol. Immunol. 236:93-111. [DOI] [PubMed] [Google Scholar]
- 9.Gan, Y. J., J. Chodosh, A. Morgan, and J. W. Sixbey. 1997. Epithelial cell polarization is a determinant in the infectious outcome of immunoglobulin A-mediated entry by Epstein-Barr virus. J. Virol. 71:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gombart, A. F., A. Hirano, and T. C. Wong. 1993. Conformational maturation of measles virus nucleocapsid protein. J. Virol. 67:4133-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horikami, S. M., and S. A. Moyer. 1995. Structure, transcription, and replication of measles virus. Curr. Top. Microbiol. Immunol. 191:35-50. [DOI] [PubMed] [Google Scholar]
- 12.Huang, Y. T., C. J. Miller, V. Wong, H. Fujioka, J. G. Nedrud, and M. E. Lamm. 1999. Replication and budding of simian immunodeficiency virus in polarized epithelial cells. Virology 257:24-34. [DOI] [PubMed] [Google Scholar]
- 13.Jackson, S., J. Mestecky, Z. Moldoveanu, and P. Spearman. 1999. Collection and processing of human mucosal secretions, p. 1567-1575. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology, 2nd ed. Academic Press, San Diego, Calif.
- 14.Kaetzel, C. S., J. K. Robinson, K. R. Chintalacharuvu, J. P. Vaerman, and M. E. Lamm. 1991. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc. Natl. Acad. Sci. USA 88:8796-8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamm, M. E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51:311-340. [DOI] [PubMed] [Google Scholar]
- 16.Low, S. H., S. J. Chapin, C. Wimmer, S. W. Whiteheart, L. G. Komuves, K. E. Mostov, and T. Weimbs. 1998. The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J. Cell Biol. 141:1503-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazanec, M. B., C. L. Coudret, and D. R. Fletcher. 1995. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 69:1339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazanec, M. B., C. S. Kaetzel, M. E. Lamm, D. Fletcher, and J. G. Nedrud. 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 89:6901-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazanec, M. B., J. G. Nedrud, C. S. Kaetzel, and M. E. Lamm. 1993. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 14:430-435. [DOI] [PubMed] [Google Scholar]
- 20.Mazanec, M. B., J. G. Nedrud, and M. E. Lamm. 1987. Immunoglobulin A monoclonal antibodies protect against Sendai virus. J. Virol. 61:2624-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mestecky, J., and J. R. McGhee. 1987. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv. Immunol. 40:153-245. [DOI] [PubMed] [Google Scholar]
- 22.Murray, C. J., and A. D. Lopez. 1999. On the comparable quantification of health risks: lessons from the Global Burden of Disease Study. Epidemiology 10:594-605. [PubMed] [Google Scholar]
- 23.Palombo, G., S. De Falco, M. Tortora, G. Cassani, and G. Fassina. 1998. A synthetic ligand for IgA affinity purification. J. Mol. Recognit. 11:243-246. [DOI] [PubMed] [Google Scholar]
- 24.Polack, F. P., S. H. Lee, S. Permar, E. Manyara, H. G. Nousari, Y. Jeng, F. Mustafa, A. Valsamakis, R. J. Adams, H. L. Robinson, and D. E. Griffin. 2000. Successful DNA immunization against measles: neutralizing antibody against either the hemagglutinin or fusion glycoprotein protects rhesus macaques without evidence of atypical measles. Nat. Med. 6:776-781. [DOI] [PubMed] [Google Scholar]
- 25.Renegar, K. B., and P. A. Small, Jr. 1991. Passive transfer of local immunity to influenza virus infection by IgA antibody. J. Immunol. 146:1972-1978. [PubMed] [Google Scholar]
- 26.Robinson, J. K., T. G. Blanchard, A. D. Levine, S. N. Emancipator, and M. E. Lamm. 2001. A mucosal IgA-mediated excretory immune system in vivo. J. Immunol. 166:3688-3692. [DOI] [PubMed] [Google Scholar]
- 27.Ruggeri, F. M., K. Johansen, G. Basile, J. P. Kraehenbuhl, and L. Svensson. 1998. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J. Virol. 72:2708-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheshberadaran, H., S. N. Chen, and E. Norrby. 1983. Monoclonal antibodies against five structural components of measles virus. I. Characterization of antigenic determinants on nine strains of measles virus. Virology 128:341-353. [DOI] [PubMed] [Google Scholar]
- 29.Tamer, C. M., M. E. Lamm, J. K. Robinson, J. F. Piskurich, and C. S. Kaetzel. 1995. Comparative studies of transcytosis and assembly of secretory IgA in Madin-Darby canine kidney cells expressing human polymeric Ig receptor. J. Immunol. 155:707-714. [PubMed] [Google Scholar]
- 30.Vaerman, J. P., and J. F. Heremans. 1970. Origin and molecular size of immunoglobulin-A in the mesenteric lymph of the dog. Immunology 18:27-38. [PMC free article] [PubMed] [Google Scholar]
- 31.Weltzin, R., V. Traina-Dorge, K. Soike, J. Y. Zhang, P. Mack, G. Soman, G. Drabik, and T. P. Monath. 1996. Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J. Infect. Dis. 174:256-261. [DOI] [PubMed] [Google Scholar]