Abstract
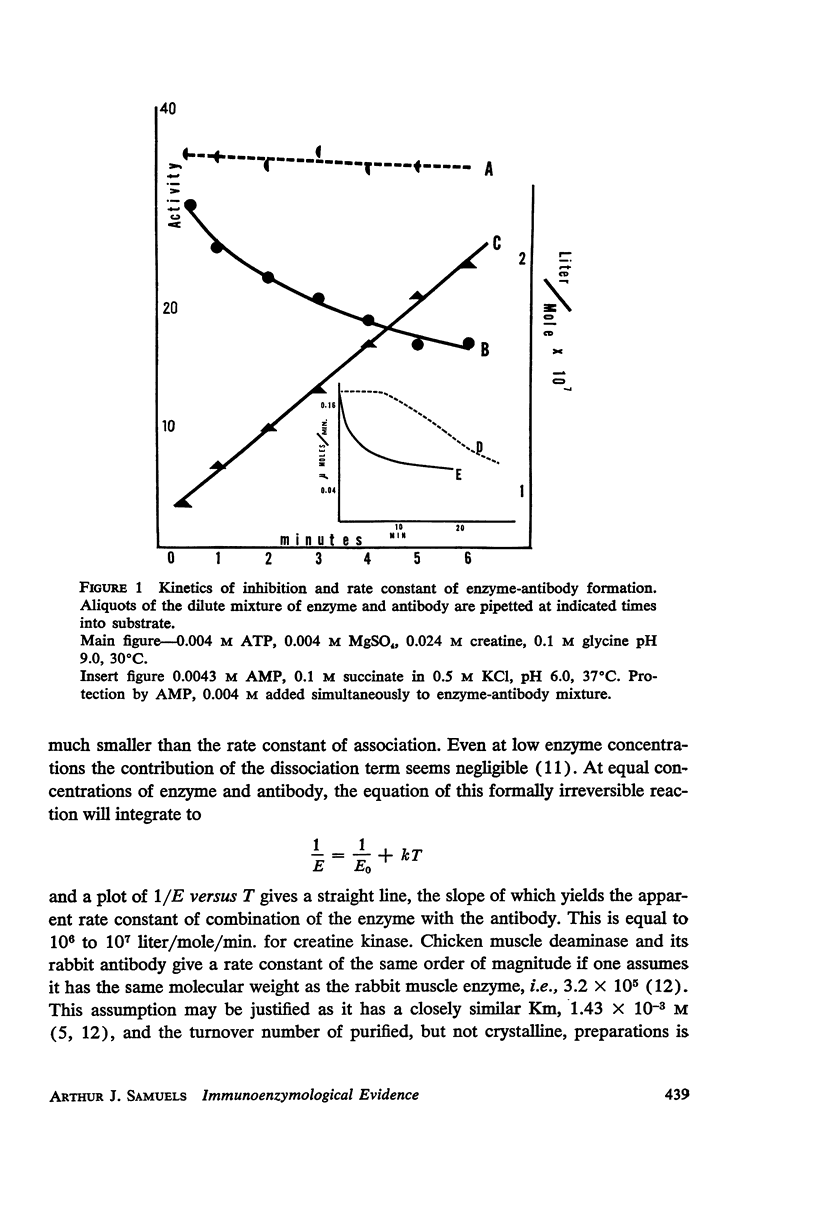
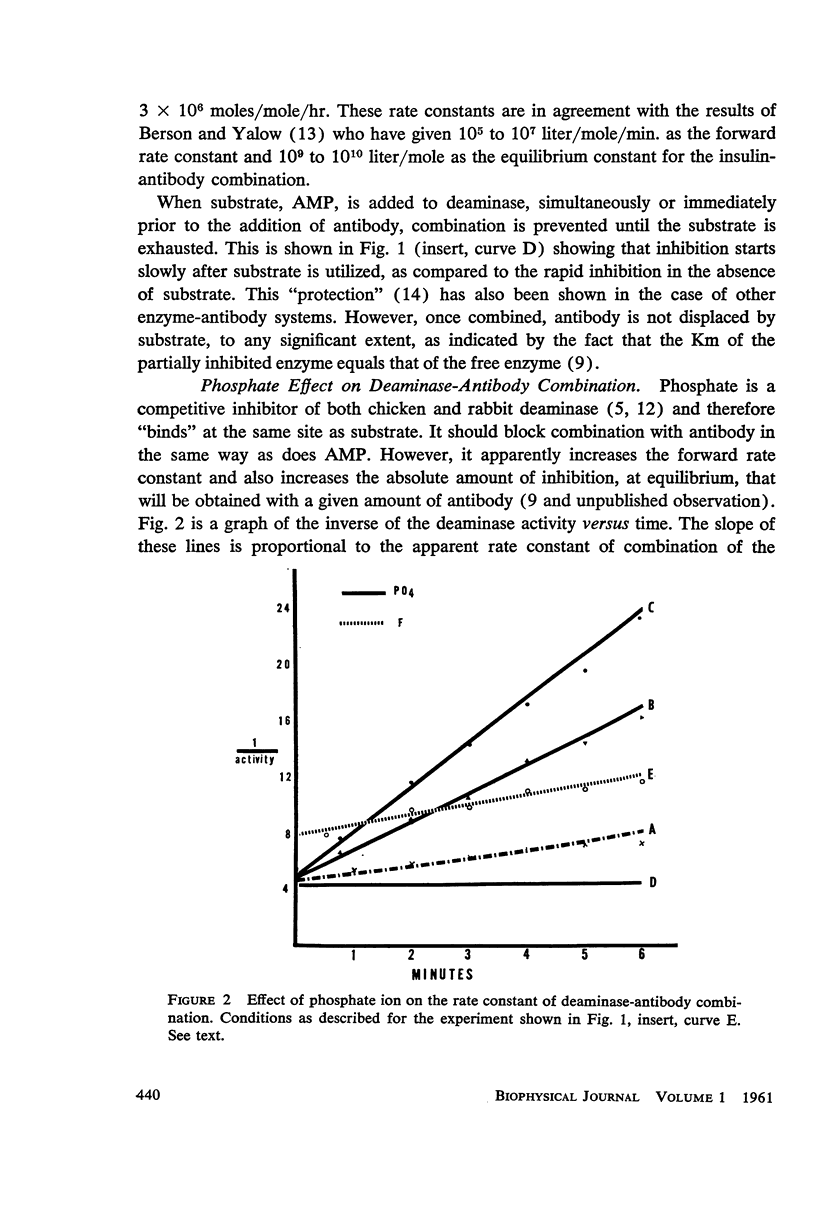
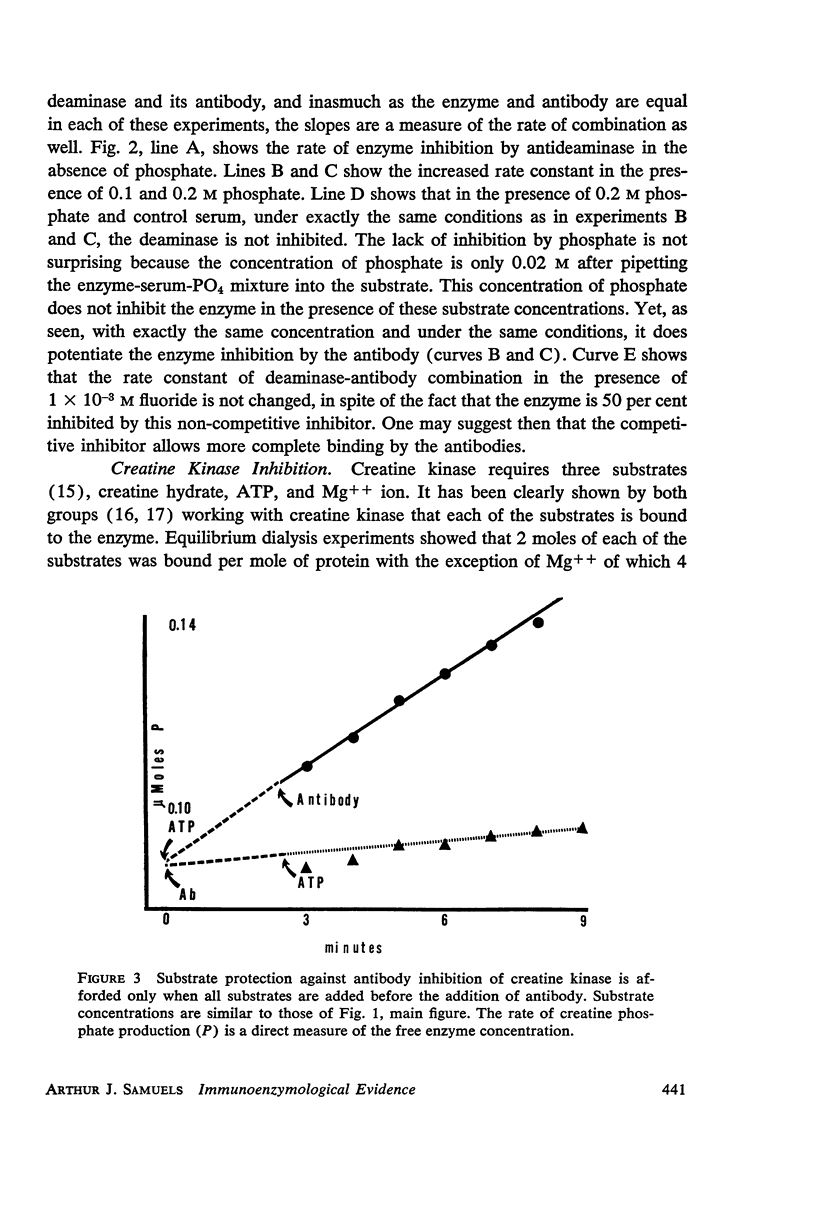
The kinetics of inhibition of 5′-adenylic acid deaminase and creatine-ATP transphosphorylase by their respective antibodies are studied and rate constants of combination are ascertained. It is shown that the single substrate 5′-adenylic acid (AMP) of deaminase “protects” the enzyme against antibody inhibition. However, phosphate, a competitive inhibitor of the highly specific deaminase, enhances combination with antibody. With creatine kinase, however, addition of either of the substrates, alone or in combination with the required magnesium, each of which separately bind to the enzyme, does not prevent inhibition of the enzyme by its antibody. However, the “working” enzyme combined with all substrates is “protected” against antibody inhibition.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERSON S. A., YALOW R. S. Quantitative aspects of the reaction between insulin and insulin-binding antibody. J Clin Invest. 1959 Nov;38:1996–2016. doi: 10.1172/JCI103979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUM J. J. Interaction between myosin and its substrates. Arch Biochem Biophys. 1960 Mar;87:104–119. doi: 10.1016/0003-9861(60)90130-2. [DOI] [PubMed] [Google Scholar]
- Blout E. R., Stryer L. ANOMALOUS OPTICAL ROTATORY DISPERSION OF DYE: POLYPEPTIDE COMPLEXES. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1591–1593. doi: 10.1073/pnas.45.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CINADER B. Antibodies against enzymes. Annu Rev Microbiol. 1957;11:371–390. doi: 10.1146/annurev.mi.11.100157.002103. [DOI] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. III. Kinetic studies. J Biol Chem. 1954 Sep;210(1):65–82. [PubMed] [Google Scholar]
- LEE Y. P. 5'-Adenylic acid deaminase. III. Properties and kinetic studies. J Biol Chem. 1957 Aug;227(2):999–1007. [PubMed] [Google Scholar]
- MANSOUR T. E., BUEDING E., STAVITSKY A. B. The effect of a specific antiserum on the activities of lactic dehydrogenase of mammalian muscle and of Schistosoma mansoni. Br J Pharmacol Chemother. 1954 Jun;9(2):182–186. doi: 10.1111/j.1476-5381.1954.tb00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAJJAR V. A., FISHER J. The mechanism of antibody-antigen reaction. Biochim Biophys Acta. 1956 Apr;20(1):158–169. doi: 10.1016/0006-3002(56)90274-8. [DOI] [PubMed] [Google Scholar]
- SAMUELS A. Alterations in metabolism during electrical excitation of frog nerve. Am J Physiol. 1957 Aug;190(2):377–382. doi: 10.1152/ajplegacy.1957.190.2.377. [DOI] [PubMed] [Google Scholar]
- SAMUELS A. The immuno-enzymology of muscle proteins. I. General features of myosin and 5'-adenylic acid deaminase. Arch Biochem Biophys. 1961 Mar;92:497–506. doi: 10.1016/0003-9861(61)90390-3. [DOI] [PubMed] [Google Scholar]
- SELA M., ANFINSEN C. B., HARRINGTON W. F. The correlation of ribonuclease activity with specific aspects of tertiary structure. Biochim Biophys Acta. 1957 Dec;26(3):502–512. doi: 10.1016/0006-3002(57)90096-3. [DOI] [PubMed] [Google Scholar]


