Abstract
The human immunodeficiency virus type 1 (HIV-1) Tat protein is essential for viral replication and stimulates transcription of the integrated provirus by recruiting the kinase complex TAK/P-TEFb, composed of cyclin T1 (CycT1) and Cdk9, to the viral TAR RNA element. TAK/P-TEFb phosphorylates the RNA polymerase II complex and stimulates transcriptional elongation. In this report, we investigated the regulation of TAK/P-TEFb in primary human macrophages, a major target cell of HIV infection. While Cdk9 levels remained constant, CycT1 protein expression in freshly isolated monocytes was very low, increased early during macrophage differentiation, and, unexpectedly, decreased to very low levels after about 1 week in culture. The kinase activity of TAK/P-TEFb paralleled the changes in CycT1 protein expression. RNA analysis indicated that the transient induction of CycT1 protein expression involves a posttranscriptional mechanism. In transient transfection assays, the ability of Tat to transactivate the HIV long terminal repeat (LTR) in the late differentiated macrophages was greatly diminished relative to its ability to transactivate the HIV LTR in early differentiated cells, strongly suggesting that CycT1 is limiting for Tat function in late differentiated macrophages. Interestingly, lipopolysaccharide, a component of the cell wall of gram-negative bacteria, reinduced CycT1 expression late in macrophage differentiation. These results raise the possibility that regulation of CycT1 expression may be involved in establishing latent infection in macrophages and that opportunistic infection may reactivate the virus by inducing CycT1 expression.
The two major cell types targeted by human immunodeficiency virus type 1 (HIV-1) are helper T cells and macrophages, as these cell types express CD4 and a chemokine receptor, either CCR5 or CXCR4, on their surfaces. CD4 is the primary receptor for the virus, while CCR5 or CXCR4 serves as a coreceptor that mediates fusion of the enveloped virion with the cell membrane. In most individuals, HIV-1 infection leads to an inevitable erosion of the immune system and development of AIDS. Analyses of patients treated with drugs that effectively block HIV-1 replication in vivo have revealed that infected CD4+ T cells produce the majority (>90%) of virus during the prolonged infection period before the onset of AIDS (reviewed in reference 4). These infected CD4+ T cells are short-lived, with a half-life estimated to be <1 day due to direct cytopathic effects of infection and to clearance of infected cells by immune-mediated mechanisms, especially that of cytotoxic CD8+ T lymphocytes. A second population of productively infected cells has a half-life estimated to be 1 to 4 weeks; this population produces a few percent of virus in infected individuals prior to AIDS. Although the nature of this longer-lived population of infected cells is uncertain, macrophages are a likely candidate cell type, as they are resistant to the cytopathic effects of infection in vitro and the half-life of tissue macrophages has been estimated to be about 2 weeks (19, 28, 41). Interestingly, at late stages of AIDS the percentage of infected cells that are macrophages increases significantly in patients who have opportunistic infections (31).
Replication of HIV-1 requires the virus-encoded transactivator protein known as Tat. Tat acts to stimulate RNA polymerase II (Pol II) transcription directed by the 5′ long terminal repeat (LTR) of the integrated provirus (reviewed in references 9, 20, and 40). The transactivation function of Tat is mediated by a cellular protein kinase complex termed TAK/P-TEFb (Tat-associated kinase/positive transcription elongation factor b), which binds along with Tat to TAR RNA, a 59-nucleotide stem-loop structure located at the 5′ ends of all nascent HIV-1 transcripts. Upon binding to TAR RNA, TAK/P-TEFb is believed to stimulate the elongation of transcription by hyperphosphorylating the carboxyl-terminal domain (CTD) of Pol II and perhaps other substrates of the transcription complex (for reviews, see references 9, 20, 34, and 40).
TAK/P-TEFb is composed of cyclin T1 (CycT1) and cyclin-dependent kinase 9 (Cdk9). The CycT1 subunit of the TAK/P-TEFb complex interacts directly with the transactivation domain of Tat to form a zinc-dependent complex that is important for the species-specific activation of HIV-1 transcription (3, 8, 11). CycT1 and Cdk9 are also subunits of the general positive transcription elongation activity termed P-TEFb (24, 32, 43, 44, 47). There are multiple P-TEFb complexes in human cells that contain Cdk9 but that differ according to their cyclin regulatory subunits, CycT1, -T2a, -T2b, and -K; only CycT1 is capable of interaction with the Tat protein. Cdk9, CycT1, CycT2a, and CycT2b localize to splicing-factor-rich nuclear speckle structures in HeLa cells (17, 25; data not shown), suggesting that the P-TEFb complex may couple transcription elongation with mRNA processing.
Although macrophages and CD4+ T lymphocytes are the major targets for HIV-1 infection, the susceptibilities to HIV-1 infection are different in these two cell types and depend on the activation state of cells (30). Activated CD4+ T lymphocytes are more susceptible to HIV-1 infection than quiescent T cells. Likewise, monocyte-derived macrophages (MDMs) are more susceptible to productive HIV-1 infection than freshly isolated monocytes (36). The limiting factors for productive infection of HIV-1 in different cell types have not been clearly defined. Because TAK/P-TEFb is essential for Tat function, under some circumstances it may be one of the limiting factors for HIV transcription and replication in these cell types. Therefore, an important topic for investigation is the regulation of TAK/P-TEFb function in CD4+ T lymphocytes and monocytes/macrophages.
Previous studies have shown that the levels of CycT1 and CDK9 mRNA and protein increase when quiescent primary T cells are activated by either phytohemagglutinin, phorbol 12-myristate 13-acetate (PMA), or antibodies (Abs) to CD3 and CD28 (12, 15). Additionally, recent results have shown that combinations of cytokines can induce CycT1 and Cdk9 protein levels and TAK/P-TEFb kinase activity in quiescent CD4+ T cells with only minimal effects on T-cell activation markers and no induction of cellular proliferation (13).
TAK/P-TEFb is also induced in activated promonocytic cell lines studied as model systems of macrophage differentiation, and the induction correlates with increased HIV-1 replication in those cell lines (44). The induction of TAK/P-TEFb in promonocytic cell lines occurs by a mechanism distinct from that in activated primary T lymphocytes (15). The expression of Cdk9 in promonocytic cell lines HL-60 and U937 is high and remains at a high level when these cells are activated to differentiate into macrophage-like cells by PMA treatment. However, CycT1 is expressed at a low constitutive level in these two cell lines, but it is induced to a high level following PMA treatment. Interestingly, CycT1 mRNA levels decrease slightly during differentiation, indicating that the induction of CycT1 expression in promonocytic cell lines involves a posttranscriptional mechanism. However, U937 and HL-60 cells are transformed cell lines that may show regulatory patterns different from those of primary monocytes/macrophages. Therefore, it is important to investigate the regulation of TAK/P-TEFb function in the natural target cells, human primary monocytes/macrophages.
In this report, we present the unexpected observation based on results for numerous blood donors that CycT1 protein levels, and therefore TAK/P-TEFb function, are induced transiently during MDM differentiation in vitro. In freshly isolated monocytes, CycT1 protein levels are low and CycT1 is induced during the first few days of MDM differentiation. However, after approximately 7 to 10 days in culture, CycT1 protein levels decline until they are very low. In agreement with this transient induction of the CycT1 protein, TAK/P-TEFb kinase function and Tat transactivation of the HIV-1 LTR are also transiently induced during MDM differentiation. Reverse transcription-PCR (RT-PCR) analysis of CycT1 mRNA levels revealed that a posttranscriptional mechanism is involved in the transient induction of the CycT1 protein during differentiation. We further found that lipopolysaccharide (LPS), a component of the cell wall in gram-negative bacteria, can induce CycT1 expression after it has declined in late MDMs. Our results suggest that fluctuation in CycT1 levels during human macrophage differentiation may be involved in the regulation of HIV-1 replication.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation (Isolymph [Gallard/Schlesinger] or Ficoll-Paque [Pharmacia]) from healthy HIV-seronegative blood donors (obtained from the Gulf Coast Regional Blood Center) (5). Monocytes were isolated either by an adherence method or by positive or negative selection. For the adherence method, 3 × 106 PBMC/ml in RPMI 1640 (Life Technologies) supplemented with 1% human serum (Sigma) and antibiotics were seeded onto 10-cm-diameter plastic cell culture dishes (Sarstedt) and incubated at 37°C for 1 h. Nonadherent cells were removed by washing vigorously with prewarmed phosphate-buffered saline (PBS). Adherent cells were then incubated with RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and antibiotics for another 2 h. After washes with prewarmed PBS, cells were cultured in the medium described above plus granulocyte-macrophage colony-stimulating factor (GM-CSF; 10 U/ml; R&D Systems) and then incubated at 37°C until various time points of the experiments. Positive and negative selections were performed according to the manufacturer's protocols (Miltenyi Biotec). Briefly, positive selection was performed by binding to an anti-CD14 Ab conjugated to magnetic beads and passing through a magnetic column. CD14-positive cells that were retained on the column were released from the column by removal of the magnet adapter. Negative selection used a combination of monoclonal Abs (Abs against CD3, CD7, CD19, CD45RA, and CD56 and an anti-immunoglobulin E [IgE] Ab) to deplete B and T lymphocytes and NK cells. Monocytes isolated by positive and negative selection were incubated with RPMI 1640 supplemented with 1% human serum and antibiotics at 37°C for 1 h to allow adherence to tissue culture dishes and then incubated in RPMI 1640 supplemented with 10% FBS, antibiotics, and GM-CSF (10 U/ml) at 37°C. To ensure the reproducibility of our observations, we examined monocytes isolated from more than 30 different donors, and typical results are presented here. Because of limiting amounts of sample obtained from a single donor, it was often not possible to perform all assays with the same donor. To stimulate MDMs with LPS (Sigma), LPS at a final concentration of 0.1 μg/ml was added in the culture medium.
To generate dendritic cells (DC) from monocytes, day 1 monocytes were incubated within 800 U of GM-CSF/ml and 1,000 U of interleukin-4 (IL-4)/ml. After 2 days of incubation, culture media were replaced by RPMI 1640 medium supplemented with 10% FBS and antibiotics containing 800 U of GM-CSF/ml and 500 U of IL-4/ml. For maturation of DC, cells were treated at day 5 with 200 U of tumor necrosis factor alpha (TNF-α)/ml and then incubated for three additional days.
Flow cytometry analysis of monocyte preparations.
To monitor the purity of monocyte preparations, several surface markers were analyzed by flow cytometry. Monocytes/macrophages were incubated for 15 min at 4°C in PBS containing 2% FBS, with fluorescein isothiocyanate- or phycoerythrin-conjugated Abs for monocyte and macrophage markers: CD11c (the α subunit of integrin complement receptor 4, CR4), CD14 (LPS receptor), and CD68 (gp110). Additionally, CD3 was analyzed to monitor contamination of T lymphocytes in monocyte/macrophage preparations. Abs against CD3, CD14, CD11c were purchased from Pharmingen, and Abs against CD68 was purchased from DAKO. Stained cells were analyzed by flow cytometry using a Coulter XL-MCL flow cytometer. By the adherence method, approximately 50 to 60% of cells in the population were found to be monocytes on the day of isolation (day 0), and the percentage of monocytes increased to 70 to 80% by 1 day of culture (day 1); after day 3, cultures contained >90% monocytes. Monocytes isolated by negative selection were found to be >90% pure at day 0.
Immunoblots.
Cell extracts were prepared by incubating cells in lysis buffer (50 mM Tris [pH 8.0], 120 mM NaCl, 0.5% NP-40) containing protease inhibitors (2 μg of aprotinin/ml, 1 μg of leupeptin/ml, and 2.5 mM phenylmethylsulfonyl fluoride) as described previously (18). Protein concentrations were determined by a Bio-Rad protein assay, and 20 μg of total protein was loaded onto 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels. The procedure for immunoblotting using enhanced chemiluminescence for detection was described previously (16). The Ab against β-actin was purchased from Sigma and used at a dilution of 1:3,000. Other Abs were purchased from Santa Cruz Biotechnology. CycT1 and CycC Abs were used at a dilution of 1:1,000, and Cdk7, Cdk8, and Cdk9 Abs were used at a dilution of 1:5,000. Cell extracts were also prepared by lysing cells directly in 2× SDS sample buffer, and results for immunoblots were identical to those for cell extracts prepared as described above.
Kinase assays.
Kinase assays were performed as described previously (18). Briefly, HIV-2 Tat (Tat-2) protein was expressed in Escherichia coli as a glutathione S-transferase (GST) fusion protein and purified by binding to glutathione-Sepharose beads. The GST-Tat-2-Sepharose bead complexes were incubated with 50 μg of cell extracts for 60 min at 4°C. After being washed with buffer (50 mM Tris [pH 7.4], 120 mM NaCl, 0.5% NP-40, 0.03% SDS), the complexes were incubated for 60 min at room temperature in reaction buffer (50 mM Tris-HCl [pH 7.4], 5 mM MgCl2, 2.5 mM MnCl2, 5 μM ATP, 5 μCi of [γ-32P]ATP [3,000 Ci/mmol]) in the presence of GST-CTD (200 ng) substrate. Products of kinase reactions were analyzed on SDS-9% polyacrylamide gels.
Transfection and luciferase assay.
Lipofectamine 2000 (Invitrogen Life Technology) was used as recommended by the manufacturer for plasmid transfections in MDM cultures (6-cm-diameter culture dishes). For each plasmid, 5 μg was used. Plasmids used in the study encoding HIV-1 LTR-luciferase, cytomegalovirus (CMV) immediate-early (IE) vector and CMV IE wild-type Tat-1 (86-residue protein), and thymidine kinase (TK)-Renilla luciferase (Promega) have been described previously (45). Cell lysates were prepared with passive lysis buffer at 24 h posttransfection. The dual-luciferase assay was performed according to the manufacturer's protocol (Promega), and the products were measured by a luminometer (Turner).
RNA analysis.
Total RNA was isolated with TRIzol reagent (Life Technologies), and the concentrations of RNA preparations were determined by measuring the absorbance at 260 nm. Total RNA (1 μg) was reverse-transcribed to cDNA with avian myeloblastosis virus (Life Technologies) or Omniscript reverse transcriptase (Qiagen) and a random hexanucleotide primer (Life Technologies) according to the manufacturer's protocol. PCRs were carried out in 25 μl containing 1/10 reverse-transcribed cDNA, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 0.1 mM (each) deoxynucleoside triphosphate, 1 mM MgCl2, 0.625 U of Taq polymerase, and 0.5 μM specific primers for either CycT1 (5′-TTAACCAGTGTGGAGATGTTGC-3′ and 5′-GGAAGTGAAGGGAAGGAGAAAT-3′), β-actin (5′-TCCTGTGGCATCCACGAAACT-3′ and 5′-GAAGCATTTGCGGTGGACGAT-3′), or CD11c (5′-TCTACCAGTGTGGCTACAGCAC-3′ and 5′-CCCTCAATGGCAAAGATCTTCT-3′). DNA amplification was performed with a thermal cycler (Perkin-Elmer) for 20 or 25 cycles of denaturation (94°C for 1 min), annealing (50°C for 1 min), and extension (72°C for 1 min). Amplified products were electrophoresed through a 1.5% agarose gel and transferred to nylon membranes (NEN or Perkin-Elmer). Membranes were prehybridized at 68°C for 3 h in 6× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]), 5× Denhardt's solution, 0.25% SDS, and 100 μg of sheared salmon sperm DNA (Life Technology)/ml. Hybridizations were performed at 45°C for CycT1 and 50°C for β-actin and CD11c for at least 16 h in 5× SSC-5× Denhardt's solution-1% SDS-100 μg of sheared salmon sperm DNA/ml-0.1 nM γ-32P-labeled probe. After washes with 2× SSC-0.1% SDS and subsequently 0.2× SSC-0.1% SDS, membranes were air dried and bands were visualized by autoradiography or with a Molecular Dynamics (Sunnyvale, Calif.) Storm 860 PhosphorImager. Probes used in hybridization were 5′ end labeled with T4 polynucleotide kinase (Life Technologies) and [γ-32P]ATP (NEN or Perkin-Elmer). Probes used were oligonucleotides specific for CycT1 (5′-AGAATTGTGCTTATCAGCTGCA-3′), β-actin (5′-ACACAGTGCTGTCTGGCGGCACCAC CATG-3′), and CD11c (5′-GATGAACTTCGTGAGAGCTGTG-3′). Unincorporated [γ-32P]ATP was separated from labeled oligonucleotides by passing through a Sephadex G-50 spin column.
Pilot experiments were performed to verify that PCRs for 20 cycles were in the exponential phase of amplification for all mRNAs, indicating that the RT-PCR assay was quantitative. However, the low level of CycT1 RNA in freshly isolated monocytes (day 0) could not be readily detected with only 20 cycles of PCR, and, therefore, 25 cycles of PCR were performed for day 0 cDNA and for day 1 cDNA as a comparison.
RESULTS
CycT1 protein is induced early during MDM differentiation.
We showed previously that TAK/P-TEFb is induced when the promonocytic cell lines U937 and HL-60 are induced to differentiate to macrophage-like cells by PMA treatment (15). This induction involves an increase in CycT1 protein levels by a posttranscriptional mechanism. To investigate whether TAK/P-TEFb is similarly regulated in primary human MDMs, we examined TAK/P-TEFb during differentiation of MDMs isolated from multiple HIV-seronegative healthy blood donors. During the course of this study, we isolated monocytes by three different procedures: an adherence method and positive and negative selections (see Materials and Methods).
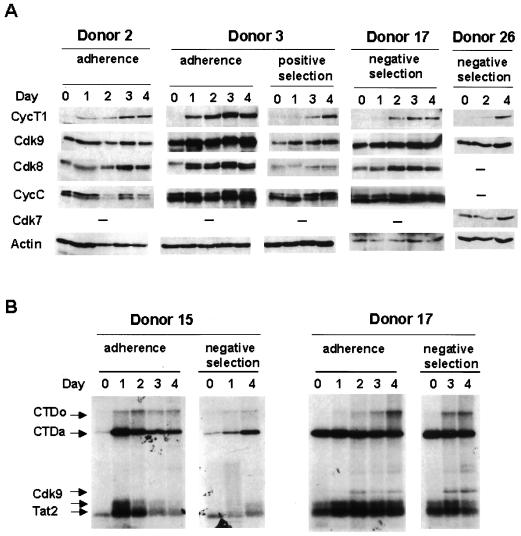
CycT1 protein levels during the early course of MDM differentiation were analyzed by immunoblotting, and results for four typical donors are shown in Fig. 1A. In donors where these early time points were examined, 21 of 26 monocyte preparations showed a very low or undetectable level of CycT1 on the day monocytes were isolated (day 0). In each of these 21 donors, a strong induction of CycT1 was observed, although the kinetics of induction varied slightly among donors. For example, donors 2 and 3 showed a strong induction at day 1, but donors 17 and 26 did not show such an induction until day 2. In 5 of 26 monocyte preparations, we observed high levels of CycT1 at day 0. The reasons for these high levels of CycT1 in day-0 monocytes from a minority of donors are not clear.
FIG. 1.
CycT1 protein expression and TAK/P-TEFb kinase activity increase during early MDM differentiation. (A) Expression of CycT1 and Cdk9 proteins during early MDM differentiation. Human monocytes were isolated as indicated from healthy blood donors and cultured for 4 days. Cell lysates were prepared at the indicated times, and the expression levels of CycT1, Cdk9, Cdk8, Cdk7, and CycC were examined by immunoblotting. Membranes were cut in four sections according to the sizes of proteins to be detected. The membrane section for Cdk9 was stripped and reprobed with an Ab against β-actin as an internal control for loading and transfer. (B) TAK/P-TEFb enzymatic activity increases during MDM differentiation. Cell extracts were prepared at the indicated days of MDM differentiation, and TAK kinase assays were performed as described in Materials and Methods. Methods used for monocyte preparation are indicated. Arrows, phosphorylated GST-Tat-2, Cdk9, and hyper- and hypophosphorylated CTD (CTDo and CTDa, respectively).
In contrast to CycT1 levels, Cdk9 protein levels in most monocyte preparations were found to be relatively high at day 0 and remained fairly constant (Fig. 1A, donors 17 and 26). These observations are consistent with our previous study using the U937 and HL-60 promonocytic cell lines, where CycT1 protein levels increased during differentiation induced by PMA treatment but Cdk9 protein levels were high before and after PMA treatment (15). In some donors, a moderate increase in Cdk9 protein levels was observed during MDM differentiation (Fig. 1A, donor 3, and data not shown). We were unable to examine two other cyclin partners of Cdk9, CycT2a and CycT2b, due to an apparent low level of expression that was below the limits of detection by immunoblotting.
Circulating monocytes are quiescent cells, and induction of differentiation to macrophages likely activates the expression of a large number of genes, suggesting that transcription may be activated globally. To examine other Cdk complexes known to be involved in transcriptional regulation, immunoblotting was performed to analyze the levels of CycC, Cdk8, and Cdk7. CycC/Cdk8 and CycH/Cdk7 complexes are thought to regulate transcription by phosphorylating the CTD of RNA Pol II (37). Protein levels of CycC and Cdk7 were constant during MDM differentiation in most donors (Fig. 1A). Cdk8 showed a doublet pattern, which may be the result of differential phosphorylation. The protein level of Cdk8 showed a slight increase in most donors (Fig. 1A, donors 2, 3, and 17). Additionally, the relative amounts of the Cdk8 doublet changed for some donors during differentiation. The reasons and significance of these relatively modest changes in Cdk8 are unclear. We conclude from these data that, relative to other Cdk and cyclin proteins involved in transcription regulation, CycT1 is strongly upregulated early during MDM differentiation, suggesting that CycT1 may be involved in regulation of expression of specific genes during differentiation.
TAK kinase activity is induced during early MDM differentiation.
To examine if the increase in CycT1 protein levels early during MDM differentiation is reflected in an increase in TAK/P-TEFb enzymatic activity, we performed in vitro kinase assays (see Materials and Methods). Kinase activity was assayed by determining the ability to generate the hyperphosphorylated form of a recombinant CTD substrate. The hyperphosphorylated form of the CTD, termed CTDo, migrates significantly more slowly in SDS-polyacrylamide gels than the hypophosphorylated CTDa form (18). Additionally, TAK/P-TEFb phosphorylates the GST-Tat-2 protein, resulting in two forms that migrate differently in SDS-polyacrylamide gels. Cdk9 is also autophosphorylated in the assay, although the level of Cdk9 autophosphorylation varies among assays. The results of kinase assays for two donors are shown in Fig. 1B. An increase in TAK/P-TEFb activity, as determined by generation of the CTDo form and an increase in Cdk9 autophosphorylation in donor 17, was observed as MDMs differentiated from day 0 to day 4. These results indicate that the increase in TAK/P-TEFb enzymatic activity correlates with the induction of CycT1 protein levels during differentiation of human MDMs and suggests that TAK/P-TEFb activity may be important for the terminal differentiation of MDMs.
CycT1 protein expression and TAK/P-TEFb activity decline at late time points during MDM differentiation.
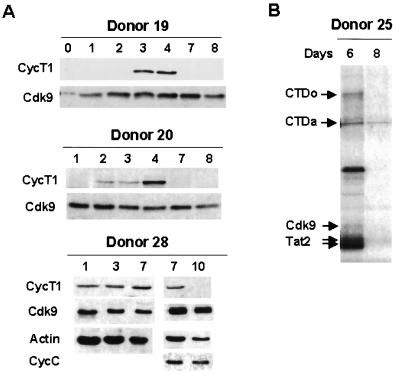
During our analysis of early events during MDM differentiation, we observed that, for 4 of 26 donors, the CycT1 protein was induced for the first 3 days but that induction decreased significantly at day 4. To examine this unexpected transient induction of CycT1, monocytes isolated from additional healthy blood donors were analyzed for extended times in culture. Remarkably, although CycT1 expression was induced early during differentiation, it decreased to very low levels at late time points for all 14 donors analyzed. Typical results from three donors are shown in Fig. 2A (also see Fig. 5 to 7). For donors 19 and 20, CycT1 expression declined by day 7, while it declined by day 10 in donor 28. The kinetics of CycT1 decline were found to vary among the donors analyzed for extended periods in culture: CycT1 decreased by day 7 in 5 of 14 donors, between days 8 and 10 in 6 donors, and after day 10 in 3 donors. In contrast, Cdk9 and CycC protein levels remained constant during extended times of macrophage differentiation (Fig. 2A and data not shown).
FIG. 2.
Transient induction of CycT1 protein and kinase activity during differentiation. (A) Expression of CycT1 and Cdk9 proteins during late MDM differentiation. MDMs with extended culturing time were analyzed for the expression of CycT1 and Cdk9. Cell lysates were prepared and immunoblotting was carried out as described in Materials and Methods. (B) Kinase activity of TAK/P-TEFb decreases while CycT1 declines at late time points of differentiation. Kinase assays were carried out with cell extracts made from MDMs with a high level of CycT1 (day 6) or undetectable levels of CycT1 (day 8). The CycT1 protein levels from the same donor at these time points are shown in Fig. 7.
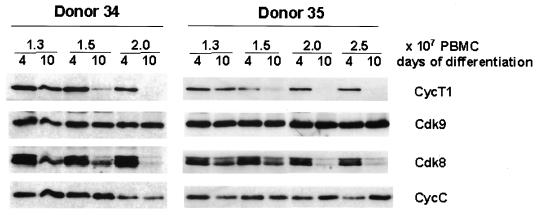
FIG. 5.
Cyclin T1 protein decline is accelerated at high culture density. PBMCs were seeded in culture dishes at the indicated densities, and monocytes were prepared by the adherence methods. After 4 or 10 days, cell extracts were prepared and CycT1, Cdk9, Cdk8, and CycC were analyzed by immunoblotting.
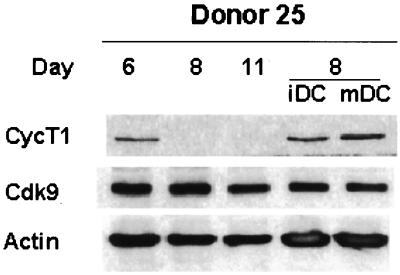
FIG. 7.
CycT1 level is maintained during differentiation of monocyte-derived DC. Isolated monocytes were cultured under conditions to induce macrophage differentiation or differentiation to immature DC (iDC) or mature DC (mDC) as described in Materials and Methods. DC were treated with TNF-α to induce maturation. On the indicated days, cell extracts were prepared and CycT1, Cdk9, and β-actin protein levels were determined by immunoblotting. Similar results were obtained with DC from three additional donors.
As CycT1 is an obligatory regulatory subunit of TAK/P-TEFb, we carried out kinase assays to examine whether the downregulation of CycT1 at late time points during MDM differentiation was reflected in a decrease in enzymatic activity. As expected, the kinase activity was undetectable when CycT1 protein expression declined (Fig. 2B). This observation suggests that the transient induction of CycT1 during MDM differentiation regulates HIV Tat function, and this is likely to be important for HIV-1 replication levels in macrophages.
CycT1 protein induction in MDM involves a posttranscriptional mechanism.
The results shown in Fig. 1 and 2 demonstrate that the CycT1 protein and TAK/P-TEFb enzymatic activity are transiently induced during MDM differentiation. To examine whether CycT1 protein expression is regulated by a posttranscriptional mechanism as has been seen in promonocytic cell lines (15), CycT1 RNA levels were examined by RT-PCR assays of total RNA isolated at various time points during MDM differentiation. β-Actin RNA was used as a reference RNA, and CD11c (α subunit of complement receptor 4) RNA was used as positive control for an RNA known to be induced during macrophage differentiation (29). As an additional control, we also measured GAPDH (glyceraldehyde-3-phosphate dehydrogenase) RNA levels, and the same patterns of expression as those for β-actin RNA were seen (data not shown).
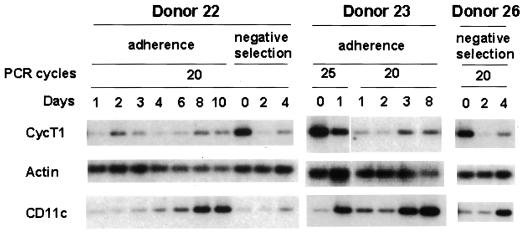
RT-PCR analyses for three donors are shown in Fig. 3. The CD11c RNA levels increased as expected after day 0, indicating that the RT-PCR assays used here are capable of detecting changes in RNAs. CycT1 RNA levels were higher at day 0 than at day 1 in monocytes isolated by either the adherence or negative-selection method but changed little from days 1 to 4. These measurements of CycT1 RNA levels contrast dramatically with a strong induction of CycT1 protein after day 0 (Fig. 1A). An induction of CycT1 protein with a concurrent reduction in CycT1 RNA levels was also seen in our previous study using HL-60 cells (15). Additionally, CycT1 RNA levels were still high at late time points during differentiation even though CycT1 protein expression was undetectable (Fig. 3, donor 22, days 6 to 10, and donor 23, day 8). These results indicate that the induction of the CycT1 protein early during MDM differentiation involves a posttranscriptional mechanism, as does the decline in CycT1 protein expression at late time points.
FIG. 3.
Cyclin T1 RNA levels during monocyte differentiation. RNA analyses were performed by an RT-PCR method as described in Materials and Methods. Methods used to isolate monocytes from donors are indicated.
Levels of Tat transactivation of HIV-1 LTR in MDMs correlates with the transient expression of CycT1.
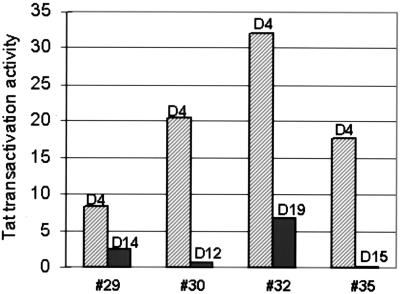
The unexpected decline of CycT1 protein expression late in MDM differentiation predicts that the HIV Tat transactivation function will be low or absent at these late time points. To test this prediction, we performed plasmid cotransfection assays at early and late time points of MDM differentiation. For these assays, a Tat expression or vector control plasmid was cotransfected with an HIV-1 LTR-luciferase reporter plasmid and an internal reference plasmid expressing Renilla luciferase from the herpes simplex virus TK promoter. Plasmid transfections at the early time point were done at day 4, and luciferase activity was measured at day 5. As shown in Fig. 4, Tat transactivation activity in four donors ranged from 8-fold to 30-fold at this early time point of differentiation. Immunoblot assays were performed at late time points for the MDM cultures from the four donors to confirm the decline in CycT1 (data not shown). Plasmid cotransfections were performed at the days indicated, and luciferase expression was measured on the following day. For each of the four MDM preparations, Tat transactivation activity was low to absent at late time points when CycT1 protein levels were very low. This result suggests that the level of CycT1 expression during MDM differentiation regulates Tat transactivation of the HIV-1 LTR, and this is likely to be crucial for HIV-1 replication levels in MDMs.
FIG. 4.
Tat transactivation activity of HIV-1 LTR during MDM differentiation. MDMs from the indicated donors were cotransfected with Tat expression or vector plasmids and HIV-1 LTR-luciferase and TK-Renilla luciferase reporter plasmids. Luciferase activities were measured 24 h posttransfection and normalized to Renilla luciferase activity.
Increased monocyte density accelerates CycT1 decline.
During the course of this work, we observed that the kinetics of CycT1 decline varied among donors. Additionally, CycT1 protein levels appeared to be maintained longer in donors in which freshly isolated monocytes were plated at lower densities. Therefore, to investigate the effects of cell density on CycT1 decline, we plated PBMC from two donors at different densities and examined CycT1 levels at days 4 and 10 in immunoblots (Fig. 5). For both donors, MDM cultures plated at the higher density showed greater reduction in CycT1 expression at day 10 than the less-densely plated cultures. CycT1 levels in donor 35 were examined on day 15 and found to be very low for all cell densities (data not shown). This result indicates that increased monocyte density strongly accelerates the decline in CycT1. Neither Cdk9 nor CycC levels were significantly altered during MDM differentiation where cells were plated at different densities. However, expression of Cdk8 declined in parallel with that of CycT1. We observed a similar parallel decline of Cdk8 and CycT1 during MDM differentiation in preparations from multiple independent donors.
LPS reinduces CycT1 expression at late time points in MDMs.
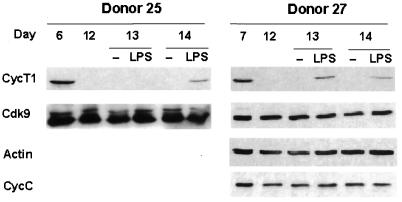
Circulating monocytes migrate to tissues and undergo differentiation to become resident macrophages. These tissue macrophages can be activated by pathogens or proinflammatory cytokines and consequently serve as effector cells of the innate immune system. We were interested to examine if the decline in CycT1 protein expression late in MDM differentiation could be reversed by macrophage activation. We therefore treated MDM cultures at late time points with either gamma interferon, TNF-α, or LPS and carried out immunoblotting to measure CycT1 levels (data not shown). Of these activation agents, LPS was found to be able to reinduce CycT1 expression. Typical results for LPS treatment are shown for two MDM preparations in Fig. 6. In these donors, CycT1 expression was at low-to-undetectable levels on day 12; following LPS treatment on day 12, CycT1 expression was detected at day 13 in donor 27 and at day 14 in donor 25. The induction of CycT1 in these MDM cultures was, however, below the peak levels seen at day 6 or 7 in the same donors. These results indicate that LPS can partially reverse the decline of CycT1 expression late in MDM differentiation and suggest that a pathogen activator of macrophages can induce TAK/P-TEFb function. This may contribute to an increase in HIV-1 replication in tissue macrophages.
FIG. 6.
LPS induces CycT1 expression at late time points of differentiation. After confirming that CycT1 expression had declined, MDMs were treated with LPS at the indicated days. Cell extracts were prepared either 1 or 2 days after addition of LPS. CycT1, Cdk9, and β-actin were detected by immunoblotting. Similar results were observed for nine additional donors.
Expression of CycT1 protein is maintained in monocyte-derived DC.
Blood-derived monocytes can be cultured under conditions that induce differentiation to either monocytes (low GM-CSF) or DC (high GM-CSF plus IL-4). We were interested to examine if culture conditions that induced differentiation to DC showed a transient induction of CycT1 similar to that seen for MDMs. Therefore, monocytes were cultured under conditions that allowed differentiation to either macrophages or DC, and CycT1 expression was measured by immunoblotting. The results shown in Fig. 7 indicate that differentiation to DC results in sustained CycT1 expression until at least day 8, in contrast to MDM differentiation, where CycT1 expression declined to undetectable levels by day 8. We also treated a culture of DC with TNF-α to induce DC maturation, but this had no effect in CycT1 expression. Results similar to those shown in Fig. 7 were obtained for DC prepared from three other donors. These results indicate that CycT1 expression declines at late time points in MDM but not DC differentiation.
DISCUSSION
Transient induction of CycT1 during macrophage differentiation: implications for viral latency.
In this study we have shown that CycT1 protein expression is transiently induced during MDM differentiation. A major implication of our study is that the shutoff of CycT1 may be a mechanism that contributes to establishing latent infection in macrophages, especially in macrophages in the central nervous system, which may exist as a viral reservoir that is resistant to highly active antiretroviral therapy. Our identification of this transient induction of CycT1 is a very unexpected observation in light of the literature showing that HIV-1 replication occurs in MDMs for at least 2 weeks in culture. However, there have been conflicting reports on this point, and some studies have presented evidence that HIV-1 replicates at significantly lower levels in MDMs after 10 to 14 days of differentiation (26, 28, 36). Because the density at which MDMs are cultured has a strong effect on the kinetics of CycT1 shutoff (Fig. 5), it is possible that disparities in the literature could be due, at least in part, to differences in culture conditions that influence CycT1 expression.
We have shown that the transient induction of CycT1 protein expression during the course of MDM differentiation is reflected in a corresponding transient induction of TAK/P-TEFb kinase activity. It is unclear if the increase in CycT1 protein levels early in MDM differentiation can fully account for the induction in TAK/P-TEFb enzymatic activity. It is possible that other regulatory events are also involved in regulating kinase activity, such as the phosphorylation of Cdk9 (6, 10) or inhibitory molecules that repress kinase function such as 7SK snRNA (27, 46). These potential regulatory factors could be themselves regulated during macrophage differentiation and could contribute to the level of TAK/P-TEFb activity.
Tat transactivation activity for the HIV-1 LTR is high when CycT1 expression is at a peak level during early macrophage differentiation, and it decreases to low or undetectable levels at late time points when CycT1 protein levels decline. This result strongly suggests that the CycT1 protein is limiting for Tat function and therefore also for replication of HIV-1 in macrophages. We attempted plasmid cotransfections to address whether CycT1 alone is limiting for Tat function late in MDM differentiation. However, no significant enhancement of Tat activity for the HIV-1 LTR could be observed in MDMs transfected with a CycT1 expression plasmid at late times when CycT1 expression was low (data not shown). The inability to restore Tat function by using the CycT1 expression plasmid may be due to low transfection efficiencies and/or rapid degradation of exogenously expressed CycT1. To investigate whether CycT1 and therefore TAK/P-TEFb may be limiting for HIV-1 replication in late differentiated MDMs, lentivirus expression vectors may be useful to transduce and express CycT1 cDNAs so that effects on HIV-1 replication levels can be monitored.
We attempted to determine if the relative levels of RNA Pol II transcription of the integrated HIV-1 provirus corresponded to CycT1 protein levels during MDM differentiation. For this purpose, we used a vesicular stomatitis virus G-pseudotyped reporter virus containing the enhanced yellow fluorescent protein (eYFP) gene in place of the nef gene. MDM cultures were infected early during differentiation (day 1) or at a late time point when immunoblots demonstrated that CycT1 expression had declined. After 5 days of infection, eYFP fluorescence intensities were measured by flow cytometry. We observed a decrease in mean fluorescence intensities at late time points of differentiation when CycT1 levels declined (unpublished results), suggesting that proviral transcription is reduced when CycT1 levels are shut off. However, interpretation of these experiments is complicated because macrophage differentiation could affect several stages of the HIV-1 replication cycle, such as reverse transcription, nuclear import of the preintegration complex, and integration.
The finding that CycT1 is induced only transiently during macrophage differentiation has implications for lentiviruses in addition to HIV-1, notably bovine immunodeficiency virus and equine infectious viruses (EIAV), both of which also use a viral Tat protein/TAR RNA mechanism, and therefore CycT1, to activate transcription directed by the viral LTR (1, 2, 39). Interestingly, EIAV targets predominantly only monocytes/macrophages during infection. EIAV infection consists of an initial period of high levels of viral replication and viremia that in most cases are controlled by an immune response; infected horses then become persistently infected, and although low levels of EIAV-infected monocytes/macrophages can be detected throughout the lifetimes of infected animals, no disease develops (38). It is possible that the transient induction of CycT1 in equine macrophages limits the levels of EIAV replication and allows the immune system to contain viral replication to nonpathogenic levels.
LPS reinduction of CycT1 expression: implications for reactivation of latent HIV-1.
We observed that LPS can reinduce CycT1 after it has declined late in MDM differentiation. This may be relevant to the finding that AIDS patients with opportunistic infections have increased HIV-1 replication in macrophages (31, 42). It seems probable that infections by gram-negative bacteria induce CycT1 in HIV-infected macrophages, and this may enhance viral replication. An important area in HIV-1 pathogenesis for future investigation will be to determine whether other pathogen-associated molecular patterns can induce CycT1 in macrophages. It will also be important to elucidate signal transduction pathways that induce CycT1 expression following activation by LPS or other agents. It is possible that proteins, especially kinases, that mediate such signal transduction pathways can be used as targets for drug development.
Why is CycT1 transiently induced during macrophage differentiation?
There is some evidence suggesting that induction of TAK/P-TEFb during macrophage differentiation may serve to protect cells from apoptosis. The ability to readily undergo apoptosis is crucial to monocyte homeostasis, since monocytes circulate in blood for a few days, during which time they either emigrate to tissues and differentiate or die through apoptosis (14, 21). Apoptosis in tissue macrophages must be equally crucial for homeostasis, as the normal emigration of macrophages to tissues must be balanced by the loss of existing macrophages. Additionally, after recruitment into tissues as the result of inflammatory signals, macrophages must be able to undergo apoptosis to limit immune responses. Expression of a dominant-negative Cdk9 protein in the promonocytic cell line U937 results in increased sensitivity to apoptosis following PMA treatment to induce macrophage-like differentiation (7). Additionally, a genomic-scale analysis of a B-cell line treated with flavopiridol, a relatively specific inhibitor of Cdk9, found that the levels of mRNAs encoding proteins that regulate apoptosis were particularly reduced (22). Thus, the absence of CycT1 in monocytes may allow them to rapidly undergo apoptosis if they fail to receive a differentiation signal. Likewise, the decline in CycT1 expression late in differentiation may allow macrophages to undergo apoptosis unless they receive an activation signal, such as LPS.
DC are another cell lineage important for HIV pathogenesis that can also differentiate from peripheral blood monocytes. However, unlike the course in MDMs, CycT1 expression in DC is sustained at a relatively high level throughout differentiation and maturation. The high expression of CycT1 in DC may be due to GM-CSF and IL-4 stimulation or may be the result of a distinct regulatory mechanism that is absent in MDMs. CycT1 regulation may be different in different cell types depending on the local environment and functional role of TAK/P-TEFb in a particular cell type.
Mechanisms involved in the transient induction of CycT1 protein expression.
The CycT1 RNA levels in freshly isolated monocytes were found to be high, and they decreased by day 1 of differentiation, even though there was a dramatic increase in CycT1 protein levels during this period. We observed a similar decrease in CycT1 RNA levels, while CycT1 protein levels in PMA-treated HL-60 promonocytic cells increased (15). One potential explanation for the decrease in CycT1 RNA levels, with a concurrent increase in CycT1 protein expression, is that the turnover rates of many mRNAs may increase upon monocyte differentiation. The storage of large amounts of CycT1 RNA in quiescent monocytes may allow a rapid induction of protein expression in the presence of high levels of mRNA turnover. Our analyses of CycT1 mRNA at late time points during macrophage differentiation showed that CycT1 RNA was still present at relatively high levels when the protein level decreased, indicating that shutoff of CycT1 expression also involves a posttranscriptional mechanism.
It is possible that CycT1 expression in MDMs may be regulated by translational control and/or ubiquitin-mediated proteolysis. There are precedents for control of cyclin protein, such as the yeast Cln3 protein, at the translation level (33). Translational regulation of CycT1 may involve the 5′ or 3′ untranslated region (UTR), or both, of CycT1 mRNA, which contains a 5′ UTR of approximately 300 nucleotides and a 3′ UTR estimated to be 5,000 nucleotides (23). Ubiquitin-mediated proteolysis may also regulate CycT1 expression, especially given that the carboxyl terminus of CycT1 contains a PEST sequence, a motif implicated in targeting proteins, such as cyclin D, for rapid degradation via ubiquitin-mediated proteolysis (35).
In summary, we have shown here that the transient expression of the CycT1 protein during MDM differentiation is regulated by a posttranscriptional mechanism. The transient induction of CycT1 appears to regulate Tat transactivation function in these cells, and this may play a critical role in the establishment of latency and the subsequent reactivation of HIV-1 in infected macrophages. The signals and molecular mechanisms that regulate CycT1 expression in macrophages are important future research topics with potentially important implications for HIV-1 pathogenesis and development of therapeutics.
Acknowledgments
We thank Dorothy Lewis for helpful discussions and critical comments on the manuscript.
This work was supported by grants AI42558 (C.H.H.), AI35381 (A.P.R.), and AI45374 (A.P.R.). Flow cytometry was supported by the Center for AIDS Research at Baylor College of Medicine Center (AI36211).
REFERENCES
- 1.Barboric, M., R. Taube, N. Nekrep, K. Fujinaga, and B. M. Peterlin. 2000. Binding of Tat to TAR and recruitment of positive transcription elongation factor b occur independently in bovine immunodeficiency virus. J. Virol. 74:6039-6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieniasz, P. D., T. A. Grdina, H. P. Bogerd, and B. R. Cullen. 1999. Highly divergent lentiviral Tat proteins activate viral gene expression by a common mechanism. Mol. Cell. Biol. 19:4592-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieniasz, P. D., T. A. Grdina, H. P. Bogerd, and B. R. Cullen. 1998. Recruitment of a protein complex containing Tat and cyclin T1 to TAR governs the species specificity of HIV-1 Tat. EMBO J. 17:7056-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankson, J. N., D. Persaud, and R. F. Siliciano. 2002. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53:557-593. [DOI] [PubMed] [Google Scholar]
- 5.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 97:77-89. [PubMed] [Google Scholar]
- 6.Fong, Y. W., and Q. Zhou. 2000. Relief of two built-in autoinhibitory mechanisms in P-TEFb is required for assembly of a multicomponent transcription elongation complex at the human immunodeficiency virus type 1 promoter. Mol. Cell. Biol. 20:5897-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foskett, S. M., R. Ghose, D. N. Tang, D. E. Lewis, and A. P. Rice. 2001. Antiapoptotic function of Cdk9 (TAK/P-TEFb) in U937 promonocytic cells. J. Virol. 75:1220-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujinaga, K., R. Taube, J. Wimmer, T. P. Cujec, and B. M. Peterlin. 1999. Interactions between human cyclin T, Tat, and the transactivation response element (TAR) are disrupted by a cysteine to tyrosine substitution found in mouse cyclin T. Proc. Natl. Acad. Sci. USA 96:1285-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber, M. E., and K. A. Jones. 1999. HIV-1 Tat: coping with negative elongation factors. Curr. Opin. Immunol. 11:460-465. [DOI] [PubMed] [Google Scholar]
- 10.Garber, M. E., T. P. Mayall, E. M. Suess, J. Meisenhelder, N. E. Thompson, and K. A. Jones. 2000. CDK9 autophosphorylation regulates high-affinity binding of the human immunodeficiency virus type 1 Tat-P-TEFb complex to TAR RNA. Mol. Cell. Biol. 20:6958-6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garber, M. E., P. Wei, V. N. KewalRamani, T. P. Mayall, C. H. Herrmann, A. P. Rice, D. R. Littman, and K. A. Jones. 1998. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 12:3512-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garriga, J., J. Peng, M. Parreno, D. H. Price, E. E. Henderson, and X. Grana. 1998. Upregulation of cyclin T1/CDK9 complexes during T cell activation. Oncogene 17:3093-3102. [DOI] [PubMed] [Google Scholar]
- 13.Ghose, R., L. Y. Liou, C. H. Herrmann, and A. P. Rice. 2001. Induction of TAK (cyclin T1/P-TEFb) in purified resting CD4+ T lymphocytes by combination of cytokines. J. Virol. 75:11336-11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidenreich, S. 1999. Monocyte CD14: a multifunctional receptor engaged in apoptosis from both sides. J. Leukoc. Biol. 65:737-743. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann, C. H., R. G. Carroll, P. Wei, K. A. Jones, and A. P. Rice. 1998. Tat-associated kinase, TAK, activity is regulated by distinct mechanisms in peripheral blood lymphocytes and promonocytic cell lines. J. Virol. 72:9881-9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann, C. H., M. O. Gold, and A. P. Rice. 1996. Viral transactivators specifically target distinct cellular protein kinases that phosphorylate the RNA polymerase II C-terminal domain. Nucleic Acids Res. 24:501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann, C. H., and M. A. Mancini. 2001. The Cdk9 and cyclin T subunits of TAK/P-TEFb localize to splicing factor-rich nuclear speckle regions. J. Cell Sci. 114:1491-1503. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann, C. H., and A. P. Rice. 1995. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J. Virol. 69:1612-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho, D. D., T. R. Rota, and M. S. Hirsch. 1986. Infection of monocyte/macrophages by human T lymphotropic virus type III. J. Clin. Investig. 77:1712-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karn, J. 1999. Tackling Tat. J. Mol. Biol. 293:235-254. [DOI] [PubMed] [Google Scholar]
- 21.Lagasse, E., and I. L. Weissman. 1997. Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell 89:1021-1031. [DOI] [PubMed] [Google Scholar]
- 22.Lam, L. T., O. K. Pickeral, A. C. Peng, A. Rosenwald, E. M. Hurt, J. M. Giltnane, L. M. Averett, H. Zhao, R. E. Davis, M. Sathyamoorthy, L. M. Wahl, E. D. Harris, J. A. Mikovits, A. P. Monks, M. G. Hollingshead, E. A. Sausville, and L. M. Staudt. 13September2001, posting date. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2:research0041.1-research0041.11. [Online.] http://genomebiology.com/2001/2/10/research/0041. [DOI] [PMC free article] [PubMed]
- 23.Liu, H., and A. P. Rice. 2000. Isolation and characterization of the human cyclin T1 promoter. Gene 252:39-49.d [DOI] [PubMed] [Google Scholar]
- 24.Mancebo, H. S., G. Lee, J. Flygare, J. Tomassini, P. Luu, Y. Zhu, J. Peng, C. Blau, D. Hazuda, D. Price, and O. Flores. 1997. P-TEFb kinase is required for HIV Tat transcriptional activation in vivo and in vitro. Genes Dev. 11:2633-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcello, A., R. A. Cinelli, A. Ferrari, A. Signorelli, M. Tyagi, V. Pellegrini, F. Beltram, and M. Giacca. 2001. Visualization of in vivo direct interaction between HIV-1 TAT and human cyclin T1 in specific subcellular compartments by fluorescence resonance energy transfer. J. Biol. Chem. 276:39220-39225. [DOI] [PubMed] [Google Scholar]
- 26.Naif, H. M., S. Li, M. Alali, A. Sloane, L. Wu, M. Kelly, G. Lynch, A. Lloyd, and A. L. Cunningham. 1998. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J. Virol. 72:830-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen, V. T., T. Kiss, A. A. Michels, and O. Bensaude. 2001. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414:322-325. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson, J. K., G. D. Cross, C. S. Callaway, and J. S. McDougal. 1986. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J. Immunol. 137:323-329. [PubMed] [Google Scholar]
- 29.Noti, J. D., and B. C. Reinemann. 1995. The leukocyte integrin gene CD11c is transcriptionally regulated during monocyte differentiation. Mol. Immunol. 32:361-369. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien, W. A., and R. J. Pomerantz. 1997. HIV infection and associated diseases, p. 815. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, Pa.
- 31.Orenstein, J. M., C. Fox, and S. M. Wahl. 1997. Macrophages as a source of HIV during opportunistic infections. Science 276:1857-1861. [DOI] [PubMed] [Google Scholar]
- 32.Peng, J., Y. Zhu, J. T. Milton, and D. H. Price. 1998. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 12:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polymenis, M., and E. V. Schmidt. 1997. Coupling of cell division to cell growth by translational control of the G1 cyclin CLN3 in yeast. Genes Dev. 11:2522-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price, D. H. 2000. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol. 20:2629-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rechsteiner, M., and S. W. Rogers. 1996. PEST sequences and regulation by proteolysis. Trends Biochem. Sci. 21:267-271. [PubMed] [Google Scholar]
- 36.Rich, E. A., I. S. Chen, J. A. Zack, M. L. Leonard, and W. A. O'Brien. 1992. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J. Clin. Investig. 89:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riedl, T., and J. M. Egly. 2000. Phosphorylation in transcription: the CTD and more. Gene Expr. 9:3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sellon, D. C., F. J. Fuller, and T. C. McGuire. 1994. The immunopathogenesis of equine infectious anemia virus. Virus Res. 32:111-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taube, R., K. Fujinaga, D. Irwin, J. Wimmer, M. Geyer, and B. M. Peterlin. 2000. Interactions between equine cyclin T1, Tat, and TAR are disrupted by a leucine-to-valine substitution found in human cyclin T1. J. Virol. 74:892-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taube, R., K. Fujinaga, J. Wimmer, M. Barboric, and B. M. Peterlin. 1999. Tat transactivation: a model for the regulation of eukaryotic transcriptional elongation. Virology 264:245-253. [DOI] [PubMed] [Google Scholar]
- 41.van Furth, R. 1989. Origin and turnover of monocytes and macrophages. Curr. Top. Pathol. 79:125-150. [PubMed] [Google Scholar]
- 42.Wahl, S. M., T. Greenwell-Wild, G. Peng, H. Hale-Donze, and J. M. Orenstein. 1999. Co-infection with opportunistic pathogens promotes human immunodeficiency virus type 1 infection in macrophages. J. Infect. Dis. 179 (Suppl. 3):S457-S460. [DOI] [PubMed] [Google Scholar]
- 43.Wei, P., M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451-462. [DOI] [PubMed] [Google Scholar]
- 44.Yang, X., M. O. Gold, D. N. Tang, D. E. Lewis, E. Aguilar-Cordova, A. P. Rice, and C. H. Herrmann. 1997. TAK, an HIV Tat-associated kinase, is a member of the cyclin-dependent family of protein kinases and is induced by activation of peripheral blood lymphocytes and differentiation of promonocytic cell lines. Proc. Natl. Acad. Sci. USA 94:12331-12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, X., C. H. Herrmann, and A. P. Rice. 1996. The human immunodeficiency virus Tat proteins specifically associate with TAK in vivo and require the carboxyl-terminal domain of RNA polymerase II for function. J. Virol. 70:4576-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, Z., Q. Zhu, K. Luo, and Q. Zhou. 2001. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414:317-322. [DOI] [PubMed] [Google Scholar]
- 47.Zhu, Y., T. Pe'ery, J. Peng, Y. Ramanathan, N. Marshall, T. Marshall, B. Amendt, M. B. Mathews, and D. H. Price. 1997. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 11:2622-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]