Abstract
The E2 proteins of papillomaviruses (PV) bind to the coactivator CBP/p300 as do many other transcription factors, but the precise role of CBP/p300 in E2-specific functions is not yet understood. We show that the E2 protein of human PV type 8 (HPV8) directly binds to p300. Activation of HPV8 gene expression by low amounts of HPV8 E2 was stimulated up to sevenfold by coexpression of p300. The interaction between E2 and p300 may play a role in differentiation-dependent activation of PV gene expression, since we can show that the expression level of p300 increases during keratinocyte differentiation. Surprisingly, sequence-specific binding of E2 to its recognition sites within the regulatory region of HPV8 is not necessary for this cooperation, indicating that E2 can be recruited to the promoter via protein-protein interaction. HPV8 E2 binds via its N-terminal activation domain (AD), its C-terminal DNA binding domain (DBD), and its internal hinge region to p300 in vitro. Transient-transfection assays revealed that the AD is necessary and sufficient for cooperative activation with p300. However, we provide evidence that the interaction of the hinge and the DBD of HPV8 E2 with p300 may contribute. Our data suggest an important role of p300 in regulation of HPV8 gene expression and reveal a new mechanism by which E2 may be recruited to a promoter to activate transcription without sequence specific DNA binding.
Papillomaviruses (PV) infect the basal cells of the skin or the mucosa, causing proliferative lesions like warts or dysplasias. PV require differentiating keratinocytes to replicate their DNA. The expression of the structural proteins is restricted to some of the most-differentiated keratinocytes. This cell tropism is due to the involvement of transcription factors specifically expressed in these cells. In addition to ubiquitously expressed and keratinocyte-specific cellular transcription factors, viral gene expression is also modulated by the viral E2 protein. The E2 protein binds via its carboxy (C)-terminal DNA binding domain (DBD) and dimerization domain to the 12-bp palindromic sequence ACCN6GGT mostly located within the regulatory region, also called the long control region or noncoding region (NCR) of the PV genome. In the case of bovine PV type 1 (BPV1), the long control region contains 12 E2 binding sites, which mediate a strong activation of several BPV1 promoters by E2 (48-50). Human PV (HPV) types infecting the genital mucosa contain four E2 binding sites in conserved positions. Here, only a moderate activation by E2 could be detected, and E2 repressed HPV gene expression in most cases. This repression is mediated by binding to two promoter-proximal E2 binding sites, as revealed by transient transfections of cervical carcinoma cell lines and immortalized skin keratinocytes (10, 52, 58, 59). Repression of HPV gene expression by the E2 activator protein occurs when E2 binding sites are overlapping with the binding sites for cellular transcription factors necessary for promoter activity. In contrast to natural HPV promoter-enhancer constellations E2 can strongly activate synthetic promoters containing several E2 binding sites upstream of a promoter, although specific differences in the mode of activation between the E2 proteins of different types may exist (18, 27, 61). In addition to its role in regulation of gene expression, E2 is also necessary for viral DNA replication. Both activation of transcription and replication depend on the amino (N)-terminal activation domain (AD). During replication, E2 enhances the binding of E1 to the origin of replication, which requires the interaction of the AD as well as the DBD with the viral protein E1, a DNA binding helicase essential for viral DNA replication (2, 4, 13, 15, 31, 37). Activation of transcription involves protein-protein interactions of the AD and the transcriptional machinery. The TATA-binding protein (TBP) and transcription factor IIB (TFIIB) (3, 34, 66), as well as the AD modulation factor AMF-1/Gps2 (6) are bound by the AD of E2. Furthermore, E2 was shown to interact with the cellular coactivator p300 and its cellular homologue CREB binding protein (CBP) (28, 42). However, a role of this interaction in E2 specific function has not been addressed. In addition to the viral E2 protein, also the E6 protein was shown to interact with CBP/p300. The binding of E6 of HPV16 to the central cysteine-histidine-rich region CH3 of p300 was correlated with repression of CBP/p300-mediated transcription (41, 69, 70).
CBP/p300 are coactivators for a growing number of transcription factors involved both in proliferation and differentiation (46, 62). CBP/p300 possess intrinsic histone acetyltransferase (HAT) activity (38). In addition to their own HAT activity, CBP/p300 are also associated with other HATs, e.g., the p300/CBP-associated factor (P/CAF) and the coactivators SRC1 and ACTR (7, 51, 64). These complexes can acetylate the N termini of histones, which may loosen the chromatin structure, facilitating the binding of transcription factors. This relief from repressive effects of the chromatin by histone acetylation requires the presence of a DNA-binding activator protein, which recruits the HAT-containing coactivators via protein-protein interaction (25). In addition to histones, transcription factors, like p53, Myo D, and YY1 are also acetylated by CBP/p300, modulating their activity as well (17, 30, 68). Furthermore, CBP/p300 bind to components of the preinitiation complex, like TBP and TFIIB. These interactions are also essential for CBP/p300-mediated activation (1, 62). Many of the activators functionally interacting with CBP/p300 are regulated by diverse signals. This may result in cooperation since factors interacting with CBP/p300 can synergize with each other when bound to the same promoter in cis (35). On the other hand, competition for limiting amounts of CBP/p300 may be the basis for inhibition of AP1-mediated activation by nuclear receptors (24), indicating that CBP/p300 are able to integrate multiple transcriptional signals.
Usually, the DBD of transcription factors has the function to direct the AD to the correct position on the DNA via sequence-specific DNA binding. However, it has also been reported that E2 proteins are able to activate transcription in the absence of E2 binding sites, e.g., promoters of lymphokine genes (21), and various viral promoters like the simian virus 40 (SV40) early promoter and the cytomegalovirus (CMV) immediate-early promoter. The AD of BPV1 E2 was shown to be sufficient for this effect (20). The mechanism of this so-called nonspecific activation is unknown.
Here, we show that the E2 protein of HPV8, which infects the skin and is associated with the rare disease epidermodysplasia verruciformis, directly binds to p300 via at least three domains: its AD, internal hinge, and DBD. HPV8 E2 (8 E2) and overexpressed p300 also functionally interact, since they cooperate in activation of HPV8 gene expression. Surprisingly, cooperation between E2 and p300 can also occur without sequence-specific DNA binding by E2. Although the N-terminal AD of 8 E2 is required and sufficient for this cooperativity, its DBD and internal hinge region may contribute. This is supported by the observation that the hinge and DBD of 8 E2 enable a heterologous AD to cooperate with coexpressed p300. Thus, E2 may use p300 as a classical coactivator and as a platform to gain access to promoters, which do not encode E2 binding sites.
MATERIALS AND METHODS
Plasmid constructs.
To express p300 as glutathione S-transferase (GST) fusion proteins, five fragments covering the entire p300 open reading frame (ORF) except the C-terminal 63 amino acids, as indicated in Fig. 1A, were amplified by PCR with appropriate primers hybridizing to the p300 ORF and encoding in addition sites for restriction enzymes to allow the cloning of the PCR products into the vector pGEX2T (Pharmacia). The plasmids pET14B-HPV18 E2, described in reference 9 and pET14B-HPV8 E2 and pET14B-BPV1 E2, which both were obtained by cloning PCR products encoding the respective E2 ORF, were used for translation of the various E2 proteins in vitro. pGEX-HPV8 E2 has been described previously (12). To express the HAT domain of p300 in bacteria, the p300 fragment from nucleotide 4782 to 6482, amplified by PCR, was cloned into pET14B (Novagen) as well. The reporter construct 4E2-Sp1 Luc (53); the eukaryotic expression vectors for 8 E2, 18 E2, and BPV1 E2 (52, 57, 65); and the expression vector for p300 (11) were used previously. The luciferase reporter construct NCR8-Luc wild type (wt), harboring the NCR of HPV8 from position 7077 to 558 in front of the luciferase gene, is described in reference 57, and the plasmids allowing the expression of the various domains of the E2 proteins as GST fusion proteins are described in reference 53. The expression vectors coding for truncated 8 E2 mutants have been generated by cloning appropriate PCR products into the eukaryotic expression vector pCB6 (56). To create a fusion protein between the AD of transcriptional enhancer factor 1 (TEF1) and the C-terminal half of 8 E2, PCR products encoding TEF1, lacking amino acids 55 to 121 and 143 to 204 (TEF1 Δ55-121/Δ143-204) as well as 8 E2 Δ1-328 were cloned into pXJ41 (63). The construct was kindly provided by M. May. TEF1 AD Δ55-121/Δ143-204, encoding the TEF1 AD alone, was obtained in a similar way, and an oligonucleotide encoding the nuclear localization signal (NLS) of the SV40 T antigen (amino acid sequence PPKKKRKV) was inserted at the 3′ end to fuse the AD with the NLS. The quick site change mutagenesis kit (Stratagene) was used to introduce point mutations into the DBD of 8 E2 and into the various E2 binding sites within the reporter construct NCR8-Luc wt, as indicated in the figures. All constructs obtained by in vitro mutagenesis and by cloning of PCR products have been verified by sequencing.
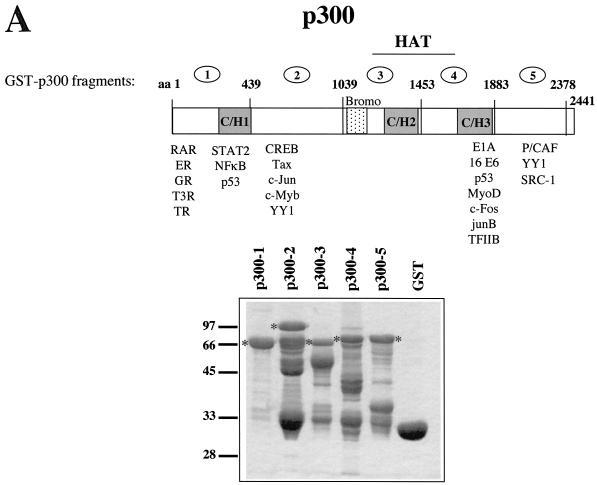
FIG.1.
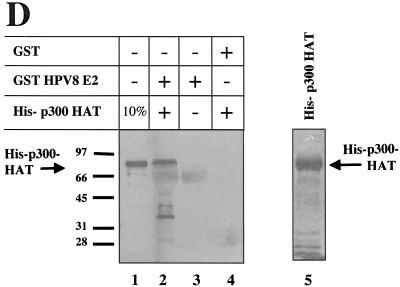
E2 proteins bind to p300 in vitro. (A) Schematic representation of p300. The regions encoding the HAT, the bromodomain (Bromo), and the cysteine- and histidine-rich regions (C/H1, C/H2, and C/H3) are indicated. Some of the transcription factors binding to specific domains of p300 are shown beneath. The ORF of p300 has been cloned in five subfragments into pGEX2T, to allow the production of p300 fused to GST. The numbers above the ORF refer to the amino acids (aa) present in the five GST-p300 fragments, respectively. An SDS-PAG stained by Coomassie brilliant blue with the five GST-p300 fusion proteins (p300-1 to p300-5) as well as GST after purification with glutathione-Sepharose from bacteria is shown. The positions of the full-length GST-p300 fusion proteins are indicated, respectively. Marker proteins are shown on the left. (B) Purified GST-p300 fusion proteins and GST, as shown on the gel in panel A have been incubated with 35S-labeled E2 proteins of HPV8, HPV18, and BPV1, as indicated, obtained by translation in vitro with a rabbit reticulocyte lysate. The autoradiograms of the SDS gels analyzing the bound proteins are shown. The lane labeled “10% Input” represents 1/10 of the labeled E2 proteins used in each assay. The position of the full-length E2 and the marker proteins (in kilodaltons) are indicated. (C) 8 E2 fused to GST (lane 2) or GST (lane 3) has been incubated with HeLa nuclear extracts. The presence of p300 was monitored by a Western blot with an antibody directed against p300. Lane 1 represents one third of the nuclear extracts used in this assay. (D) GST-pull down assay using a bacterially expressed, purified His-tagged fragment of p300 encoding the HAT region (His-p300 HAT) and purified GST-8 E2 (lane 2) or GST (lane 4). The presence of His-p300 HAT was analyzed by Western blotting with a Ni-NTA conjugate recognizing the six histidines. To demonstrate the specificity of the antibody, the same amount of purified GST-8 E2 used in lane 2, however without adding His-p300-HAT, was included (lane 3), as well as 10% of the input His-p300-HAT (lane 1). Purified His-p300-HAT is shown on the right in an SDS-PAG, stained by Coomassie blue (lane 5).
Cell culture and transient transfections.
293T and C33A cells were maintained in Dulbecco's modified Eagle medium, supplemented with 7.5% fetal calf serum. The skin keratinocyte cell line RTS3b (44) was cultivated in E-medium (33). Approximately 2 × 105 or 1 × 105 C33A or RTS3b cells, respectively, were seeded into six-well plates the day before transfection. The next day, the cells were transfected with the FuGene reagent (Roche Diagnostics) in accordance with the manufacturer's recommendations. About 40 h later, the cells were washed twice in ice-cold phosphate-buffered saline (PBS), scraped in 50 μl of luciferase extraction buffer (0.1 M potassium phosphate buffer, pH 8.0; 1 mM dithiothreitol) and lysed by four freeze-thaw cycles. Lysates were cleared by centrifugation and measured in a Luminometer as described in the manufacturer's manual. The results (in relative light units) have been normalized by the protein concentration of the samples.
Protein expression and protein-protein and DNA-protein interaction studies.
BL21 pLys S bacteria (Stratagene) were used to express the GST fusion proteins, as well as His-tagged proteins. Bacteria were resuspended in LSDB buffer (50 mM Tris-HCl, 10% glycerol, 1 mM dithiothreitol, and 0.1% NP-40, including the protease inhibitors phenylmethylsulfonyl fluoride, aprotinin, pepstatin, and leupeptin) containing 500 mM KCl and sonicated, and the lysate was cleared by centrifugation. Lysates of bacteria expressing GST fusion proteins were incubated with 50 μl of glutathione-Sepharose (Pharmacia) for 1 h at 4°C in LSDB-500 mM KCl. The beads were washed four times in 1 ml of LSDB-1 M KCl and twice in LSDB-100 mM KCl. About 1 to 2 μg of immobilized GST fusion protein was incubated with 10 μl of 35S-labeled protein, which was obtained by in vitro transcription and translation with a TNT kit (Promega) and incubated for 2 h at 4°C with agitation. Thereafter the beads were washed three times in LSDB-250 mM KCl and once in LSDB-100 mM KCl. Bound proteins were eluted in 2× sodium dodecyl sulfate (SDS) loading buffer and analyzed on an SDS-polyacrylamide gel (SDS-PAG). The expression and purification of His-tagged proteins were performed as previously described (22). The concentration of the purified proteins was estimated by a Western blot developed with a Ni-nitrilotriacetic acid (NTA) conjugate (Qiagen). To demonstrate a direct protein-protein interaction, the 8 E2-GST fusion protein or GST, coupled to glutathione-Sepharose, respectively, were incubated with 1 to 2 μg of purified His-p300-HAT and further treated as described above. For precipitation of 8 E2ΔN, 100 μg of extracts of 293T cells transiently transfected with an expression vector for pCMV2-FLAG-HPV8E2ΔN or the pCMV2-FLAG (Kodak) were incubated with GST fusion proteins and also further treated as described above. Bound proteins were analyzed by Western blotting with the anti-FLAG M5 antibody (Kodak). Gel shift analysis and preparation of nuclear extracts have been performed as described previously (52).
Immunohistochemical staining of skin sections.
Biopsy specimens were cut into 5-μm-thick sections, placed onto 3-aminopropyltriethyloxysilane-coated slides, and fixed in ice-cold acetone for 10 min. After a 5-min wash in PBS-0.5% bovine serum albumin (BSA), the sections were incubated with mouse monoclonal antibody (1.5 μg/ml) directed against p300 (Calbiochem) diluted in PBS-1% BSA for 30 min. After three washes for 5 min in PBS-0.5% BSA, bound antibodies were detected with the kit from BioGenex (Hamburg, Germany) according to the manufacturer's instructions.
RESULTS
8 E2 directly binds to the HAT domain of p300.
In order to characterize the binding of different E2 proteins to p300, the cellular homologue of CBP, we divided the ORF of p300 into five subfragments and expressed them as GST fusion proteins. As seen on the SDS PAG in Fig. 1A, after purification of the five GST-p300 fusion proteins we obtained proteins corresponding in size to the full-length products and several faster-migrating bands in variable amounts, indicating that the p300 fusion proteins are degraded to a certain degree. The binding of 35S-labeled E2 proteins of HPV8, HPV18, and BPV1, respectively, to similar amounts of the GST-p300 proteins and GST is shown in Fig. 1B. Although 8 E2, obtained by in vitro translation, appeared in several bands, the major product corresponding in size to the full-length protein and a faster-migrating form were retained by GST-p300-4. This fragment of p300 spans the C-terminal half of the HAT domain, including the region bound by E1A and a variety of cellular transcription factors (for an overview, see Fig. 1A and reference 62). The E2 proteins of HPV18 and BPV1 in addition bound to GST-p300-1 and GST-p300-5. GST on its own as well as GST-p300-2 and GST-p300-3 were not bound by any of the E2 proteins, pointing to a specificity of the binding reactions. The subdivision of p300 into five fragments may have led to the exposition of protein sequences on the surface not accessible on the native protein, which may affect the binding of E2 to p300. In order to exclude this, a GST-8 E2 fusion protein was incubated with HeLa nuclear extracts, and the binding of endogenous p300 was analyzed by Western blotting with antiserum directed against p300 (Santa Cruz). Figure 1C reveals that 8 E2 is able to precipitate p300 from HeLa nuclear extracts.
To exclude the possibility that a protein present in the reticulocyte lysate or the nuclear extracts mediates the interaction between p300 and E2, we carried out GST-pull down experiments with purified proteins expressed in bacteria. A p300 fragment encoding the HAT domain (His-p300-HAT), which includes the domain bound by 8 E2, was expressed in bacteria fused with a tag of six histidines at the N terminus and purified on a nickel-coupled agarose column. The binding of His-p300-HAT to purified GST-8 E2 protein was analyzed by Western blotting with a Ni-NTA conjugate recognizing the histidine tag (Qiagen). Figure 1D shows that about 10% of the His-p300 HAT was bound by GST-8 E2. In summary, the in vitro interaction studies presented in Fig. 1 show that the E2 protein of HPV8 directly interacts with a region colocalizing with the HAT domain of p300. In addition, the E2 proteins of HPV18 and BPV1 bind to domains present within the N- and the C-terminal part of p300.
E2 and p300 cooperatively stimulate transcription.
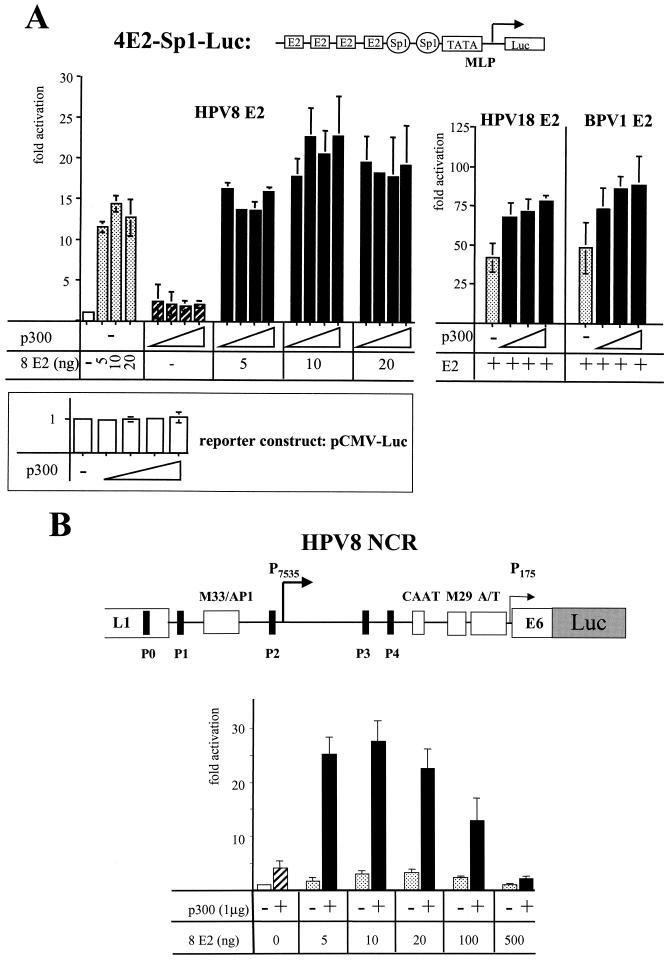
In order to address a role of the direct interaction between E2 and p300 in activation of transcription by E2, we analyzed the effect of increasing amounts of p300 on activation by E2. This is conceivable since it has been suggested that p300 is limiting within the cell (24, 67). We used a synthetic promoter composed of the TATA box and the initiator element of the adenovirus major late promoter following two high-affinity Sp1 binding sites and four classical E2 binding sites (Fig. 2A). We could show previously that the E2 proteins of different PV types strongly stimulate this promoter (19, 53). As shown in Fig. 2A, transfection of the HPV-negative cervical carcinoma cell line C33A with an expression vector for p300 (11) increased the basal activity of this promoter twofold. Ten nanograms of expression vector for 8 E2 led to a 14-fold activation. After cotransfection of 125 ng of p300 expression vector with the 8 E2 expression vector the promoter was stimulated 23-fold. This level of activation could not be reached by any E2 concentration tested. p300 also enhanced activation in the presence of saturating amounts of E2 (after transfection of 20 ng of expression vector) to a similar degree indicating that the binding of E2 to p300 may represent a rate-limiting step for activation by E2. Higher p300 concentration did not further increase activation by E2. Other factors may become rate limiting now. The immediate-early promoter of CMV linked to the luciferase gene was not significantly affected by the concentrations of p300 expression vector used here, as also shown in Fig. 2A. Furthermore, the protein level of a FLAG-tagged BPV1 E2 under control of the CMV promoter was not affected by overexpression of p300 as well (data not shown). This supports the notion that the synergy between 8 E2 and p300 does not result from elevated concentrations of E2 by overexpression of p300 due to activation of the CMV promoter, which drives the expression of 8 E2. A similar level of cooperativity could be revealed for the E2 proteins of HPV18 and BPV1 (Fig. 2A).
FIG. 2.
E2 and p300 cooperate in activation of transcription. (A) C33A cells have been transiently transfected with a synthetic reporter construct, 4E2-Sp1-Luc, containing a promoter that is activated efficiently by E2, as indicated at the top. The activity of the promoter on its own was set as 1. A vector (5, 10 or 20 ng) expressing 8 E2 under control of the CMV promoter and 50, 125, 250, or 500 ng of an expression vector for p300 (indicated by triangles) have been cotransfected alone or together with the reporter construct. As a control, a reporter construct expressing the luciferase gene under control of the CMV immediate-early promoter was transfected with the same amounts of expression vector for p300. In addition, 375 ng of a CMV promoter based expression vector for HPV18 E2 or 125 ng of an SV40 early promoter-driven expression vector for BPV1 E2, respectively, have been transfected alone or in combination with 250, 500, or 750 ng of p300 expression vector, also indicated by triangles. The bars represent the activation of at least three independent experiments, and the standard deviations (error bars) are shown. (B) A reporterconstruct encoding the luciferase under control of the NCR of HPV8 has been transfected into the skin keratinocyte cell line RTS3b. A schematic representation of the NCR of HPV8 with the E2 binding sites P0 to P4 and the sequence motifs M33/AP1, CAAT, M29, and an A/T-rich region, which are conserved among the PV types associated with epidermodysplasia verruciformis, is shown at the top. Increasing amounts of an expression vector for 8 E2 have been transfected either alone or in combination with 1 μg of expression vector for p300. The bars represent the results of four independent experiments. The activity of the NCR-Luc construct on its own was set as 1.
Next we analyzed the effect of overexpression of p300 and E2 on a natural PV promoter, which is composed in addition to E2 binding sites of a cluster of binding sites for ubiquitous and keratinocyte specific transcription factors, which in part also use CBP/p300 as coactivators. In HPV8, the E2 protein regulates HPV8 gene expression in a dose-dependent manner by binding to five E2 binding sites (P0 to P4) (for an overview, see Fig. 2B). The binding of E2 to P0, P1, P3, and P4 confers a fourfold activation of the promoter in position 7535 (P7535), which is supposed to be the late promoter. Higher amounts of E2 lead to occupancy of the low-affinity E2 binding site P2, resulting in promoter repression (57). P2 overlaps with the binding sites for RUNX1 and papillomavirus-binding factor, which are required for the basal activity of the promoter and which are displaced by E2 (5, 45). The luciferase reporter construct we used here contains the NCR of HPV8 from position 7077 to 558. This fragment also harbors the promoter in position 175, presumably the early promoter. This promoter is rather weak, and the dose-dependent regulation by E2 was supposed to concern the P7535 (55-57). We used the immortalized keratinocyte cell line RTS3b, derived from a squamous cell carcinoma of a renal transplant patient (44). Cotransfection of the p300 expression vector stimulated HPV8 promoter activity about fourfold. A maximal activation of fourfold was obtained by transfecting 20 ng of 8 E2 expression vector. Further increasing the E2 concentrations resulted in promoter repression. The coexpression of p300 and subsaturating amounts of 8 E2 increased activation up to 28-fold, pointing to a strong cooperation between E2 and p300 in activation of HPV8 gene expression. In the presence of high amounts of E2 the cooperativity decreased (Fig. 2B).
Sequence-specific DNA binding by E2 is not required for cooperativity with p300.
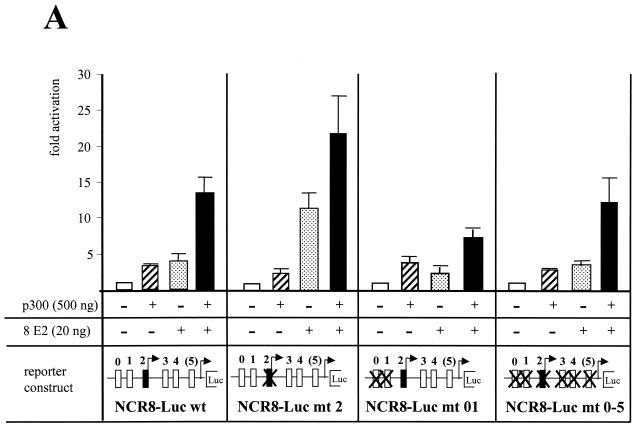
In order to identify the E2 binding sites required for the cooperativity between 8 E2 and p300 in activation of the HPV8 P7535, we mutated either single or several E2 binding sites in various combinations. In addition to the five previously characterized E2 motifs (P0 to P4) (57), E2 also binds to an aberrant E2 binding site from position 100 to 110 within the NCR of HPV8 in vitro (B. Akgül, personal communication). Also this site, called P(5), was mutated. All mutations have been shown to result in loss of binding of 8 E2 (reference 57 and data not shown). For transient transfection we used 20 ng of 8 E2 expression vector, which yielded maximal activation and a strong cooperation with p300 in the experiment described in Fig. 2B. In the experiments shown in Fig. 3A, E2 and p300 also cooperated in P7535 activation; the promoter was stimulated 14-fold by both proteins. A point mutation within P2 increased activation by E2 on its own and cooperation with p300 (Fig. 3A). It has to be noted that p300 on its own only activated 2.5-fold when using reporter constructs with mutations in the repression-mediating E2 binding site P2. The mutation in P2 affects the binding of RUNX1, which has been shown to use p300 as a cofactor (26). Previously, the E2 binding sites P0 and P1 have been found to play a major role in E2-mediated activation (57). In accordance, the mutations of these sites almost eliminated cooperation between E2 and p300 (Fig. 3A). Thus, the results described in Fig. 3A suggest that the binding of E2 to P2 limits E2- and p300-mediated activation of the P7535, which occurs mostly by binding of E2 to the sites P0 and P1. However, when all known E2 binding sites had been mutated, E2 and p300 still retained cooperation in activation (Fig. 3A). We certainly cannot exclude the possibility that this activation by E2 and p300, while all known E2 binding sites are mutated, may be related to not-yet-characterized E2 binding sites or to residual binding of E2 to the mutated binding sites. In order to address this we used an E2 protein defective in DNA binding due to a point mutation within the C-terminal DBD. The E2 proteins are rather conserved within their N-terminal AD and C-terminal DBD, in contrast to the hinge region (16). The amino acids necessary for DNA contact have been identified in the case of BPV1 E2. A cysteine at position 340 has been shown to be essential for DNA binding. Converting this cysteine into an arginine abolished activation of transcription by BPV1 E2 (43). This cysteine is conserved within all E2 proteins (16). The change of this cysteine of 8 E2, here in position 430, into an arginine (8 E2 mt430), abolished binding to a high-affinity E2 binding site, as demonstrated by a gel shift with a bacterially expressed purified 8 E2DBD mt430 (Fig. 3C). Full-length 8 E2 mt430 could activate gene expression from the HPV8 NCR-Luc wt reporter fourfold. Coexpression of p300 enhanced this activation up to 14-fold (Fig. 3B), indicating that cooperativity can still be observed between 8 E2 mt430 and p300. Also a C-terminally truncated E2 protein was able to cooperate with coexpressed p300 (Fig. 3B), supporting the notion that DNA binding by E2 is not essential. The N-terminal AD is sufficient to activate and cooperate with p300, although in this case a greater variability from experiment to experiment was observed, which is also reflected by the error bars. The 8 E2ΔN, which lacks the AD, did not activate the HPV8 promoter in all concentrations tested. Also, the low activation by p300 alone was not increased after coexpression of 8 E2ΔN (Fig. 3B). Thus, the N-terminal AD is absolutely required and sufficient for cooperativity with p300 in vivo.
FIG. 3.
DNA binding by E2 is dispensable for cooperativity with p300. (A) RTS3b cells have been transfected with luciferase reporter constructs containing either the wt NCR of HPV8 (NCR8-Luc wt), an NCR with point mutations within the E2 binding sites P0 and P1 (NCR8-Luc mt 01), with a point mutation in P2 (NCR8-Luc mt 2), or with point mutations in all six E2 binding sites (NCR8-Luc mt0-5). Twenty nanograms of expression vector for 8 E2 or 500 ng of expression vector for p300 were transfected either alone or together, as indicated. The activities of the reporter constructs in the absence of E2 and p300 were set as 1, respectively. The bars represent the results of four independent experiments. The standard deviations (error bars) are given. (B) The reporter construct NCR8-Luc wt was cotransfected with expression vectors encoding either the wt E2 protein of HPV8 (E2), an E2 protein with a point mutation at position 430, abolishing DNA binding (E2 mt430); an E2 protein lacking the C-terminal DBD (E2ΔC); an E2 protein encoding the N-terminal AD (E2N); or an E2 protein lacking the N-terminal AD (E2ΔN). The amounts of the various E2 expression vectors are indicated in the figure. A plus sign indicates the addition of 500 ng expression vector for p300. The results represent the means of six independent experiments. (C) Gel shift analysis with 1 ng (lanes 5 and 9), 0.33 ng (lanes 4 and 8), 0.11 ng (lanes 3 and 7), and 0.037 ng (lanes 2 and 6) of His-tagged, bacterially expressed purified 8 E2 DBD (His-8 E2-C, lanes 2 to 5), or DBD with an exchange of the cysteine at position 430 into an arginine (His-E2-C mt 430, lanes 6 to 9). The probe was the promoter distal high-affinity E2 binding site of HPV18 (18-E2-4).
8 E2 binds via its AD, hinge, and DBD to p300.
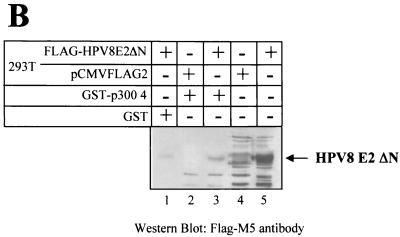
In order to get more insight into the mechanism of the cooperativity between E2 of HPV8 and p300 we mapped the domain within 8 E2 which is bound by p300 by performing GST-pull down experiments. GST fusion proteins encoding the AD, the hinge, and the DBD of 8 E2, respectively, were incubated with a 35S-labeled p300-HAT fragment, obtained by in vitro translation. As shown in Fig. 1B, this p300 part harbors the 8 E2 binding motif. p300-HAT was retained by the DBD and the hinge of 8 E2, fused to GST, respectively (Fig. 4A). Weak binding to the GST-AD fusion protein and only background binding to GST alone were observed. These interactions could be confirmed in a vice versa experiment with GST-p300-4 and in vitro-translated 35S-labeled AD, hinge, or DBD of 8 E2, respectively (data not shown). A weak binding of the AD of BPV1 E2 to p300 has already been reported (42) and may be conserved among E2 proteins, since the AD of 18 E2 also behaves similarly (Fig. 4A). 18 E2 also bound with its DBD to p300, in contrast to its hinge. Thus, the interaction of the nonconserved hinge with p300 may be specific for 8 E2. In order to confirm p300-binding mediated by domains C-terminal to the AD of 8 E2, we transiently transfected 293T cells with a vector encoding FLAG-tagged 8 E2ΔN and prepared whole cell extracts. As shown by the Western blot in Fig. 4B, in contrast to GST, the GST-p300-4 fusion protein was able to specifically precipitate the 8 E2ΔN protein from cell extracts.
FIG. 4.
8 E2 binds to p300 via its AD, hinge, and DBD. (A) 8 E2-GST fusion proteins encoding the AD (lane 2), the hinge (lane 3), and the DBD (lane 4), as well as HPV18 E2-GST fusion proteins of the AD (lane 7), the hinge (lane 8), and the DBD (lane 9) or GST alone (lanes 5 and 10), were incubated with 35S-labeled p300-HAT spanning the amino acids from 1195 to 1761 of p300. 10% of the p300-HAT used in each interaction assay was included on the SDS gel in lanes 1 and 6. Bound proteins were analyzed by autoradiography. The structures of the two E2 proteins, including the three domains fused to GST, are shown at the top. The numbers above the ORF refer to the amino acids of each E2 proteins. (B) 293T cells have been transfected with an expression vector for FLAG-tagged 8 E2ΔN, which lacks the AD (lanes 1, 3, and 5), or the empty vector pCMV2-FLAG (lanes 2 and 4), and whole-cell extracts were prepared 48 h later. These were incubated with GST (lane 1) or GST-p400-4 (lanes 2 and 3) bound to glutathione-Sepharose. The precipitated E2 protein was detected by Western blotting with the M5 monoclonal antibody directed against the FLAG epitope. 10% of the cell extracts used in the GST-pull down assay are shown in lanes 4 and 5, respectively.
The hinge and DBD of 8 E2 are able to mediate cooperativity between p300 and a heterologous AD.
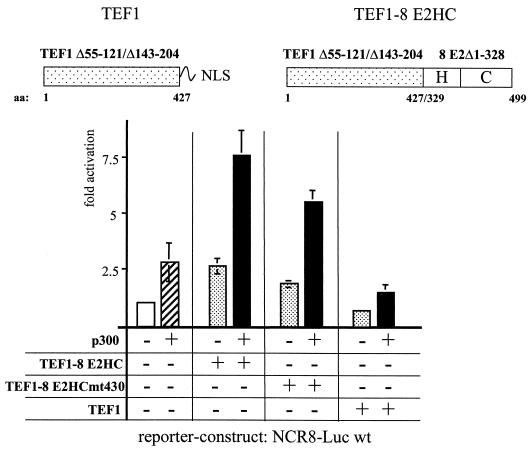
The data presented in Fig. 3B show that the AD on its own is necessary and sufficient to cooperate with p300, which correlates with a weak binding to p300 in vitro. We cannot draw any conclusion as to whether the hinge and the DBD play a role in functional cooperation with p300 in spite of their interaction in vitro, since an AD is absolutely required for activation and the AD of 8 E2 functionally interacts with p300, masking a possible contribution of the hinge and/or the DBD. To dissect the roles of the AD and the hinge and/or DBD in functional cooperation with p300, we replaced the AD of 8 E2 by a heterologous AD, for which p300 does not serve as a coactivator. It has been shown that the cellular transcription factor TEF1 neither binds to p300 in vitro nor does it cooperate with p300 (reference 47 and data not shown). We used a fusion protein composed of the AD of TEF1 (TEF1 Δ55-121/Δ143-204) (63) and part of the hinge and the DBD of 8 E2. This hybrid protein, TEF1-8 E2HC, stimulated transcription 2.5-fold from the HPV8-NCR wt reporter construct. Coexpression of p300 led to 7.4-fold enhancement, indicating that TEF1-8 E2HC is able to cooperate with p300. Also, a TEF1-8 E2HC mt430, defect in DNA binding cooperated with coexpressed p300. In contrast, the AD of TEF1 on its own, here called TEF1, was not able to activate nor could synergize with coexpressed p300 (Fig. 5). The lack of activation was not due to reduced expression or incorrect localization since we used a TEF1 AD fused to the NLS of the SV40 T antigen and confirmed the expression level and nuclear localization by immunofluorescence tests of transiently transfected cells (data not shown). Thus, the hinge and the DBD of 8 E2 enable the TEF1 AD to cooperate with p300.
FIG. 5.
The hinge and the DBD of 8 E2 are able to mediate cooperativity with p300. RTS3b cells have been transiently cotransfected with the NCR8-Luc wt reporter construct and a pXJ41-based expression vector (63), allowing the production of a fusion protein composed of the AD of TEF1 (comprising amino acids 1 to 427 lacking amino acids 55 to 121 and 143 to 204 [61]) and the C-terminal 171 amino acids of 8 E2, which represents part of the hinge and the wt DBD (TEF1-8 E2HC). The constructs TEF1-8 E2HC mt430, encoding the same fusion protein but with a point mutation within the DBD abolishing DNA binding, and TEF1, encoding the AD of TEF1 alone, were included, as indicated. In order to ensure proper nuclear localization, we fused the NLS of the SV40 T antigen to the TEF1 AD. The structures of the TEF1 and TEF1-8 E2HC proteins are shown at the top. The transfection of 500 ng of p300 expression vector is indicated. The results represent the average of four independent experiments, and the standard deviations (error bars) are given.
p300 is upregulated during keratinocyte differentiation.
Our data presented until now have demonstrated that E2 requires high amounts of p300 to strongly activate the P7535 promoter of HPV8, which is supposed to be the late promoter (56). Although CBP/p300 are believed to be expressed ubiquitously, tissue-specific changes in protein levels have been described (40). p300 was shown to be required during terminal keratinocyte differentiation in mouse by inducing the expression of the cyclin-dependent kinase inhibitor p21CIP1/WAF1 (36). However, the expression pattern of p300 within the human skin has not been examined. In order to determine the expression of p300 in the differentiating keratinocytes we analyzed the protein level of p300 by immunohistochemistry using a monoclonal antibody directed against p300 and cryo-sections of normal skin. As shown in Fig. 6, a negative control serum did not reveal any staining. The p300 antibody weakly stained the nuclei of the basal cells. Strong nuclear staining of the uppermost intact cell layer, belonging to the stratum granulosum could be observed, i.e., of the cells to which the expression of the late genes of HPV is restricted. This clearly demonstrates that the protein level of p300 increases during terminal differentiation of skin keratinocytes.
FIG. 6.
Differentiation-dependent up-regulation of p300 expression in normal skin. Immunohistochemical staining of 5-μm-thick cryo-sections from normal skin, derived from the upper arm, using either a monoclonal antibody directed against p300 or, as a negative control, IgG fraction from mouse. Sections were counterstained with Mayer's hematoxylin solution. Some positively stained cells are indicated.
DISCUSSION
The transcriptional coactivator p300 and CBP function to regulate transcription and chromatin. Through their interaction with many transcriptional regulators, particularly those involved in proliferation and differentiation, CBP/p300 integrate diverse differentiation and proliferation supporting signals. The data provided here suggest that also the E2 proteins of PV use these cellular integrators to regulate PV gene expression. We show that the E2 proteins of HPV8 and -18 bind p300 in vitro, in addition to BPV1 E2, as shown previously (42). Our mapping experiments reveal that the E2 proteins may differ in their interaction with p300 in vitro. In contrast to 8 E2, which only binds to one domain colocalizing with the HAT domain, the E2 proteins of BPV1 and HPV18 in addition recognize regions in the N and the C terminus of p300. In a previous study it has been shown that 18 E2 binds to the KIX region of CBP (28). We could not detect any interaction of 18 E2 and the corresponding region of p300, which should be present in our GST-p300-2 fusion protein. It remains to be tested whether E2 proteins differ in their binding to p300 and CBP. Here, we did not address the role of the interaction of E2 of HPV18 and BPV1 with the N- and the C-terminal regions of p300. We rather focused on the E2 protein of HPV8, since it only binds one domain of p300 in vitro. This interaction is direct, as demonstrated by the use of purified proteins produced in bacteria. We were not able to reveal a complex formation between 8 E2 and p300 by coimmunoprecipitation after transiently transfecting the corresponding expression vectors (data not shown). This failure might be due to the low expression level of both proteins, particularly of 8 E2, in the transfected cells. On the other side, the interaction between the two proteins might be too weak to be observed in such an assay. Also, activator-TAF interactions such as VP16-TAF31II or CREB-TAF130II are comparably weak, and complex formation between those proteins was not detectable in vivo by coimmunoprecipitation (1, 60). However, in GST-pull down experiments, GST-8 E2 bound endogenous p300 and vice versa, GST-p300 precipitated 8 E2ΔN from extracts of transiently transfected cells (Fig. 1C, and Fig. 4B), indicating that the interaction is not restricted to proteins produced in bacteria.
E2 and p300 also functionally interact, as demonstrated by transient-transfection assays. p300 may be a rate-limiting factor for activation by 8 E2, since an increase in the intracellular p300 concentration enhances activation by E2 up to a level beyond that obtained by saturating amounts of E2. The cooperation between 8 E2 and p300 was much stronger on a natural HPV8 promoter than on a synthetic minimal promoter composed of E2 and Sp1 binding sites only. This implies that cellular factors binding to the NCR of HPV8 play a role in mediating cooperativity between 8 E2 and p300. The NCR of HPV8 encodes binding sites for a variety of cellular transcription factors, like AP1, NF1, YY1, RUNX1, and papillomavirus-binding factor (5, 14, 39, 45). Most of these factors also use CBP/p300 as coactivators (26; reviewed in references 46 and 62). It is possible that one or several of these cellular factors and E2, bound to the NCR, cooperate in recruiting p300 leading to increased transcription. A synergism between activator proteins binding to different domains of p300 has been described (35). Our mutational analysis demonstrates that the E2 binding sites P0 and P1 are mostly responsible for cooperative activation with p300, whereas binding of E2 to P2 leads to its inhibition (Fig. 3B). The sites P0 and P1 have been identified previously as mediating the stimulatory effect of E2 on the P7535 of HPV8 (57), indicating that the activation by E2 may involve binding to endogenous p300. Strange to say, the cooperativity between E2 and p300 was dependent on the passage of cells used for the transient-transfection assays. In cells which have been kept for many passages in culture, overexpression of p300 did not lead to activation. This correlated with a reduced cooperation between E2 and p300 (unpublished observation). We speculate that the level of endogenous p300 will change with increasing cell culture time with the consequence that elevated concentrations of endogenous p300 may mask the effect of overexpression of p300. However, other control mechanisms involving p300 may become affected after prolonged proliferation of these cell lines.
Surprisingly, in the absence of DNA binding, E2 can still activate and cooperate with coexpressed p300 (Fig. 3A and B). Residual DNA binding by E2 can be excluded since we used an E2 with a mutation abolishing DNA binding (8 E2 mt 430) to a high-affinity site in vitro (Fig. 3C) and two E2 deletion mutants lacking the DBD (E2AD and E2ΔC). In these cases, E2 must be tethered to the promoter via protein-protein interaction. It is possible that activator proteins, like YY1, which interact with E2 and their sites within the HPV8 NCR, recruit E2 (29, 39; M. Adam, personal communication). Alternatively, E2 may be tethered to the promoter via p300, complexed by the cellular factors, which bind to the NCR of HPV8 and use p300 as cofactor, like YY1, RUNX1, and AP1 (reviewed in references 46 and 62). We favor the latter possibility, since we can correlate interactions in vitro and cooperation with coexpressed p300 in transient transfections. The N-terminal AD of 8 E2 is able to cooperate with p300, although its binding to p300 seems to be weak in our in vitro assay. However, within the cells, the interaction might be stronger due to the presence of enhancing factors. For example the interaction between the AD and p300 may be enhanced by AMF-1/Gps2, as shown for BPV1 E2 (42). The E2 protein lacking the AD, 8 E2ΔN, is not able to activate, although the hinge and the C terminus of HPV8 bind to p300 in vitro, indicating that interaction with p300 is not sufficient for activation. This is in line with a previous report demonstrating the recruitment of p300 to the enhanceosome to be required for synergistic activation but not sufficient. In addition, the ADs of the transcription factors binding to the enhancer may contribute with critical interactions with basal factors (32; reviewed in reference 62). Thus, the failure of 8 E2ΔN to cooperate may be due to the lack of domains essential for activation localized within the AD. For example, the contacts with components of the PIK, like TBP or TFIIB, or with other factors which have been shown for of other E2 proteins may be essential (3, 34, 66). These functions may also be important when E2 is tethered to the promoter via interaction with activator bound p300. The involvement of the hinge and DBD of 8 E2 in cooperation with p300 was evaluated by a hybrid protein composed of part of the hinge and the DBD of 8 E2 and the AD of TEF1 (TEF1-8 E2HC). This is suitable since the TEF1-AD does not bind to p300 (47; also data not shown) nor is it able to cooperate with coexpressed p300, in contrast to the AD of E2 (Fig. 3B and 5). Thus, the cooperation of the hybrid protein must be mediated by the hinge and/or DBD of 8 E2. Also here, DNA binding is not necessary, since the mutant TEF1-8 E2HC mt430, defective in DNA binding, still activates and cooperates with p300. The lower effect of TEF1-8 E2HC or TEF1-8 E2HC mt430 compared to wt E2 may be due to an impaired function of the TEF1 AD in RTS3b cells, the cells suitable for HPV8 gene expression. This is indicated by our observation that TEF1-8 E2HC is able to activate a synthetic promoter containing E2 binding sites much stronger in Cos 7 cells than in RTS3b cells (data not shown). Taken together, complex formation between E2 and p300 may be stabilized by multiple surface contacts, allowing 8 E2 to be tethered to the promoter when sequence specific DNA binding is not possible. Thus, E2 may use p300 as a classical coactivator and as an anchor to reach promoters in the absence of E2 binding sites. However, p300 may not be the only platform to gain access to promoters without E2 binding sites. We were able show recently that the hinge of 8 E2 directly binds to Sp1 and can activate the promoter of the cyclin dependent kinase inhibitor p21WAF1/CIP1 via Sp1 (53).
Previously, it has been suggested that E2 is a moderate activator of HPV promoters under control of their homologous regulatory sequences. This activation turns into repression when the amount of E2 increases, since repression-mediating E2 binding sites usually reveal a lower affinity for E2 compared to activating sites (8, 23, 52, 54, 57). Repression occurs by binding to promoter proximal binding sites which overlap with sites for cellular transcription factors involved in promoter activity (5, 10, 23, 45, 52, 55, 57-59). We can show here that the slight activation of the HPV8 P7535 by low amounts of E2 observed previously in the keratinocyte cell line RTS3b (57) seems to be determined by the concentration of cellular factors required for activation by E2 in these non differentiated cells. Low amounts of E2 may activate much more strongly when the amount of intracellular p300 increases. Again, at high E2 concentrations E2 binds to the low affinity binding site P2, resulting in promoter repression by displacement of cellular factors necessary for promoter activity. As suggested by the data shown in Fig. 6, the expression level of p300 in normal skin strongly increases during keratinocyte differentiation, with the highest expression level within the stratum granulosum. Thus, the overexpression of p300 in RTS3b cells, resulting in strong activation of P7535 by E2, may mimic the situation in differentiating skin regarding p300 levels. Our data provide the first time evidence for a role of E2 in significant activation of HPV gene expression. The P7535 of HPV8 was shown to encode in addition to E2 specific transcripts also late transcripts (56). Activation of HPV late gene expression is restricted to the differentiated cells of the stratum spinosum and stratum granulosum and thus correlates with increased expression levels of p300 in normal skin.
Acknowledgments
We thank David M. Livingston, Michael May, and Frank Stubenrauch for providing plasmid DNAs; Carsta Schnabel for excellent technical assistance in some experiments; and Herbert Pfister for critical reading of the manuscript and helpful discussions.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 274, A8, STE 604/3-1).
REFERENCES
- 1.Asahara, H., B. Santoso, E. Guzman, K. Du, P. A. Cole, I. Davidson, and M. Montminy. 2001. Chromatin-dependent cooperativity between constitutive and inducible activation domains in CREB. Mol. Cell. Biol. 21:7892-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson, J. D., and P. M. Howley. 1995. Amino-terminal domains of the bovine papillomavirus type 1 E1 and E2 proteins participate in complex formation. J. Virol. 69:4364-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson, J. D., R. Lawande, and P. M. Howley. 1997. Conserved interaction of the papillomavirus E2 transcriptional activator proteins with human and yeast TFIIB proteins. J. Virol. 71:8041-8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, M., and A. Stenlund. 1997. Functional interactions between papillomavirus E1 and E2 proteins. J. Virol. 71:3853-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeckle, S., H. Pfister, and G. Steger. 2002. A new cellular factor recognizes E2 binding sites which mediate transcriptional repression by E2. Virology 293:103-117. [DOI] [PubMed] [Google Scholar]
- 6.Breiding, D. E., F. Sverdrup, M. J. Grossel, N. Moscufo, W. Boonchai, and E. J. Androphy. 1997. Functional interaction of a novel cellular protein with the papillomavirus E2 transactivation domain. Mol. Cell. Biol. 17:7208-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, H., R. J. Lin, R. L. Schiltz, D. Chakravarti, A. Nash, L. Nagy, M. L. Privalski, Y. Nakatani, and R. M. Evans. 1997. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569-580. [DOI] [PubMed] [Google Scholar]
- 8.Demeret, C., C. Desaintes, M. Yaniv, and F. Thierry. 1997. Different mechanisms contribute to the E2-mediated transcriptional repression of human papillomavirus type 18 viral oncogenes. J. Virol. 71:9343-9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demeret, C., M. Yaniv, and F. Thierry. 1994. The E2 transcriptional repressor can compensate for SP1 activation of the human papillomavirus type 18 early promoter. J. Virol. 68:7075-7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong, G., T. R. Broker, and L. T. Chow. 1994. Human papillomavirus type 11 E2 proteins repress the homologous E6 promoter by interfering with the binding of host transcription factors to adjacent elements. J. Virol. 68:1115-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckner, R., M. E. Ewen, D. Newsome, M. Gerdes, J. A. DeCaprio, J. B. Lawrence, and D. M. Livingston. 1994. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 8:869-884. [DOI] [PubMed] [Google Scholar]
- 12.Enzenauer, C., G. Mengus, A.-C. Lavigne, I. Davidson, H. Pfister, and M. May. 1998. Interaction of human papillomavirus 8 regulatory proteins E2, E6 and E7 with components of the TFIID complex. Intervirology 41:80-90. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson, M. K., and M. R. Botchan. 1996. Genetic analysis of the activation domain of bovine papillomavirus protein E2: its role in transcription and replication. J. Virol. 70:4193-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs, P. G., and H. Pfister. 1997. Molecular biology of HPV and mechanisms of keratinocyte transformation. CRC Press, Boca Raton, Fla.
- 15.Gillitzer, E., G. Chen, and A. Stenlund. 2000. Separate domains in E1 and E2 proteins serve architectural and productive roles for cooperative DNA binding. EMBO J. 19:3069-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giri, I., and M. Yaniv. 1988. Structural and mutational analysis of E2 trans-activating proteins of papillomaviruses reveals three distinct functional domains. EMBO J. 7:2823-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595-606. [DOI] [PubMed] [Google Scholar]
- 18.Ham, J., N. Dostatni, F. Arnos, and M. Yaniv. 1991. Several different upstream promoter elements can potentiate transactivation by the BPV-1 E2 protein. EMBO J. 10:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ham, J., G. Steger, and M. Yaniv. 1994. Cooperativity in vivo between the E2 transactivator and the TATA box binding protein depends on core promoter structure. EMBO J. 13:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugen, T. H., L. Turek, F. M. Mercurio, T. P. Cripe, B. J. Olson, R. D. Anderson, D. Seidl, M. Karin, and J. Schiller. 1988. Sequence-specific and general transcriptional activation by the bovine papillomavirus-1 E2 trans-activator require an N-terminal amphipathic helix-containing E2 domain. EMBO J. 7:4245-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heike, T., S. Miyatake, M. Yoshida, K.-i. Arai, and A. Naoka. 1989. Bovine papilloma virus encoded E2 protein activates lymphokine genes through DNA elements, distinct from the consensus motif, in the long control region of its own genome. EMBO J. 8:1411-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann, A., and R. G. Roeder. 1991. Purification of his-tagged proteins in non-denaturing conditions suggests a convenient method for protein interaction studies. Nucleic Acids Res. 19:6337-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson, M. E., and M. S. Campo. 1995. Both viral E2 protein and the cellular factor PEBP2 regulate transcription via E2 consensus sites within the bovine papillomavirus type 4 long control region. J. Virol. 69:6038-6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamei, Y., L. Xu, T. Heinzel, J. Torchia, R. Kurokawa, B. Gloss, S.-C. Lin, R. A. Heyman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414. [DOI] [PubMed] [Google Scholar]
- 25.Kingston, R. E., and G. J. Narlikar. 1999. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 13:2339-2352. [DOI] [PubMed] [Google Scholar]
- 26.Kitabayashi, I., A. Yokoyama, K. Shimizu, and M. Ohki. 1998. Interaction and functional cooperation of the leukemia-associated factors AML-1 and p300 in myeloid cell differentiation. EMBO J. 17:2994-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovelman, R., G. K. Bilter, E. Glezer, A. Y. Tsou, and M. S. Barbosa. 1996. Enhanced transcriptional activation by E2 proteins from the oncogenic human papillomaviruses. J. Virol. 70:7549-7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, D., B. Lee, J. Kim, D. W. Kim, and J. Choe. 2000. cAMP response element binding protein-binding protein binds to human papillomavirus E2 protein and activates E2 dependent transcription. J. Biol. Chem. 275:7045-7051. [DOI] [PubMed] [Google Scholar]
- 29.Lee, K. Y., T. R. Broker, and L. T. Chow. 1998. Transcription factor YY1 represses cell-free replication from human papillomavirus origins. J. Virol. 72:4911-4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mal, A., M. Sturniolo, R. L. Schiltz., M. K. Ghosh, and M. L. Harter. 2001. A role for histone deacetylase HDAC1 in modulating the transcriptional activity of MoyD: inhibition of the myogenic program. EMBO J. 20:1739-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masterson, P. J., M. A. Stanley, A. P. Lewis, and M. A. Romanos. 1998. A C-terminal helicase domain of the human papillomavirus E1 protein binds E2 and the DNA polymerase a-primase p68 subunit. J. Virol. 72:7407-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merika, M., A. J. Williams, G. Chen, T. Collins, and D. Thanos. 1998. Recruitment of CBP/p300 by the IFNβ enhanceosome is required for synergistic activation of transcription. Mol. Cell 1:277-287. [DOI] [PubMed] [Google Scholar]
- 33.Meyers, C., M. G. Frattini, and L. A. Laimins. 1994. Tissue culture techniques for the study of human papillomaviruses in stratified epithelia. Academic Press, Orlando, Fla.
- 34.Miller Rank, N., and P. F. Lambert. 1995. Bovine papillomavirus type 1 E2 transcriptional regulators directly bind two cellular transcription factors, TFIID and TFIIB. J. Virol. 69:6323-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mink, S., B. Haenig, and K.-H. Klempnauer. 1997. Interaction and functional collaboration of p300 and C/EBPβ. Mol. Cell. Biol. 17:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Missero, C., E. Calautti, R. Eckner, J. Chin, L. H. Tsai, D. M. Livingston, and G. P. Dotto. 1995. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc. Natl. Acad. Sci. USA 92:5451-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohr, I. J., R. Clark, S. Sun, E. J. Androphy, P. MacPherson, and M. R. Botchan. 1990. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science 250:1694-1699. [DOI] [PubMed] [Google Scholar]
- 38.Ogryzko, V. V., R. L. Schiltz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 39.Pajunk, H. S., C. May, H. Pfister, and P. G. Fuchs. 1997. Regulatory interactions of transcription factor YY1 with control sequences of the E6 promoter of human papillomavirus type 8. J. Gen. Virol. 78:3287-3295. [DOI] [PubMed] [Google Scholar]
- 40.Partanen, A., J. Motoyama, and C. C. Hui. 1999. Developmentally regulated expression of the transcriptional cofactors/histone acetyltransferases CBP and p300 during mouse embryogenesis. Int. J. Dev. Biol. 43:487-494. [PubMed] [Google Scholar]
- 41.Patel, D., S.-M. Huang, L. A. Baglia, and D. J. McCance. 1999. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 18:5061-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng, Y.-C., D. E. Breiding, F. Sverdrup, J. Richard, and E. J. Androphy. 2000. AMF-1/Gps2 binds p300 and enhances its interaction with papillomavirus E2 proteins. J. Virol. 74:5872-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakash, S. S., S. R. Grossman, R. B. Pepinsky, L. A. Laimins, and E. J. Androphy. 1992. Amino acids necessary for DNA contact and dimerization imply novel motifs in the papillomavirus E2 trans-activator. Genes Dev. 6:105-116. [DOI] [PubMed] [Google Scholar]
- 44.Purdie, K. J., C. J. Sexton, C. M. Proby, M. T. Glover, A. T. Williams, J. N. Stables, and I. M. Leigh. 1993. Malignant transformation of cutaneous lesions in renal allograft patients: a role for human papillomavirus. Cancer Res. 53:5328-5333. [PubMed] [Google Scholar]
- 45.Schmidt, H.-M., G. Steger, and H. Pfister. 1997. Competitive binding of viral E2 protein and mammalian core-binding factor to transcriptional control sequences of human papillomavirus type 8 and bovine papillomavirus type 1. J. Virol. 71:8029-8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shikama, N., J. Lyon, and N. Thangue. 1997. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 7:230-236. [DOI] [PubMed] [Google Scholar]
- 47.Slepak, T. I., K. A. Webster, J. Zang, H. Prentice, A. O'Dowd, M. N. Hicks, and N. H. Bishopric. 2001. Control of cardiac-specific transcription by p300 through myocyte enhancer factor 2D. J. Biol. Chem. 276:7575-7585. [DOI] [PubMed] [Google Scholar]
- 48.Spalholz, B. A., P. F. Lambert, C. L. Lee, and P. M. Howley. 1987. Bovine papillomavirus transcriptional regulation: localization of the E2-responsive elements of the long control region. J. Virol. 61:2128-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spalholz, B. A., S. B. Vande Pol, and P. M. Howley. 1991. Characterization of the cis elements involved in basal and E2 transactivated expression of the bovine papillomavirus P2443 promoter. J. Virol. 65:743-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spalholz, B. A., Y. C. Yang, and P. M. Howley. 1985. Transactivation of a bovine papillomavirus transcriptional regulatory element by the E2 gene product. Cell 42:183-191. [DOI] [PubMed] [Google Scholar]
- 51.Spencer, T. E., G. Jenster, M. M. Burcin, C. D. Allis, J. Zhou, C. A. Mizzen, N. J. McKenna, S. A. Onate, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1997. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389:194-198. [DOI] [PubMed] [Google Scholar]
- 52.Steger, G., and S. Corbach. 1997. Dose-dependent regulation of the early promoter of human papillomavirus type 18 by the viral E2 protein. J. Virol. 71:50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steger, G., C. Schnabel, and H.-M. Schmidt. 2002. The hinge region of the human papillomavirus type 8 E2 protein activates the human p21 WAF1/CIP1 promoter via interaction with Sp1. J. Gen. Virol. 83:503-510. [DOI] [PubMed] [Google Scholar]
- 54.Stenlund, A., and M. R. Botchan. 1990. The E2 trans-activator can act as a repressor by interfering with a cellular transcription factor. Genes Dev. 4:123-136. [DOI] [PubMed] [Google Scholar]
- 55.Stubenrauch, F., I. Leigh, and H. Pfister. 1996. E2 represses the late gene promoter of human papillomavirus type 8 at high concentrations by interfering with cellular factors. J. Virol. 70:119-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stubenrauch, F., J. Malejczyk, P. G. Fuchs, and H. Pfister. 1992. Late promoter of human papillomavirus type 8 and its regulation. J. Virol. 66:3485-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stubenrauch, F., and H. Pfister. 1994. Low-affinity E2 binding site mediates downmodulation of E2 transactivation of the human papillomavirus type 8 late promoter. J. Virol. 68:6959-6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan, S. H., B. Gloss, and H.-U. Bernard. 1992. During negative regulation of the human papillomavirus-16 E6 promoter, the viral E2 protein can displace Sp1 from a proximal promoter element. Nucleic Acids Res. 20:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thierry, F., and P. Howley. 1991. Functional analysis of E2-mediated repression of the HPV18 P105 promoter. New Biol. 3:90-100. [PubMed] [Google Scholar]
- 60.Uesugi, M., O. Nyanguile, H. Lu, A. J. Levine, and G. L. Verdine. 1997. Induced alpha helix in the VP16 activation domain upon binding to human TAF. Science 277:1310-1313. [DOI] [PubMed] [Google Scholar]
- 61.Ushikai, M., M. J. Lace, Y. Yamakawa, M. Kono, J. Anson, et al. 1994. trans activation by the full-length E2 protein of human papillomavirus type 16 and bovine papillomavirus type 1 in vitro and in vivo: cooperation with activation domains of cellular transcription factors. J. Virol. 68:6655-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vo, N., and R. H. Goodman. 2001. CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 276:13505-13508. [DOI] [PubMed] [Google Scholar]
- 63.Xiao, J. H., I. Davidson, H. Matthes, J.-M. Garnier, and P. Chambon. 1991. Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell 65:551-568. [DOI] [PubMed] [Google Scholar]
- 64.Yang, X. J., V. V. Ogryzko, J. Nishikawa, B. H. Howard, and Y. Nakatani. 1996. A p300/CBP-associated factor that competes with adenoviral oncoprotein E1A. Nature 382:319-324. [DOI] [PubMed] [Google Scholar]
- 65.Yang, Y.-C., H. Okayama, and P. M. Howley. 1985. Bovine papillomavirus contains multiple transforming genes. Proc. Natl. Acad. Sci. USA 82:1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao, J.-M., D. E. Breiding, and E. J. Androphy. 1998. Functional interaction of the bovine papillomavirus E2 transactivation domain with TFIIB. J. Virol. 72:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao, T.-P., S. P. Oh, M. Fuchs, N.-D. Zhou, L.-E. Ch'ng, D. Newsome, R. T. Bronson, E. Li, D. M. Livingston, and R. Eckner. 1998. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93:361-372. [DOI] [PubMed] [Google Scholar]
- 68.Yao, Y.-L., W.-M. Yang, and E. Seto. 2001. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol. Cell. Biol. 21:5979-5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmermann, H., R. Degenkolbe, H.-U. Bernard, and M. J. O'Connor. 1999. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J. Virol. 73:6209-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmermann, H., C.-H. Koh, R. Degenkolbe, M. J. O'Connor, A. Müller, G. Steger, J. J. Chen, Y. Lui, E. J. Androphy, and H.-U. Bernard. 2000. Interaction with CBP/p300 enables the bovine papillomavirus type 1 E6 protein to downregulate CBP/p300 mediated transcativation by p53. J. Gen. Virol. 81:2617-2623. [DOI] [PubMed] [Google Scholar]