Abstract
Human cytomegalovirus (HCMV) establishes persistent lifelong infections and replicates slowly. To withstand robust immunity, HCMV utilizes numerous immune evasion strategies. The HCMV gene cassette encoding US2 to US11 encodes four homologous glycoproteins, US2, US3, US6, and US11, that inhibit the major histocompatibility complex class I (MHC-I) antigen presentation pathway, probably inhibiting recognition by CD8+ T lymphocytes. US2 also inhibits the MHC-II antigen presentation pathway, causing degradation of human leukocyte antigen (HLA)-DR-α and -DM-α and preventing recognition by CD4+ T cells. We investigated the effects of seven of the US2 to US11 glycoproteins on the MHC-II pathway. Each of the glycoproteins was expressed by using replication-defective adenovirus vectors. In addition to US2, US3 inhibited recognition of antigen by CD4+ T cells by a novel mechanism. US3 bound to class II α/β complexes in the endoplasmic reticulum (ER), reducing their association with Ii. Class II molecules moved normally from the ER to the Golgi apparatus in US3-expressing cells but were not sorted efficiently to the class II loading compartment. As a consequence, formation of peptide-loaded class II complexes was reduced. We concluded that US3 and US2 can collaborate to inhibit class II-mediated presentation of endogenous HCMV antigens to CD4+ T cells, allowing virus-infected cells to resist recognition by CD4+ T cells.
Human cytomegalovirus (HCMV) is ubiquitous and normally causes relatively benign clinical manifestations (6). HCMV infections are lifelong and are characterized by low-level persistence, latency, and bouts of reactivation. However, HCMV causes serious disease in immunosuppressed or immunocompromised individuals, e.g., patients undergoing transplant-related therapy or patients with AIDS (54). HCMV infection can cause neurologic sequelae, including hearing loss and mental retardation, and organ failure in young children (54, 56). HCMV infects many cell types, including epithelial cells, fibroblasts, endothelial cells, glial cells, and monocytes/macrophages. Infection of epithelial cells of the upper respiratory tract, salivary gland, and kidney allows excretion and spread to other hosts (54), while infection of monocytes/macrophages provides a site for virus latency (53) and a means for dissemination throughout the body.
HCMV replicates slowly, often at low levels, without causing rapid cell destruction. The virus escapes vigorous host defenses by establishing a latent state in which gene expression is highly restricted. However, following reactivation from latency, the virus replicates in the face of fully primed cell-mediated immunity. Nevertheless, the virus often persists in the body for long periods of time, with an astonishing ability to withstand the strong anti-HCMV immune response. Apparently, HCMV uses a variety of immune evasion tactics to allow virus replication in certain cell types and at defined times in the virus life cycle (reviewed in references 15, 28, 30, and 60). Among these are a panel of inhibitors that apparently reduce recognition by T lymphocytes and NK cells. HCMV expresses four glycoproteins, all encoded by homologous genes in the US2-to-US11 gene region of the viral genome, that can independently inhibit various steps in the major histocompatibility complex class I (MHC-I) antigen presentation pathway. US2 and US11 cause retrotranslocation of class I proteins from the endoplasmic reticulum (ER) and proteasome-mediated degradation (33, 62, 63). US3 causes retention of class I proteins in the ER, although US3 itself apparently dissociates from MHC-I and moves through the Golgi apparatus in time (2, 19, 20, 32). US6 binds the transporter associated with antigen presentation (TAP) in the ER (3, 25, 37) and allosterically affects the cytosolic ATPase activity of TAP (26, 34) so that peptides are not available to assemble class I molecules. The HCMV tegument protein pp65 reduces presentation of viral protein pp72 to CD8+ T cells (18). Additionally, HCMV encodes two membrane glycoproteins, UL16 and UL18, that mediate resistance to NK cells (10, 47).
We recently described a novel form of immune evasion: inhibition of the MHC-II antigen presentation pathway by HCMV US2 (58). Cells expressing US2 are less able to present antigens via the class II pathway to CD4+ T lymphocytes. CD4+ T cells normally recognize antigens presented by “professional” antigen-presenting cells (APCs), macrophages, dendritic cells, and B cells, that take up extracellular viral proteins by phagocytosis or endocytosis and process the proteins to antigenic peptides in endosomal compartments. MHC-II α/β heterodimers assemble with invariant chain (Ii) in the ER (reviewed in reference 42). Nonameric complexes of class II α/β/Ii are targeted to the MHC-II loading compartment (MIIC) by signals present in the N terminus of Ii (35, 39). During this transport to the MIIC, Ii is partially degraded, and, in the MIIC, a fragment of Ii is exchanged for antigenic peptides that bind the peptide-binding groove of α/β. This process of peptide loading is facilitated by the enzyme human leukocyte antigen DM (HLA-DM or DM). US2 causes rapid retrotranslocation of class II proteins DR-α and DM-α from the ER, followed by proteasome-mediated degradation, so that there are few newly synthesized class II proteins to present (7a, 58).
Since US2 is expressed exclusively within HCMV-infected cells, it appears that US2 is designed to block class II-mediated presentation of endogenous or intracellular HCMV antigens rather than exogenous or extracellular antigens (28). This inhibition of the class II pathway may allow HCMV to escape detection by CD4+ T cells, which can act cytolytically or produce antiviral cytokines and coordinate anti-HCMV immune responses. This may be especially important given that HCMV infects numerous cell types that express MHC-II, including monocytes/macrophages, glial cells, dendritic cells, endothelial cells, and epithelial cells. However, it is by no means clear where this immune evasion is important, which cells exhibit inhibition of class II presentation, when this occurs in the HCMV life cycle, or how comprehensively HCMV escapes detection by CD4+ T cells in any cell. Given the high levels of class II in several professional APCs, such as macrophages and dendritic cells, coupled with relatively poor virus gene expression in these cells, at least in vitro, it is unlikely that US2 can inhibit the class II pathway in these cells. Indeed, there was a recent report claiming that US2 does not cause degradation of class II proteins in dendritic cells (45). However, since US2 also did not cause any discernible degradation of the already-unstable class I proteins expressed in these dendritic cells, no general conclusions about the relative effects of class I versus class II are valid. In other cell types, e.g., endothelial or epithelial cells, where lower levels of class II and higher levels of US2 may be produced, inhibition of the class II pathway is likely to be more robust. Arguments about whether US2 or other HCMV US2 to US11 glycoproteins are or are not effective in particular cell types in vivo miss two salient points: (i) these viral proteins have the capacity to act at several levels and in combination in HCMV-infected cells to mediate a spectrum of immune evasion, and (ii) individual US2 to US11 genes that have more-subtle effects will be maintained even for the slightest selective advantage in a certain important host cell.
We have suggested that inhibition of the MHC-II pathway may be particularly important for HCMV and other herpesviruses because large amounts of viral proteins accumulate in the trans-Golgi network (TGN) as part of virion assembly (16, 29, 49, 59). This would be expected to deliver relatively large quantities of viral antigens to endosomes and the MIIC. Reduction in class II proteins or inhibition of intracellular transport reduces presentation of HCMV antigens to CD4+ T cells. It appears that US2's effects occur relatively early after infection (58) and are combined with other effects that reduce gamma interferon (IFN-γ)-induced upregulation of class II genes (38, 40).
Here, we expressed seven of the HCMV US2 to US11 glycoproteins by using adenovirus (Ad) vectors and analyzed the effects of these viral glycoproteins on MHC-II antigen presentation to CD4+ T cells. As expected, US2 blocked antigen recognition by CD4+ T cells. Glycoprotein US3 also blocked antigen presentation to CD4+ T cells. US3 bound newly synthesized class II heterodimers and reduced their association with Ii. Loss of Ii apparently caused class II complexes to be inefficiently sorted to MIIC, leading to reduced loading of antigenic peptides onto class II. This finding underscores the potential negative effects of class II-mediated presentation of endogenous viral antigens on survival of HCMV and illustrates a second mechanism by which the virus evades recognition by CD4+ T cells.
MATERIALS AND METHODS
Cells.
U373-CIITAHis16 (His16) (58) and U373-CIITANeo6 (Neo6) cells, which express MHC-II proteins, were derived by transfecting human glial U373 cells with the human class II transactivator (CIITA) gene and pSV2His or pSV2Neo and grown in media containing 0.5 mM Histidinol (Sigma, St. Louis, Mo.) or 200 μg of G-418 sulfate (GIBCO, Grand Island, N.Y.)/ml, respectively. Neo6 cells express approximately 50% of the amount of class II expressed by His16 cells. 293 cells that express Ad E1 genes were propagated in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum. TbH9, a CD4+ T-cell clone specific for the Mycobacterium tuberculosis antigen Mtb39 (14), was maintained and expanded by using anti-CD3 antibodies as described previously (58).
Viruses.
An Ad vector expressing US2, AdtetUS2, has been described (58). Other replication-defective (E1−) Ad vectors expressing HCMV US3 (this paper) and US7, US8, US9, US10, and US11 (27) were similarly constructed. For expression of these glycoproteins, His16 and Neo6 cells were coinfected with these US2- to US11-expressing Ad vectors and a second vector, Adtet-trans (by using 20% of the amount of AdtetUS virus), which expresses a transactivator protein that activates the tet promoter without the need for tetracycline. Throughout this study, Adtet-trans alone was used as the control Ad vector at a dose similar to the total amount of Ad in each case. All Ad vectors were propagated and titered on 293 cells as described previously (41).
Antibodies.
Rabbit antisera to class I heavy chain (HC) (4) and HLA-DR (CHAMP) (55) and monoclonal antibodies (MAbs) to HLA-DR-α (DA6.147) (22), -DR-β (HB10A) (8), -DR-α/β (L243) (36), -DM-α (5C1) (50), -DM-β (MaP.DMB/C) (13), and Ii (PIN.1) (48) have been described. Antibodies to calnexin, calreticulin, p115, GM130 (Transduction Laboratories, Lexington, Ky.), protein disulfide isomerase (PDI; StressGen, Vancouver, British Columbia, Canada), TGN46 (Serotec, Raleigh, N.C.), and lysosome-associated membrane protein 1 (LAMP-1) (Pharmingen, San Diego, Calif.) were purchased from commercial sources. Rabbit antisera to US2, US7, US8, US9, and US10 are described elsewhere (27, 58). Rabbit antiserum to N-terminal peptides of US3 (RLADSVPRPLDVVVSEIRSC) was generated by immunizing rabbits with peptides coupled to keyhole limpet hemocyanin or bovine serum albumin (BSA) according to standard protocols (24). Rabbit antiserum to US11 (31) was obtained from Thomas Jones, Wyeth-Ayerst Research, Pearl River, N.Y. The secondary antibodies for immunofluorescence were Alexa 488-conjugated donkey anti-sheep and goat anti-mouse immunoglobulin G (IgG), Alexa 594-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, Oreg.), and Cy5-conjugated goat anti-rat IgG (Jackson ImmunoResearch Laboratories, Bar Harbor, Maine).
Labeling of cells with [35S]methionine-cysteine and immunoprecipitation of proteins.
His16 and Neo6 cells were radiolabeled after 18 to 24 h of infection with recombinant Ad vectors. The cells were incubated for 1 h in starvation medium lacking methionine and cysteine, washed, and then labeled for various time periods in starvation medium supplemented with [35S]methionine-cysteine (50 to 200 μCi/ml; Amersham Pharmacia Biotech, Piscataway, N.J.). To chase the label, cells were incubated in medium containing a 10-fold excess of methionine and cysteine. Cell extracts were made with NP-40-deoxycholate (DOC) lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1 mg of BSA/ml, and a cocktail of protease inhibitors). Proteins of interest were immunoprecipitated from the cell extracts as described previously (58). Endoglycosidase H (endo H) analyses were carried out with enzyme preparations and protocols supplied by New England Biolabs (Boston, Mass.). Sequential immunoprecipitations were performed by lysing labeled cells in Tris-saline (50 mM Tris-HCl [pH 7.5], 100 mM NaCl) containing 1% digitonin (Calbiochem, San Diego, Calif.) and protease inhibitors (58). Material precipitated in the first round was denatured by boiling in 100 μl of Tris-saline containing 1% sodium dodecyl sulfate (SDS) and then diluted 10-fold with Tris-saline containing 1% Triton X-100. The samples were incubated with a secondary antibody and then with protein A-agarose (GIBCO), the beads were washed, and the bound proteins were eluted by boiling in Laemmli loading buffer. In each case, protein samples were subjected to polyacrylamide gel electrophoresis using 12% polyacrylamide gels, and the gels were fixed and incubated with Enlightning (New England Nuclear, Beverly, Mass.), dried, and then exposed to X-ray film or PhosphorImager screens (Molecular Dynamics, Sunnyvale, Calif.).
SDS stability assays.
The protocol for SDS stability assays was adapted from that previously described (17). Briefly, cells were radiolabeled with [35S]methionine-cysteine for 1 h, and the label was chased for 3 to 9 h. Cells were lysed with a solution containing 25 mM Tris, pH 7.5, 150 mM NaCl, 1% polidocanol (Sigma), 7.5 mM iodoacetamide (Sigma), 1 mg of BSA/ml, and protease inhibitors. HLA-DR was immunoprecipitated with MAb HB10A and protein A-agarose beads, and the beads were washed in Tris-saline containing 0.1% polidocanol, 7.5 mM iodoacetamide, and 1 mM phenylmethylsulfonyl fluoride and then incubated for 30 min at room temperature in 100 mM Tris-HCl, pH 6.8, containing 20% glycerol, 2% SDS, and 0.05% bromophenol blue before electrophoresis.
Subcellular fractionation and Western blotting.
Cells were fractionated, separating denser lysosomes, endosomes, and the MIIC from other cytosolic membrane fractions, by using Percoll gradients as described previously (21, 23). Briefly, cells were washed twice with phosphate-buffered saline (PBS) containing 1 mM CaCl2 and 1 mM MgCl2, suspended in 10 mM Tris, pH 7.5-1.5 mM MgCl2, and incubated for 8 min on ice. The cells were subjected to Dounce homogenization, nuclei were pelleted at 1,500 × g, and membranes were separated by using Percoll gradients centrifuged for 1 h in an SW41 rotor at 17,500 rpm, as described previously (23). Fractions were collected from the top, and the membranes were characterized for the presence of radiolabeled class II proteins by immunoprecipitation, as described in the previous section. For Western blotting, samples were solubilized in SDS loading buffer and subjected to electrophoresis, proteins were transferred onto Immobilon-P (Millipore, Bedford, Mass.) membranes, and the membranes were blocked with 3% BSA-1% fish gelatin-1% polyvinylpyrrolidone in PBS. The membranes were incubated with antibodies specific for LAMP-1, PDI, p115, DM-α, or DR-α, the blots were washed and incubated with horseradish peroxidase-conjugated secondary antibodies, and the proteins were visualized by using Renaissance Western blot chemiluminescent reagent (New England Nuclear, Boston, Mass.).
Confocal immunofluorescence microscopy.
Cells were seeded into eight-well Labtek (Nalge/Nunc, Rochester, N.Y.) dishes. After 18 to 24 h of infection with Ad vectors, the cells were washed, fixed in PBS-4% paraformaldehyde, washed, and permeabilized with PBS-0.2% Triton X-100 for 30 min. The cells were washed and blocked with PBS-0.02% Tween 20-2% goat serum-1% fish gelatin for 1 h. Cells were incubated with primary antibodies for 2 h, washed, incubated with secondary antibodies for 2 h, mounted by using Prolong Antifade (Molecular Probes), and visualized on a Bio-Rad (Hercules, Calif.) 1024 ES laser scanning confocal system attached to a Nikon Eclipse TE300 fluorescence microscope.
Flow cytometry.
His16 cells were infected with Adtet-trans (120 PFU/cell) or AdtetUS2 and Adtet-trans (100 and 20 PFU/cell, respectively) for 18 h. Infected cells were removed from plastic dishes by trypsinization and washed in fluorescence-activated cell sorter (FACS) buffer (PBS containing 1% fetal bovine serum-0.05% sodium azide). The cells were suspended in duplicate 96-well U-bottom microtiter plates and incubated with 50 μl of a 1:1,000 dilution of the anti-class II antibody L243 for 1 h at 4°C. The cells were pelleted in the plates, washed three times with FACS buffer, and stained with fluorescein isothiocyanate-conjugated goat anti-mouse antibodies for 40 min at 4°C. The cells were washed three times with FACS buffer and analyzed by using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, Calif.).
CD4+ T-cell assays.
CD4+ T-cell assays were performed as described previously (58). Briefly, 104 His16 or Neo6 cells were plated in 96-well flat-bottom microtiter plates and infected with Ad vectors expressing US2 to US11 for 18 h or left uninfected. The infected cells were then incubated for 18 to 24 h with 2 mg of M. tuberculosis antigen/ml-Mtb39 (Corixa, Seattle, Wash.) and simultaneously with 1 × 104 to 2 × 104 Mtb39-specific TbH9 CD4+ T cells (14). Secretion of IFN-γ by the T cells was detected by a sandwich enzyme-linked immunosorbent assay (ELISA) using anti-IFN-γ antibodies and the DuoSet ELISA development system (R & D Systems, Minneapolis, Minn.).
RESULTS
Effects of HCMV glycoproteins US2 to US11 on the MHC-II antigen presentation pathway.
Expression of US2, US3, US6, or US11 in HeLa cells has been shown to cause reduced cell surface class I expression (3). However, the effects of these HCMV glycoproteins on recognition by CD8+ and CD4+ T cells have not been characterized. Given that HCMV expresses at least four inhibitors of the MHC-I pathway, as well as at least one inhibitor of the class II pathway, we wondered if there might be other inhibitors of the MHC-II pathway. Previous identification of US2 and the initial observations on degradation of class II proteins involved infection of U373 cells with wild-type HCMV and an HCMV mutant lacking US2 and US3 (58). However, in these experiments, it was frequently difficult to efficiently infect U373 cells with HCMV; although cells were incubated three times consecutively with undiluted virus stocks, often a fraction of these cells remained uninfected or expressed low levels of viral proteins. Thus, it was difficult to study the biochemical effects of glycoproteins US2 to US11 on class II by using U373 cells infected with HCMV. Macrophages, dendritic cells, and endothelial cells, which can express class II, are less efficiently infected by HCMV grown in fibroblasts (53), and there are no HCMV strains carrying US2 to US11 mutants that are better able to infect these cells. Moreover, the combined low-level expression of HCMV genes and high-level expression of class II genes in cultured macrophages and dendritic cells make it difficult or impossible to observe more-subtle effects of HCMV on class II proteins. Thus, for the characterization of proteins US2 to US11 biochemically and for inhibition of T-cell recognition, we used replication-defective Ad vectors that do not express significant amounts of Ad proteins and that allow expression of an individual HCMV glycoprotein in a variety of cells (7a, 58). We used U373 cells, glial cells that express relatively high levels of MHC-II proteins after IFN-γ treatment or transfection with CIITA, a transactivator of class II genes (58). Note that U373 cells express low levels of class II proteins naturally and present antigens to CD4+ T cells without IFN-γ or CIITA, albeit less efficiently than after CIITA activation (58). HCMV infects glial cells in vivo (64), and these cells should be considered relevant. To study the biochemical aspects of the class II pathway, we previously constructed His16 cells and stably transfected CIITA-expressing U373 cells, which express relatively high levels of class II proteins and more efficiently present exogenous (58) as well as endogenous (C. Dunn, D. Lewinsohn, and D. C. Johnson, unpublished data) antigens to CD4+ T-cell clones.
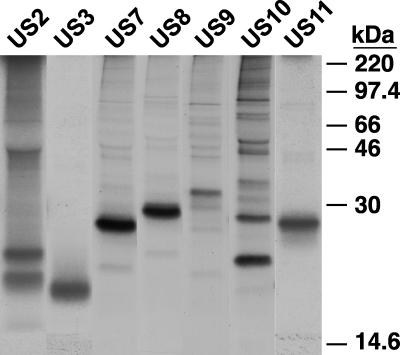
Replication-defective (E1−) Ad vectors expressing US2, US3, US7, US8, US9, US10, and US11 were constructed as described previously (7a, 27, 58). These Ad vectors do not express substantial adenoviral proteins or cause cytopathic effect in cells (41) and can be used to deliver HCMV proteins into a variety of cells at various levels of expression. Moreover, these vectors avoid the problems of overexpressing potentially toxic ER-retained glycoproteins in cells and the compensation that occurs in such transfected cells. The US2, US3, US7, US9, US10, and US11 genes were coupled to a promoter that can be induced by coinfection of cells with a second Ad vector, Adtet-trans, in the absence of tetracycline (7a, 58). AdCMV8 contains a constitutively active HCMV promoter (27). His16 cells were infected with the Ad vectors, the cells were radiolabeled, and each of the glycoproteins was immunoprecipitated with rabbit antipeptide antibodies (Fig. 1). In each case, a protein of the predicted size was detected. Modification of all of these glycoproteins with N-linked oligosaccharides was verified in other experiments (27). As previously described, the US2 glycoprotein appeared as glycosylated and nonglycosylated species (7a, 58, 63). Similarly, there were two protein bands with US10. In cells infected with AdtetUS3 only the larger, 22- to 23-kDa form of the two alternatively spliced forms of US3 (2, 32) was observed by using antibodies specific to the N terminus of US3.
FIG. 1.
Expression of HCMV glycoproteins US2 to US11 by Ad vectors. Replication-defective (E1−) Ad viruses carrying the US2, US3, US7, US8, US9, US10, and US11 genes were constructed. His16 cells were coinfected for 18 h with each of these Ad vectors and Adtet-trans where appropriate at 100 and 20 PFU/cell, respectively. The infected cells were labeled with [35S]methionine-cysteine for 1 h, and each of the respective glycoproteins US2 to US11 were immunoprecipitated with rabbit polyclonal antibodies before electrophoresis and autoradiography. Molecular masses of marker proteins are indicated at the right.
To determine whether expression of any of the individual US2 to US11 glycoproteins affected the class II pathway, we tested antigen presentation to CD4+ T cells as described previously (58). His16 cells were infected with various doses of the Ad vectors and incubated simultaneously with the Mtb39 protein. The cells were then incubated with CD4+ T-cell clone TbH9, which secretes IFN-γ in response to Mtb39 antigen derived by the endocytosis and processing of the Mtb39 protein in His16 cells (58). As expected, US2 effectively inhibited recognition of Mtb39 by TbH9 T cells in a dose-dependent manner (Fig. 2). Expression of US7, US8, US9, US10, or US11 (Fig. 2A) or combinations of these glycoproteins (not shown) did not substantially alter class II antigen presentation. However, US3 inhibited presentation of antigen by His16 cells to CD4+ T cells (Fig. 2). The effect of US3 was reproducibly less than that of US2 at a given dose. At the highest dose used here, the inhibition was 80% for US3 and 95% for US2 (Fig. 2B). US3 also effectively inhibited presentation of Mtb39 antigen in another U373-derived CIITA transfectant, Neo6, which expresses lower levels of class II (not shown). Moreover, US2 and US3 inhibited presentation of HCMV glycoprotein B (gB) to gB-specific CD4+ T cells (C. Dunn, D. Lewinsohn, and D. C. Johnson, unpublished results). We concluded that, like US2, US3 can effectively inhibit MHC-II-restricted antigen presentation to CD4+ T cells. In other experiments, US2, US3, and US11, but not US7, US8, US9, or US10, inhibited recognition of cells by CD8+ T-cell clones (results not shown).
FIG. 2.
Inhibition of antigen presentation to CD4+ T cells. His16 cells were infected with Ad vectors expressing various US2 to US11 glycoproteins and Adtet-trans at 50 and 10, 100 and 20, or 150 and 30 PFU/cell, respectively, for 18 h. Infected cells were incubated with the Mtb39 antigen and Mtb39-specific TbH9 CD4+ T cells for an additional 18 h. IFN-γ produced by the T cells was measured by ELISA. The amounts of IFN-γ were normalized to that produced by T cells incubated with antigen-exposed His16 cells infected with Adtet-trans. (A) Experiment in which US2, US3, US7, US8, US9, US10, and US11 were compared; (B) experiment involving US2 and US3. In both experiments, IFN-γ production is in arbitrary units based on a value of 100 obtained when CD4+ T cells were mixed with His16 cells infected with 150 PFU of the control Ad vector, Adtet-trans/cell.
US3 does not affect the synthesis, stability, or Golgi transport of MHC-II proteins.
US3 causes the MHC-I HC to remain for a longer period in the ER, but US3 moves slowly to the Golgi apparatus before being degraded (2, 19, 20, 32). Effects of US3 on expression, stability, and intracellular transport of HLA-DR-α and -DR-β and Ii were measured by labeling the proteins in a pulse-chase format and treating immunoprecipitated proteins with endo H, which removes N-linked oligosaccharides from immature glycoproteins resident in the ER but not from mature glycoproteins in the Golgi apparatus. In His16 cells, DR-β largely attained endo H resistance after a 6-h chase, whether or not US3 was expressed (Fig. 3A). Assembly and transport of class II proteins to the Golgi apparatus is generally slow compared with those of most other glycoproteins. DR-α attained partial endo H resistance, and again this was unaffected by US3 expression (Fig. 3A). Similarly, Ii attained partial endo H resistance, and US3 did not affect this. By contrast, the movement of class I HC was markedly reduced by US3 expression. In uninfected cells, HC was largely endo H resistant after a 1-h chase period, while in US3-expressing cells most of the HC remained endo H sensitive (Fig. 3C). After a 2-h chase, the majority of class I HC was endo H resistant in control cells, whereas over one-half of the HC in US3-expressing cells was still endo H sensitive. Neither class I nor class II proteins were lost in US3-expressing cells. Western blot analyses indicated that the total levels of DR-α were not affected, even at the highest levels of US3 expressed in these cells, for 24 h (not shown and see below). Therefore, US3 expression does not obviously affect the synthesis of class II proteins, their stability, or transport to the Golgi apparatus.
FIG. 3.
Effects of US3 on class I and class II synthesis and maturation. His16 cells were left uninfected (UN; A to C) or were infected with AdtetUS3 and Adtet-trans at 100 and 20 or 200 and 40 (A and C) or 150 and 30 PFU/cell (B), respectively, for 18 h. Infected cells were labeled with [35S]methionine-cysteine in a pulse-chase format. DR-α and DR-β (A), Ii (B), and MHC-I HC (C) were immunoprecipitated from cell extracts with MAb DA6.147, HB10A, and PIN.1, and rabbit anti-HC serum, respectively. (D) His16 cells were infected with Adtet-trans alone at 120 PFU/cell, AdtetUS2 and Adtet-trans at 100 and 20 PFU/cell, respectively, or AdtetUS3 and Adtet-trans at 100 and 20 PFU/cell, respectively. The cells were labeled for 12 min, the label was chased for 120 min, and then DM-α and DM-β were immunoprecipitated with MAbs 5C1 and MaP.DMB/C, respectively. For endo H treatment, precipitated proteins were divided in half and treated with endo H (+) or not treated (−). The proteins were subjected to SDS-polyacrylamide gel electrophoresis and visualized by autoradiography. r, endo H-resistant species of DM; s, endo H-susceptible species of DM.
Since class II antigen presentation was inhibited by US3, it was possible that US3 affected HLA-DM, which is required for efficient loading of peptide antigens. As expected from previous results (58), pulse-chase experiments on His16 cells showed that US2 caused loss of expression of DM-α, and there was little or no effect on DM-β (Fig. 3D, middle). By contrast, in cells expressing US3 both DM-α and DM-β were expressed normally and without any effect on endo H resistance (Fig. 3D, bottom). Note that DM-α showed a shift in electrophoretic mobility without acquiring endo H resistance in chase samples, as has been observed previously (13), and this processing was not affected by US3. The DM-β chain, which is complexed with DM-α, largely attained endo H resistance in cells infected with either AdUS3 or the control Adtet-trans. Steady-state levels of DM, measured by Western blotting, were not affected by US3 expression (not shown). Therefore, US3 does not obviously affect the synthesis of DM or its transport to the Golgi apparatus.
US3 binds to newly synthesized class II α/β heterodimers in the ER and reduces the assembly of Ii onto class II complexes.
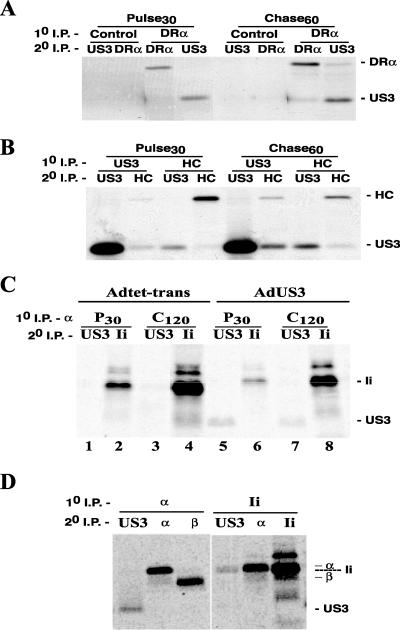
US3 binds to MHC-I protein complexes in the ER, causing ER retention or retrieval (2, 19, 32). We examined whether US3 interacted with class II proteins by immunoprecipitating class II complexes with anti-DR-α antibody DA6.147. Protein complexes were denatured and reprecipitated with anti-US3 or anti-class II antibodies. When cells were labeled for 30 min, US3 was present in material immunoprecipitated with DA6.147 but not with a control MAb (Fig. 4A). US3 was also observed with class II complexes after a 60-min chase, suggesting that this was a relatively stable interaction. As expected, US3 was found associated with MHC-I complexes (HC) in both the pulse and chase samples (Fig. 4B). Anti-US3 polyclonal antibodies did not precipitate class I and class II proteins well, possibly because anti-US3 antibodies displaced MHC proteins or because the fraction of US3 that contains class I and II proteins is relatively low. Therefore, we concluded that US3 binds to class II complexes shortly after synthesis in the ER and remains stably associated.
FIG. 4.
US3 binds HLA-DR and inhibits association of Ii with the α/β heterodimer. His16 cells were infected with 120 PFU of Adtet-trans/cell or 100 and 20 PFU of AdtetUS3 and Adtet-trans/cell, respectively. After 18 h, the cells were labeled in a pulse (30 min)-chase (60 [A and B] and 120 min [C]) format. Digitonin cell extracts were subjected to primary immunoprecipitation, followed by denaturation in SDS and reprecipitation. (A) Primary precipitation with a control mouse MAb or anti-DR-α MAb (DA6.147) followed by secondary precipitation with rabbit anti-US3 or anti-DR-α. (B) Primary and secondary precipitations with rabbit antisera to MHC-I HC and US3. (C) Primary precipitation with anti-DR-α antibody DA6.147 followed by secondary precipitation of US3 with rabbit antiserum or Ii with MAb PIN.1. (D) Primary precipitation of DR-α with MAb DA6.147 or of Ii with PIN.1 followed by secondary precipitation of US3, DR-α, DR-β, and Ii with rabbit antisera to US3 or MAbs DA6.147, HB10A, and PIN.1, respectively.
Given that US3 binds to class II α/β complexes early after their synthesis, it was reasonable that this might affect the association of the α/β dimer with Ii in the ER. Sequential immunoprecipitation experiments, in which material precipitated first with DA6.147 was denatured and reprecipitated for Ii or US3, were performed. In control cells, Ii was readily detected in association with α/β after a 30-min pulse, the amount increasing over the 120-min chase period (Fig. 4C). In US3-expressing cells, there was three- to fourfold less Ii associated with class II complexes, in both the pulse and chase samples. Note that the synthesis and maturation of Ii itself were not altered when US3 was present (Fig. 3B). Ii is intensely labeled metabolically, as it contains relatively more methionine and cysteine residues than DR-α or DR-β. We concluded that α/β/Ii complexes were formed in US3-expressing cells but that smaller quantities of α/β were assembled with Ii. To ask whether US3 was associated with the α/β/Ii complexes, immunoprecipitations were performed for US3, DR-α, and Ii on material initially precipitated with anti-Ii or anti-DR-α antibodies. US3 was detected in complexes precipitated with anti-DR-α, but not with anti-Ii (Fig. 4D). This experiment also served as a specificity control, as anti-Ii MAb PIN.1 precipitated DR-α but not US3. Unfortunately, since our anti-US3 antibodies did not coprecipitate class I or II proteins well, it was impossible to determine if Ii was present in US3 complexes. However, it was clear that US3 reduces the assembly of Ii onto DR-α/β heterodimers and that α/β/Ii complexes have little or no US3.
Subcellular localization of US3 and class II proteins in US3-expressing cells.
To determine where US3 and class II proteins were localized in cells expressing US3, we used confocal microscopy. Previous studies involving HeLa cells expressing class I and not class II proteins indicated that US3 was largely found in the ER, with some evidence for movement to the Golgi apparatus and degradation in lysosomes (19). In His16 cells that also express class II proteins, little or no US3 colocalized with ER markers PDI (Fig. 5A) or calnexin or calreticulin (not shown). US3 colocalized extensively with Golgi markers p115 (Fig. 5B) and GM130 (not shown), as well as the TGN marker TGN46 (Fig. 5C). By contrast, US3 was not found in lysosomes stained for LAMP-1 (Fig. 5D), which is also found in the MIIC (7).
FIG. 5.
US3 localizes to the Golgi apparatus. His16 cells were infected with 150 and 30 PFU of AdtetUS3 and Adtet-trans/cell, respectively. After 18 h, the cells were fixed and permeabilized and incubated simultaneously with rabbit antibodies to US3 (red) and mouse antibodies to cellular markers (green) PDI (A), p115 (B), TGN46 (C), or LAMP-1 (D). Primary antibodies were visualized with secondary fluorescent antibodies by using a laser scanning confocal microscope.
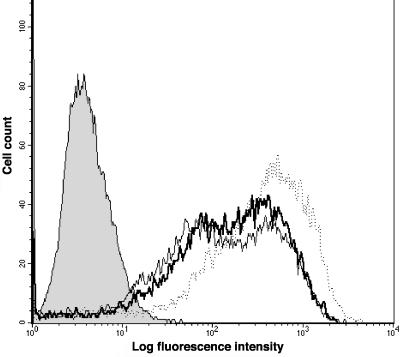
In control cells (infected with Adtet-trans), MHC-II proteins were found in the Golgi apparatus colocalizing with p115 (Fig. 6A), in the TGN (not shown), and in lysosomes colocalizing with LAMP-1 (Fig. 6B), with lesser amounts on the cell surface (Fig. 6B). In AdtetUS3-infected cells, class II was also found in the Golgi apparatus (Fig. 6C), TGN (not shown), and lysosomes (Fig. 6D). A fraction of class II colocalized with US3 in the Golgi (Fig. 6C). Note that colocalization of all three proteins produces a white image. Again there was no evidence of US3 in lysosomes (Fig. 5D). In some of the US3-expressing cells, especially those that expressed high levels of US3, class II proteins were present in small nonperinuclear vesicles distributed uniformly throughout the cytoplasm (Fig. 6C). No differences in DM localization in US3-expressing versus control cells were observed (not shown). To compare the cell surface distributions of class II proteins, FACS analyses were performed. The mean fluorescence intensities for cells infected with Adtet-trans, AdtetUS3, and AdtetUS2 were 503, 292, and 258, respectively (Fig. 7). However, in other experiments there was less-apparent or no differences in the amounts of class II on the surfaces of cells expressing US2 or US3. Therefore, the steady-state levels of surface class II are altered only very slightly in some experiments, and not at all in other experiments, by expression of either US2 or US3.
FIG. 6.
Effects of US3 on intracellular pools of class II proteins. Neo6 cells were infected with Adtet-trans alone (180 PFU/cell; A and B) or with AdtetUS3 and Adtet-trans (150 and 30 PFU/cell, respectively; C and D). After 18 h, the cells were fixed and permeabilized and incubated simultaneously with rabbit antibodies to class II (red) and mouse antibodies to cellular markers (green) p115 (A and C) and LAMP-1 (B and D), as well as rat antibodies to US3 (blue; C and D). The intracellular proteins were visualized as in Fig. 5.
FIG. 7.
Effects of US3 on cell surface class II molecules. His16 cells were infected with Adtet-trans alone (120 PFU/cell) or with AdtetUS3 and Adtet-trans (100 and 20 PFU/cell, respectively) for 18 h. Class II molecules on the surfaces of infected cells were stained by incubating suspended cells with L243 and then with fluorescein isothiocyanate-conjugated goat anti-mouse IgG. The results were assessed by flow cytometry. The designation of the histograms are as follows: shaded, secondary antibody control; dotted line, Adtet-trans-infected cells; thin line, AdtetUS2-infected cells; thick line, AdtetUS3-infected cells.
The confocal microscopy and FACS results suggested that US3 expression produced little or no change in surface class II levels. By confocal microscopy, some cells expressing high levels of US3 displayed redistribution of class II proteins into cytoplasmic vesicles that did not stain with antilysosomal antibodies, but in other cells class II was found in lysosomal compartments. It is important that there are long-lived and substantial pools of intracellular class II proteins in these cells, proteins which do not reach the cell surface for over 12 h after synthesis. The delivery of US3 in these cells was transient; significant expression of US3 began 8 to 12 h after infection with AdtetUS3, and the effects of US3 on class II proteins were measured 18 to 24 h postinfection. Thus, pools of intracellular and cell surface class II present before US3 expression were apparently less dramatically affected by US3. Under similar circumstances of US3 expression, presentation of antigens to CD4+ T cells was reduced by three- to fivefold (Fig. 2). Class II-mediated presentation of antigens largely or exclusively involves newly synthesized class II proteins (1, 12). Therefore, it appeared likely that US3 delivered by using Ad vectors affected newly synthesized MHC-II proteins, reducing association with Ii without affecting the substantial pool of preexisting class II proteins.
US3 inhibits loading of peptides onto class II proteins and movement to lysosomes.
To examine the effects of US3, as well as other US2 to US11 glycoproteins, on the loading of antigenic peptides onto class II dimers, SDS stability assays were performed. Class II α/β dimers bound by peptide antigens are relatively stable in 1 to 2% SDS, and these α/β dimers can be observed on SDS-polyacrylamide gels (17). His16 or Neo6 cells expressing US2 to US11 glycoproteins were radiolabeled for 60 min, the label was chased for 3 or 9 h, and then class II complexes were immunoprecipitated and incubated in 2% SDS at room temperature. As expected, US2 expression caused rapid loss of expression of class II complexes (Fig. 8A). Glycoproteins US7, US8, US9, US10, and US11 did not substantially affect the SDS stability of α/β complexes (Fig. 8A and B). By contrast, US3 reduced the amounts of SDS-stable α/β dimers by approximately threefold in His16 cells (Fig. 8A and B). In Neo6 cells, which express lower levels of class II proteins than His16 cells, the inhibition by US3 of SDS-stable dimers was as much as ninefold (Fig. 8C). Therefore, US3 expression reduces the loading of peptides onto class II complexes.
FIG. 8.
US3 inhibits formation of SDS-stable class II complexes. His16 (A and B) and Neo6 (C) cells were left uninfected or were coinfected with Ad vectors expressing various US2 to US11 glycoproteins and Adtet-trans at 100 and 20 PFU/cell, respectively (A and B) or the indicated amounts of virus (C). After 18 (A and B) or 30 h (C), the cells were labeled in a pulse (60 min; P)-chase (3 or 9 h) format. Class II complexes were immunoprecipitated from cell extracts with MAb HB10A, incubated with 2% SDS at room temperature for 30 min, and subjected to electrophoresis. (A) Visualization of DR-α/β complexes in His16 cells after the pulse or a 3- or 9-h chase. (B) Quantification of DR-α/β complexes in His16 cells after a 9-h chase period (from panel A). (C) Quantification of DR-α/β complexes in Neo6 cells infected with AdtetUS3 or AdtetUS9, following a 9-h chase period (in a separate experiment).
To further characterize the effects of US3, especially concentrating on class II proteins synthesized after substantial US3 expression, cell fractionation was performed. Percoll gradients have been used to separate dense lysosomal fractions, including the MIIC, from other cytoplasmic membranes (21, 23). Western blotting was used to measure the distribution of cellular markers and the steady-state levels of class II proteins. The lysosomal marker LAMP-1 accumulated predominantly in the densest fractions, 15 and 16, at the bottom of these gradients, whereas the ER marker PDI was found near the top in fractions 1 to 3 (Fig. 9A, top). Other cellular markers for the Golgi apparatus and the plasma membrane were similarly at the top of the gradient, in fractions 1 to 4 (not shown). DR-α, detected by Western blotting, was found distributed more randomly across the gradient, with higher concentrations in fractions 1 to 3 and substantial amounts in the lysosomal fractions 15 and 16 (Fig. 9A). There were no significant differences in the steady-state levels or distribution of DR-α in AdtetUS3-infected cells (US3) compared with cells infected with control Ad vector Adtet-trans. Similarly, US3 did not alter the distribution of any of the cellular markers including LAMP-1 (not shown).
FIG. 9.
US3 reduces accumulation of DR-α/β complexes in lysosomal compartments. (A) Uninfected Neo6 cells were fractionated with Percoll gradients, and fractions were subjected to electrophoresis and Western blotting for LAMP-1, PDI, and DR-α. For DR-α, cells were infected either with 120 PFU of Adtet-trans/cell alone (DR/trans) or with 100 and 20 PFU of AdtetUS3 and Adtet-trans (DR/US3)/cell, respectively, before fractionation and Western blotting. (B) Neo6 cells were infected with 120 PFU of Adtet-trans/cell alone (trans) or with 100 and 20 PFU of AdtetUS3 and Adtet-trans (US3)/cell, respectively, for 18 h. Infected cells were radiolabeled for 1 h, and the label was chased for 8 h. The cells were fractionated with Percoll gradients, and the fractions were collected from the top and numbered 1 to 16. The membrane fractions were solubilized in NP-40-DOC lysis buffer, and DR proteins were immunoprecipitated with MAb DA6.147. The samples were subjected to electrophoresis and phosphorimager analysis.
Notwithstanding these results, it was reasonable that US3 might affect transport of newly synthesized class II proteins to the MIIC. As discussed above, the newly synthesized class II molecules make up only a relatively small fraction of the total class II in the cytoplasm of these cells, as assembly and transport are slow. To test this hypothesis, infected Neo6 cells were radiolabeled, the label was chased for 8 h, and the cells were fractionated as described above. In this experiment, US3-expressing cells displayed a 3.8-fold reduction in the levels of class II DR-α and -β in fractions 15 and 16, compared with control cells (Fig. 9B). There was a reproducible reduction in class II complexes in fractions 15 and 16 in five separate experiments. However, there was not an obvious increase in class II proteins found in other fractions, probably because the sum of class II present in other fractions, e.g., 1 to 9, far exceeded that present in fractions 15 and 16 and there was often a modest (1.2-fold) increase in fractions 1 to 9. Thus the increase in class II in fractions 1 to 9 would not be expected to be threefold. We note that Western blots (Fig. 9A) and pulse-chase experiments (Fig. 3A) did not indicate alterations in the total levels of class II proteins or their stability. Therefore, we concluded that US3 can reduce accumulation of newly synthesized class II molecules in lysosomal compartments, where loading of class II with peptides occurs. This result can largely explain the observed three- to fivefold reductions in loading of peptides onto class II complexes (Fig. 8), as well as in presentation to CD4+ T cells (Fig. 2).
DISCUSSION
Our results demonstrate that US3 inhibits the MHC-II pathway, reducing presentation to CD4+ T lymphocytes and altering the intracellular events involved in loading peptides onto class II proteins. US3's effects on class II proteins can be added to a growing list of effects of glycoproteins US2 to US11 (reviewed in references 28, 30, and 60). US2 to US11 are all retained in the ER or Golgi apparatus and do not reach the cell surface (27). Collectively, these viral proteins can be viewed as proteins that bind classical as well as nonclassical (5, 57) MHC molecules, causing their destruction, retention, or mislocalization in cells. However, the consequences of the binding of any one of these glycoproteins to individual MHC substrates can differ dramatically. For example, US2 causes class I proteins to be translocated into the cytoplasm before being degraded (63), whereas class II DR-α remains membrane bound until degraded (58). We have recently produced mutant forms of US2 that show preference for class II versus class I proteins or vice versa (7a). Similarly, US3 binds to both MHC-I and -II proteins, but the results are strikingly different.
US3 binds to class I proteins early after their synthesis in the ER, causing retention or slowed onward movement to the Golgi apparatus (2, 19, 32). In transfected HeLa cells, US3 localizes largely to the ER but partially colocalizes with the Golgi marker COP1 and may be transported eventually to lysosomes before being rapidly degraded (32). Retention of class I required continued expression of US3, implying a transient interaction and a continuous exchange between US3 and class I molecules (32). It was interesting that little US3 accumulated in the ER in our His16 cells, which express both class I and class II proteins. Perhaps, a more substantial fraction of US3 in these cells moves to the Golgi apparatus in complex with class II proteins. Alternatively, US3 may alter class I or class II without remaining bound to the MHC proteins, as has been described for human herpesvirus-8 K3 and K5 proteins (9).
The effects of US3 on MHC-II proteins were quite different from those on class I. In contrast to its effects on class I, US3 altered the assembly of class II complexes by binding class II proteins before or during assembly of α/β complexes in the ER. This binding was relatively stable, as US3 was observed in association with class II complexes during subsequent chase periods, although there may also be exchange of US3 molecules onto class II complexes. The amount of Ii associated with the class II α/β heterodimers was reduced by three- to fourfold. We could not detect US3 in α/β/Ii complexes. Thus, it appears that US3 binds to class II complexes, preventing the binding of Ii. Ii acts as a chaperone in the ER, preventing illegitimate peptide loading and promoting final maturation, folding, and assembly of class II complexes (11). Thus, class II complexes lacking Ii and containing US3 may be misfolded and assembled in a different fashion from the nonameric α/β/Ii complexes that normally leave the ER. However, US3 may substitute for Ii in certain aspects of class II folding.
Ii contains sequences that affect the sorting of class II α/β/Ii complexes from the Golgi apparatus to acidic peptide loading compartments, the MIICs (35, 39). α/β complexes expressed in the absence of Ii accumulate predominantly in the ER and Golgi apparatus, but a substantial fraction of these molecules reach the cell surface without peptides (39, 51, 52). In His16 cells expressing US3, all of the class II proteins acquired endo H-resistant oligosaccharides normally. Thus, class II α/β heterodimers with bound US3 and without Ii reach the Golgi apparatus without obvious delay. It is reasonable to suggest that US3 can substitute for Ii in mediating transport as far as the Golgi apparatus, although transport beyond the Golgi apparatus to acidic loading compartments appears to be altered.
Cell fractionation experiments demonstrated that labeled class II complexes comprising newly synthesized class II proteins, as opposed to pre-existing class II proteins, were less able to accumulate in dense lysosomal compartments. Associated with reduced traffic to lysosomes, there was three- to ninefold-reduced loading of antigenic peptides onto class II dimers, as measured by SDS stability. This reduced peptide loading can entirely explain the inhibition of antigen presentation in US3-expressing cells. Obvious effects on the synthesis, maturation, or stability of DM that could account for reduced peptide loading were not observed. Therefore, it appears that class II complexes with US3 bound and lacking Ii were mislocalized and directed to other post-Golgi compartments, so that they did not reach the MIIC. US3/class II complexes lack Ii and its signals for sorting to lysosomes/MIIC. It is possible that the absence of these lysosomal targeting signals largely explains the missorting of class II complexes. Alternatively, US3 may possess motifs that sort class II complexes to post-Golgi compartments other than lysosomes/MIIC.
The exact nature of the aberrant intracellular transport of class II proteins in US3-expressing cells was difficult to investigate for two reasons. First, substantial intracellular and cell surface pools of class II proteins preexisted expression of US3. Cell surface class II is long lived, persisting for over 24 h in some cells (12), and there are often large pools of intracellular class II (Fig. 6). Thus, class II molecules synthesized after US3 was expressed at abundant levels in these cells (12 h after Ad infection) were superimposed on a relatively large background of preexisting class II. Therefore, our confocal and FACS analyses could not consistently discriminate between class II molecules that were affected by US3 and those that preexisted US3 expression. Longer periods of expression of US3 proved counterproductive because of cytotoxicity. Second, the observed effects of US3 were often more subtle than those of US2. US3 at the highest doses affected only about 70 to 80% of the class II proteins.
One could argue that US3-transfected cells might be more useful to characterize its effects on the class II pathway. However, US2 and US3 are normally expressed by HCMV, a virus that expresses these glycoproteins transiently at early stages of infection. In this sense, cells infected with Ad vectors more accurately reflect the situation in vivo, compared with stably transfected cell lines, where long-term expression can have pleiotropic effects. In HCMV-infected cells, newly synthesized proteins likely succumb to the effects of both US2 and US3, but class II proteins produced before infection would not be affected. US3 is expressed as an immediate-early gene product, while US2 is expressed somewhat later as an early protein (32). US2 and US3 may act temporally to downregulate class II, or there may be additive or synergistic effects in HCMV-infected cells.
Why does HCMV inhibit both class I and class II antigen presentation, by using US2 and US3, and transcription of class II genes (38, 40) by using unknown genes? The class II pathway normally allows professional APCs to present extracellular proteins to CD4+ T cells. These APCs would not be subject to the effects of glycoproteins US2 to US11 unless the APCs were themselves infected. HCMV is known to infect a variety of cell types that can express MHC-II proteins, either constitutively or after cytokine stimulation, including monocytes/macrophages and dendritic, endothelial, glial, and epithelial cells (54). In these cells, inhibition of class II presentation would allow virus-infected cells to escape detection by CD4+ T cells that can act as cytotoxic cells or to produce antiviral cytokines and coordinate antiviral immune responses. Consistent with this, the proposed assembly of HCMV in the TGN or acidic compartments (16, 29, 49, 59) would provide a plentiful source of viral structural proteins targeted to these compartments for presentation by class II proteins. Therefore, it makes ample sense that HCMV, a virus that grows slowly and that persists for long periods, would benefit by inhibiting class II-restricted endogenous antigen presentation.
The important question about which cells might be affected by US2 and US3 in vivo has been difficult to address in vitro. HCMV replicates poorly in most cultured cells other than normal human fibroblasts and expresses viral genes weakly or not at all in cultured cells that express class II. Monocytes/macrophages are an important cell type in vivo, but only a fraction of these cells cultured in vitro can be infected with laboratory-adapted strains of HCMV (53). Moreover, the expression of many classes of viral genes is often low in those macrophages that are infected. Clinical HCMV strains or viruses passaged in endothelial cell cultures can infect monocytes/macrophages and dendritic cells better (44, 46), but there are no strains with mutant US2 and US3 at present. Downmodulation of class II molecules has been observed with dendritic cells (46), although this may be due to multiple mechanisms. Given the high levels of class II proteins expressed in professional APCs such as dendritic cells and macrophages, it appears highly unlikely that HCMV US2 and US3 could damage the class II pathway in these cells either in vitro or in vivo. It is conceivable that US2 and US3 may more effectively damage the class II pathway in endothelial and epithelial cells in vivo since these cells express lower levels of class II than macrophages or dendritic cells. However, again, HCMV infection of these cells cultured in vitro is inefficient. Those that call for studies of the effects of US2 and US3 in biologically relevant cells by using HCMV infection should recognize the difficulties of working with HCMV in cultured cells, especially cells that express class II. The use of Ad vectors has distinct advantages compared to transfected cells (62, 63); because US2 or US3 are delivered over a relatively shorter period, as during HCMV infection, cells do not adapt to the toxic effects of accumulated ER proteins and the levels of the viral proteins can be readily adjusted in a variety of cell types.
For these reasons, we chose to study the effects of US2 and US3 in U373 glial cells transfected with CIITA by using Ad vectors. U373 cells naturally and constitutively express class II proteins at relatively low levels and, without induction of class II, can present exogenous antigens to CD4+ T cells (58). Class II is upregulated after treatment with IFN-γ or transfection with CIITA (58). Glial cells are quite relevant for studies of the effects of US2 and US3, especially because HCMV infects glial cells in the nervous system and the retina (43, 61, 64). HCMV is a leading cause of congenital birth defects, and this often involves infection in the central nervous system leading to brain damage and hearing loss (6). A recent publication (45) challenged the notion that US2 could affect MHC-II proteins and suggested that there might be aberrant expression of class II proteins in U373 cells stably transfected with CIITA, leading to an unfolded-protein response. However, this appears unlikely as class II proteins do not turn over rapidly, and US2 hastens the turnover of class II in these cells. Moreover, these cells very efficiently present exogenous tuberculosis (TB) antigens to TB-specific CD4+ T cells (58) and can also efficiently present endogenously expressed HCMV gB to gB-specific CD4+ T cells (C. Dunn, D. Lewinsohn, and D. C. Johnson, unpublished results). In both cases, this MHC-II presentation can be blocked by expression of either US2 or US3. This is probably the best evidence that both US2 and US3 can effectively block the class II pathway. It appears that Rehm et al. (45) were unable to observe the effects of US2 on MHC-II proteins in dendritic cells because US2 expression was insufficient and there were high levels of MHC-I and -II proteins. Testifying to this, they observed relatively rapid turnover of class I in control dendritic cells, and US2 did not significantly affect this (45). In our studies, US2 can cause extensive degradation of class II-α in a variety of cell types and when US2 is expressed by using Ad vectors or transfection (7, 57; N. Hegde and D. C. Johnson, unpublished data). We find that US2 shows a preference for class I over class II proteins; however this is only about twofold. For now, the cells that are affected in vivo by US2 and US3 remain unknown. However, it appears unlikely that HCMV has evolved the capability to inhibit the MHC-II pathway at two different steps entirely by chance.
Acknowledgments
N. R. Hegde and R. A. Tomazin contributed equally to this work.
We are indebted to Aurelie Snyder for assistance with confocal microscopy. We thank Betsy Mellins, John Trowsdale, Hidde Ploegh, Peter Cresswell, and Tom Jones for antibodies. We are grateful to Stan Riddell for advice and T-cell reagents and to Klaus Frueh for critical evaluation of the work.
This work was supported by an NIH grant from the National Eye Institute (grant EY11245).
REFERENCES
- 1.Adorini, L., S. J. Ullrich, E. Appella, and S. Fuchs. 1990. Inhibition by brefeldin A of presentation of exogenous protein antigens to MHC class II-restricted T cells. Nature 346:63-66. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, K., A. Angulo, P. Ghazal, P. A. Peterson, Y. Yang, and K. Fruh. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. USA 93:10990-10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn, K., A. Gruhler, B. Galocha, T. R. Jones, E. J. Wiertz, H. L. Ploegh, P. A. Peterson, Y. Yang, and K. Fruh. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. [DOI] [PubMed] [Google Scholar]
- 4.Beersma, M. F., M. J. Bijlmakers, and H. L. Ploegh. 1993. Human cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I H chains. J. Immunol. 151:4455-4464. [PubMed] [Google Scholar]
- 5.Ben-Arieh, S. V., B. Zimerman, N. I. Smorodinsky, M. Yaacubovicz, C. Schechter, I. Bacik, J. Gibbs, J. R. Bennink, J. W. Yewdell, J. E. Coligan, H. Firat, F. Lemonnier, and R. Ehrlich. 2001. Human cytomegalovirus protein US2 interferes with the expression of human HFE, a nonclassical class I major histocompatibility complex molecule that regulates iron homeostasis. J. Virol. 75:10557-10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Strauss (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 7.Calafat, J., M. Nijenhuis, H. Janssen, A. Tulp, S. Dusseljee, R. Wubbolts, and J. Neefjes. 1994. Major histocompatibility complex class II molecules induce the formation of endocytic MIIC-like structures. J. Cell Biol. 126:967-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Chevalier, M. S., G. M. Daniels, and D. C. Johnson. 2002. Binding of human cytomegalovirus US2 to major histocompatibility complex class I and II proteins is not sufficient for their degradation. J. Virol. 76:8265-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, E. A., and R. Yakoshi. 1984. Leucocyte typing. Springer-Verlag, Berlin, Germany.
- 9.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosman, D., J. Mullberg, C. L. Sutherland, W. Chin, R. Armitage, W. Fanslow, M. Kubin, and N. J. Chalupny. 2001. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 14:123-133. [DOI] [PubMed] [Google Scholar]
- 11.Cresswell, P. 1996. Invarian chain structure and MHC class II function. Cell 84:505-507. [DOI] [PubMed] [Google Scholar]
- 12.Davidson, H. W., P. A. Reid, A. Lanzavecchia, and C. Watts. 1991. Processed antigen binds to newly synthesized MHC class II molecules in antigen-specific B lymphocytes. Cell 67:105-116. [DOI] [PubMed] [Google Scholar]
- 13.Denzin, L. K., N. F. Robbins, C. Carboy-Newcomb, and P. Cresswell. 1994. Assembly and intracellular transport of HLA-DM and correction of the class II antigen-processing defect in T2 cells. Immunity 1:595-606. [DOI] [PubMed] [Google Scholar]
- 14.Dillon, D. C., M. R. Alderson, C. H. Day, D. M. Lewinsohn, R. Coler, T. Bement, A. Campos-Neto, Y. A. Skeiky, I. M. Orme, A. Roberts, S. Steen, W. Dalemans, R. Badaro, and S. G. Reed. 1999. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect. Immun. 67:2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrell, H., M. Degli-Esposti, E. Densley, E. Cretney, M. Smyth, and N. Davis-Poynter. 2000. Cytomegalovirus MHC class I homologues and natural killer cells: an overview. Microbes Infect. 2:521-532. [DOI] [PubMed] [Google Scholar]
- 16.Fish, K. N., C. Soderberg-Naucler, and J. A. Nelson. 1998. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation of state of Ser900. J. Virol. 72:6657-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Germain, R. N., and L. R. Hendrix. 1991. MHC class II structure, occupancy and surface expression determined by post-endoplasmic reticulum antigen binding. Nature 353:134-139. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert, M. J., S. R. Riddell, B. Plachter, and P. D. Greenberg. 1996. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature 383:720-722. [DOI] [PubMed] [Google Scholar]
- 19.Gruhler, A., P. A. Peterson, and K. Fruh. 2000. Human cytomegalovirus immediate early glycoprotein US3 retains MHC class I molecules by transient association. Traffic 1:318-325. [DOI] [PubMed] [Google Scholar]
- 20.Gruhler, A., and K. Fruh. 2000. Control of MHC class I traffic from the endoplasmic reticulum by cellular chaperones and viral anti-chaperones. Traffic 1:306-311. [DOI] [PubMed] [Google Scholar]
- 21.Guerra, C. B., R. Busch, R. C. Doebele, W. Liu, T. Sawada, W. W. Kwok, M. D. Chang, and E. D. Mellins. 1998. Novel glycosylation of HLA-DRα disrupts antigen presentation without altering endosomal localization. J. Immunol. 160:4289-4297. [PubMed] [Google Scholar]
- 22.Guy, K., V. Van Heyningen, B. B. Cohen, D. L. Deane, and C. M. Steel. 1982. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia α and β chains. Eur. J. Immunol. 12:942-948. [DOI] [PubMed] [Google Scholar]
- 23.Hammond, C., L. K. Denzin, M. Pan, J. M. Griffith, H. J. Geuze, and P. Cresswell. 1998. The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR, -DM, and -DO molecules. J. Immunol. 161:3282-3291. [PubMed] [Google Scholar]
- 24.Harlow, E., and D. Lane. 1999. Using antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Hengel, H., T. Flohr, G. J. Hammerling, U. H. Koszinowski, and F. Momburg. 1996. Human cytomegalovirus inhibits peptide translocation into the endoplasmic reticulum for MHC class I assembly. J. Gen. Virol. 77:2287-2296. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt, E. W., S. S. Gupta, and P. J. Lehner. 2001. The human cytomegalovirus gene product US6 inhibits ATP binding by TAP. EMBO J. 20:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber, M. T., R. Tomazin, T. Wisner, J. Boname, and D. C. Johnson. 2002. Human cytomegalovirus US7, US8, US9, and US10 are cytoplasmic glycoproteins, not found at cell surfaces, and US9 does not mediate cell-to-cell spread. J. Virol. 76:5748-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, D. C., and N. R. Hegde. 2002. Inhibition of the MHC class II antigen presentation pathway by human cytomegalovirus. Curr. Top. Microbiol. Immunol. 269:101-115. [DOI] [PubMed]
- 29.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, D. C., and G. McFadden. 2002. Viral immune evasion, p357-377. In S. H. E. Kaufmann, A. Sher, and R. Ahmed (ed.), Immunology of infectious diseases. American Society for Microbiology, Washington, D.C.
- 31.Jones, T. R., V. P. Muzithras, and Y. Gluzman. 1991. Replacement mutagenesis of the human cytomegalovirus genome: US10 and US11 gene products are nonessential. J. Virol. 65:5860-5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones, T. R., E. J. Wiertz, L. Sun, K. N. Fish, J. A. Nelson, and H. L. Ploegh. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. USA 93:11327-11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, T. R., L. K. Hanson, L. Sun, J. S. Slater, R. M. Stenberg, and A. E. Campbell. 1995. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J. Virol. 69:4830-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kyristis, C., S. Gorbulev, S. Hutschenreiter, K. Pawlitschko, R. Abele, and R. Tampe. 2001. Molecular mechanism and structural aspects of transporter associated with antigen processing inhibition by the cytomegalovirus US6. J. Biol. Chem. 276:48031-48039. [DOI] [PubMed] [Google Scholar]
- 35.Lamb, C. A., J. W. Yewdell, J. R. Bennink, and P. Cresswell. 1991. Invariant chain targets HLA class II molecules to acidic endosomes containing internalized influenza virus. Proc. Natl. Acad. Sci. USA 88:5998-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampson, L. A., and R. Levy. 1980. Two populations of Ia-like molecules on a human B cell line. J. Immunol. 125:293-299. [PubMed] [Google Scholar]
- 37.Lehner, P., J. T. Karttunen, G. W. Wilkinson, and P. Cresswell. 1997. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl. Acad. Sci. USA 94:6904-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Roy, E., A. Muhlethaler-Mottet, C. Davrinche, B. Mach, and J. L. Davignon. 1999. Escape of human cytomegalovirus from HLA-DR-restricted CD4+ T-cell response is mediated by repression of gamma interferon-induced class II transactivator expression. J. Virol. 73:6582-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lotteau, V., L. Teyton, A. Peleraux, T. Nilsson, L. Karlsson, S. L. Schmid, V. Quaranta, and P. A. Peterson. 1990. Intracellular transport of class II MHC molecules directed by invariant chain. Nature 348:600-605. [DOI] [PubMed] [Google Scholar]
- 40.Miller, D. M., B. M. Rahill, J. M. Boss, M. D. Lairmore, J. E. Durbin, W. J. Waldman, and D. D. Sedmak. 1998. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the jak/stat pathway. J. Exp. Med. 187:675-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng, P., and F. L. Graham. 2001. Construction of first-generation adenoviral vectors, p. 389-413. In J. R. Morgan (ed.), Gene therapy protocols. Humana Press Inc., Totowa, N.J.
- 42.Pieters, J. 2000. MHC class II-restricted antigen processing and presentation. Adv. Immunol. 75:159-208. [DOI] [PubMed] [Google Scholar]
- 43.Pulliam, L. 1991. Cytomegalovirus preferentially infects a monocyte-derived macrophage/microglial cell in human brain cultures: neuropathology differs between strains. J. Neuropathol. Exp. Neurol. 50:432-440. [DOI] [PubMed] [Google Scholar]
- 44.Raftery, M. J., M. Schwab, S. M. Eibert, Y. Samstag, H. Walczak, and G. Schonrich. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997-1009. [DOI] [PubMed] [Google Scholar]
- 45.Rehm, A., A. Engelsberg, D. Tortorella, I. J. Korner, I. Lehmann, H. L. Ploegh, and U. E. Hopken. 2002. Human cytomegalovirus gene products US2 and US11 differ in their ability to attack major histocompatibility class I heavy chains in dendritic cells. J. Virol. 76:5043-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riegler, S., H. Hebart, H. Einsele, P. Brossart, G. Jahn, and C. Sinzger. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81:393-399. [DOI] [PubMed] [Google Scholar]
- 47.Reyburn, H. T., O. Mandelboim, M. Vales-Gomez, D. M. Davis, L. Pazmany, and J. L. Strominger. 1997. The class I MHC homologue of human cytomegalovirus inhibits attack by natural killer cells. Nature 386:514-517. [DOI] [PubMed] [Google Scholar]
- 48.Roche, P. A., M. S. Marks, and P. Cresswell. 1991. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature 354:392-394. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanderson, F., C. Thomas, J. Neefjes, and J. Trowsdale. 1996. Association between HLA-DM and HLA-DR in vivo. Immunity 4:87-96. [DOI] [PubMed] [Google Scholar]
- 51.Schaiff, W. T., K. A. Hruska, Jr., D. W. McCourt, M. Green, and B. D. Schwartz. 1992. HLA-DR associates with specific stress proteins and is retained in the endoplasmic reticulum in invariant chain negative cells. J. Exp. Med. 176:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekaly, R. P., C. Tonnelle, M. Strubin, B. Mach, and E. O. Long. 1986. Cell surface expression of class II histocompatibility antigens occurs in the absence of the invariant chain. J. Exp. Med. 164:1490-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 54.Soderberg-Naucler, C., K. Fish, and J. A. Nelson. 1999. Cytomegalovirus, p. 209-242. In R. Ahmed and I. Chen (ed.), Persistent viral infections. John Wiley & Sons, New York, N.Y.
- 55.Stern, L. J., and D. C. Wiley. 1992. The human class II MHC protein HLA-DR1 assembles as empty αβ heterodimers in the absence of antigenic peptide. Cell 68:465-477. [DOI] [PubMed] [Google Scholar]
- 56.Streblow, D. N., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 57.Sun, Y., E. Kim, M. Jin, H. C. Sung, H. Han, D. E. Geraghty, and K. Ahn. 2000. Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules. J. Immunol. 164:805-811. [DOI] [PubMed] [Google Scholar]
- 58.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Y. Altschuler, T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Riddell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 5:1039-1043. [DOI] [PubMed] [Google Scholar]
- 59.Tooze, J., M. Hollinshead, B. Reis, K. Radsak, and H. Kern. 1993. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur. J. Cell Biol. 60:163-178. [PubMed] [Google Scholar]
- 60.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 61.van den Pol, A. N., E. Mocarski, N. Saederup, J. Vieira, and T. J. Meier. 1999. Cytomegalovirus cell tropism, replication, and gene transfer in brain. J. Neurosci. 19:10948-10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiertz, E. J., T. R. Jones, L. Sun, M. Bogyo, H. J. Geuze, and H. L. Ploegh. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84:769-779. [DOI] [PubMed] [Google Scholar]
- 63.Wiertz, E. J., D. Tortorella, M. Bogyo, J. Yu, W. Mothes, T. R. Jones, T. A. Rapoport, and H. L. Ploegh. 1996. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384:432-438. [DOI] [PubMed] [Google Scholar]
- 64.Wiley, C. A., R. D. Schrier, F. J. Denaro, J. A. Nelson, P. W. Lampert, and M. B. Oldstone. 1986. Localization of cytomegalovirus proteins and genome during fulminant central nervous system infection in an AIDS patient. J. Neuropathol. Exp. Neurol. 45:127-139. [DOI] [PubMed] [Google Scholar]