Abstract
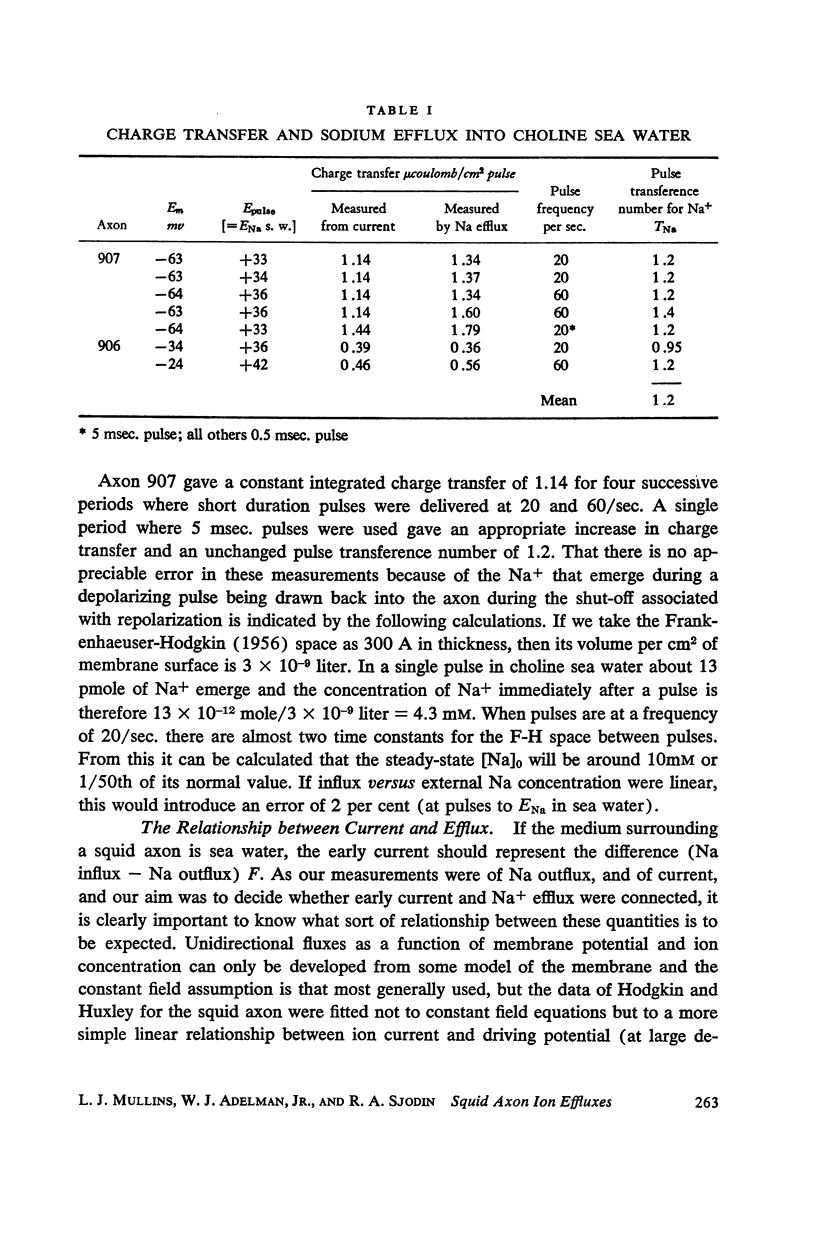
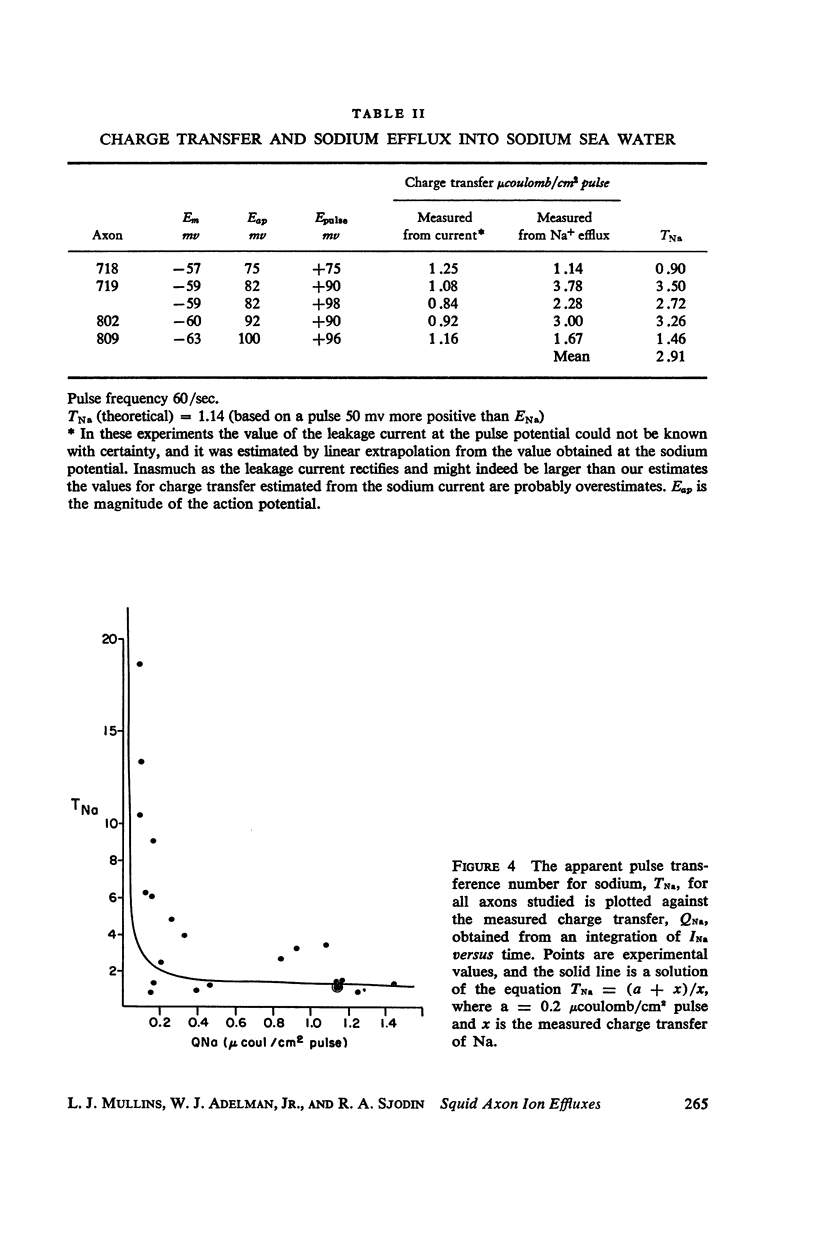
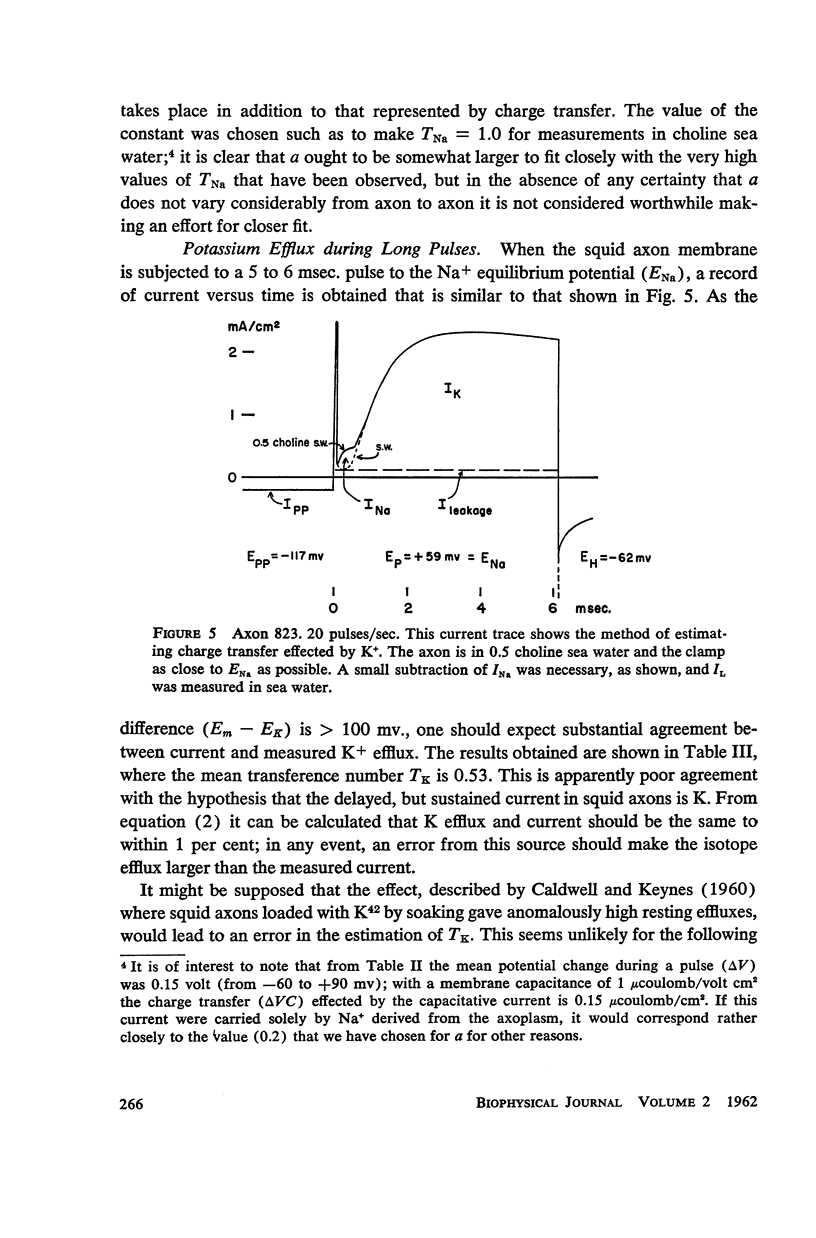
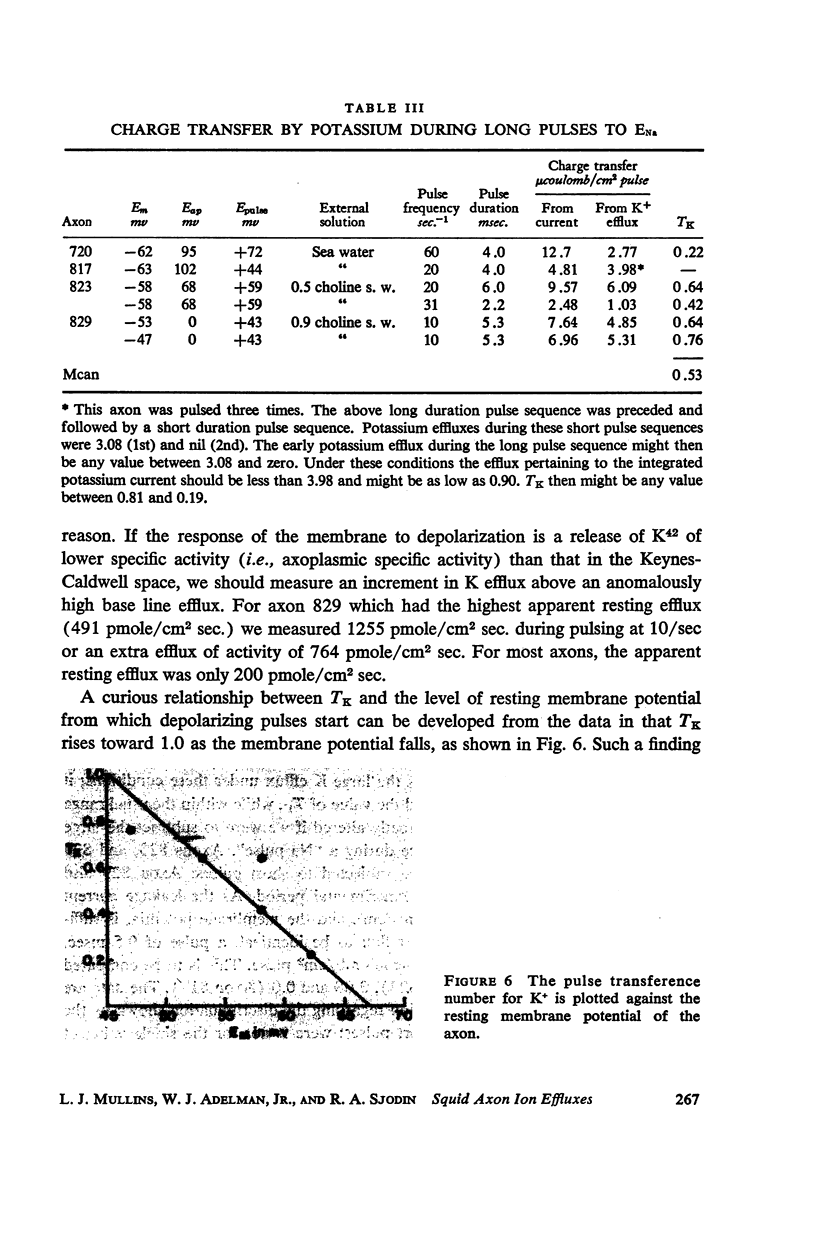
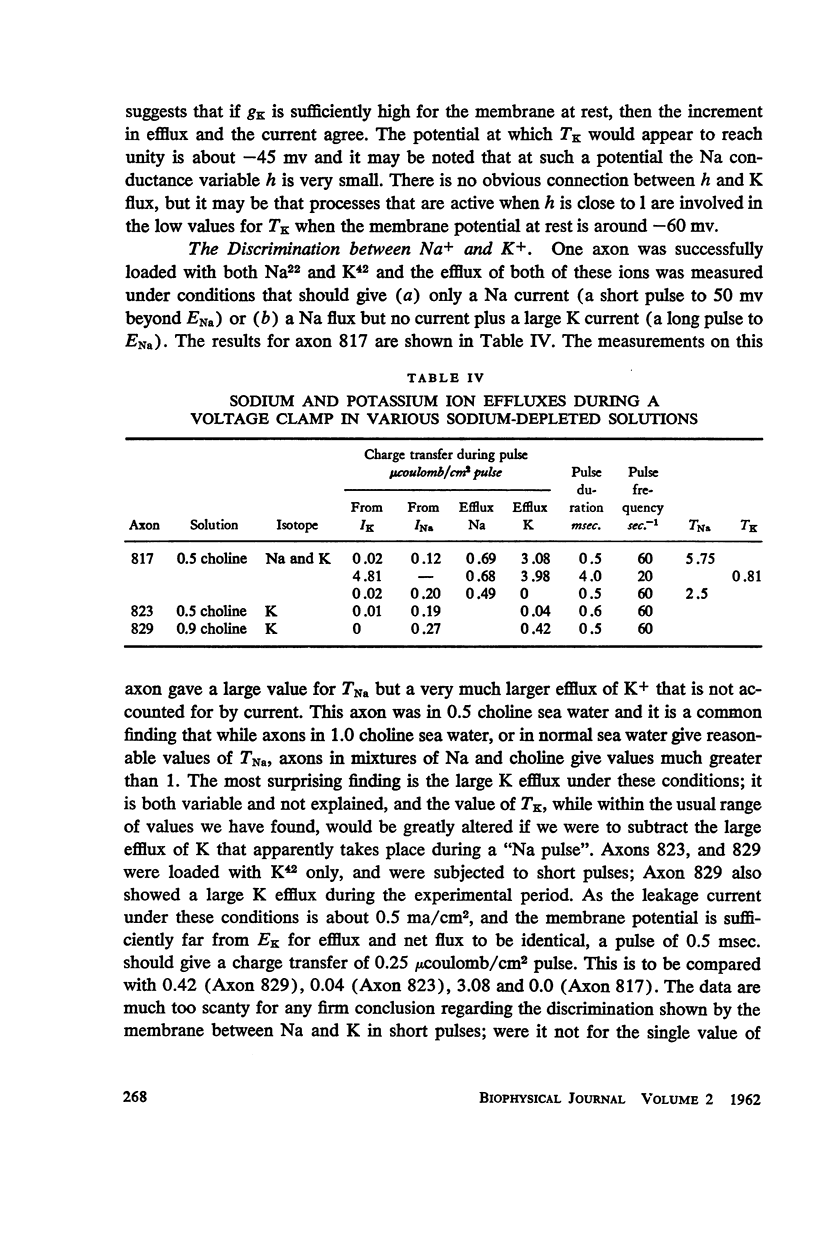
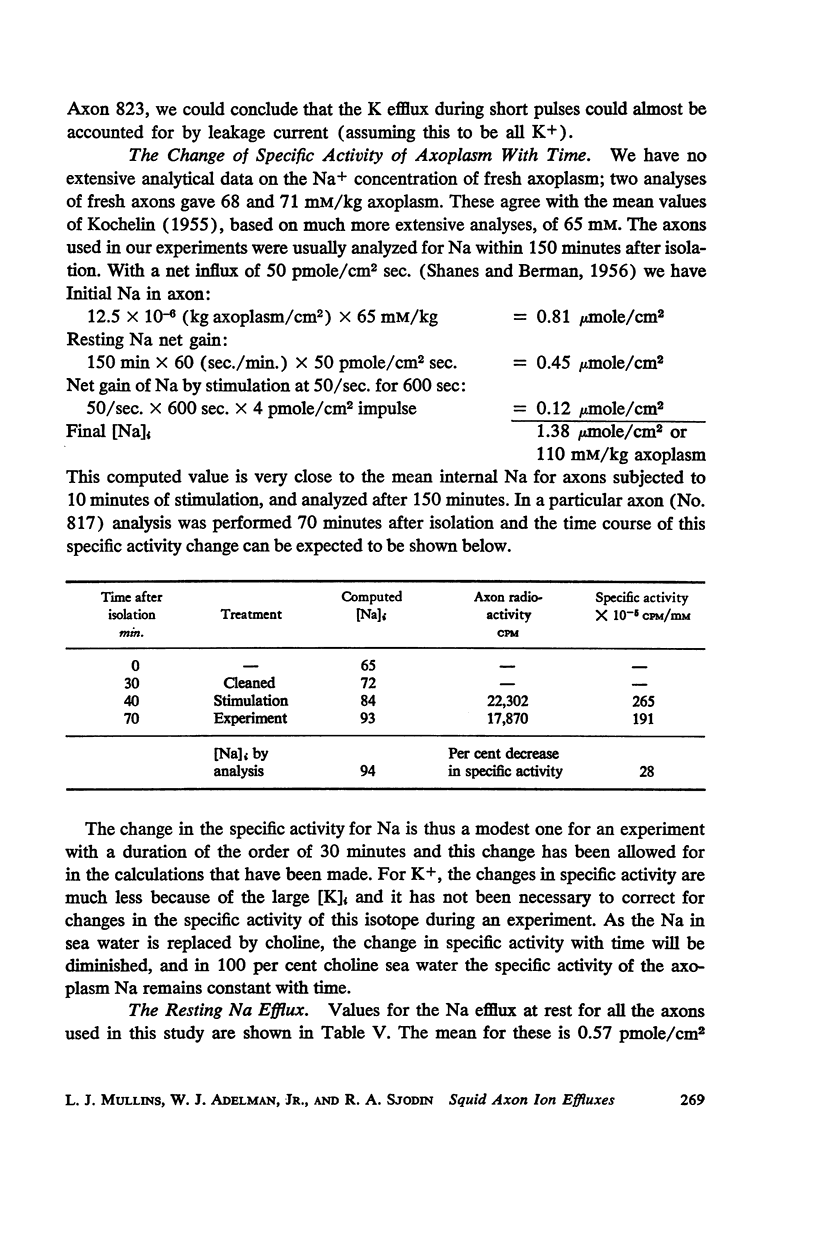
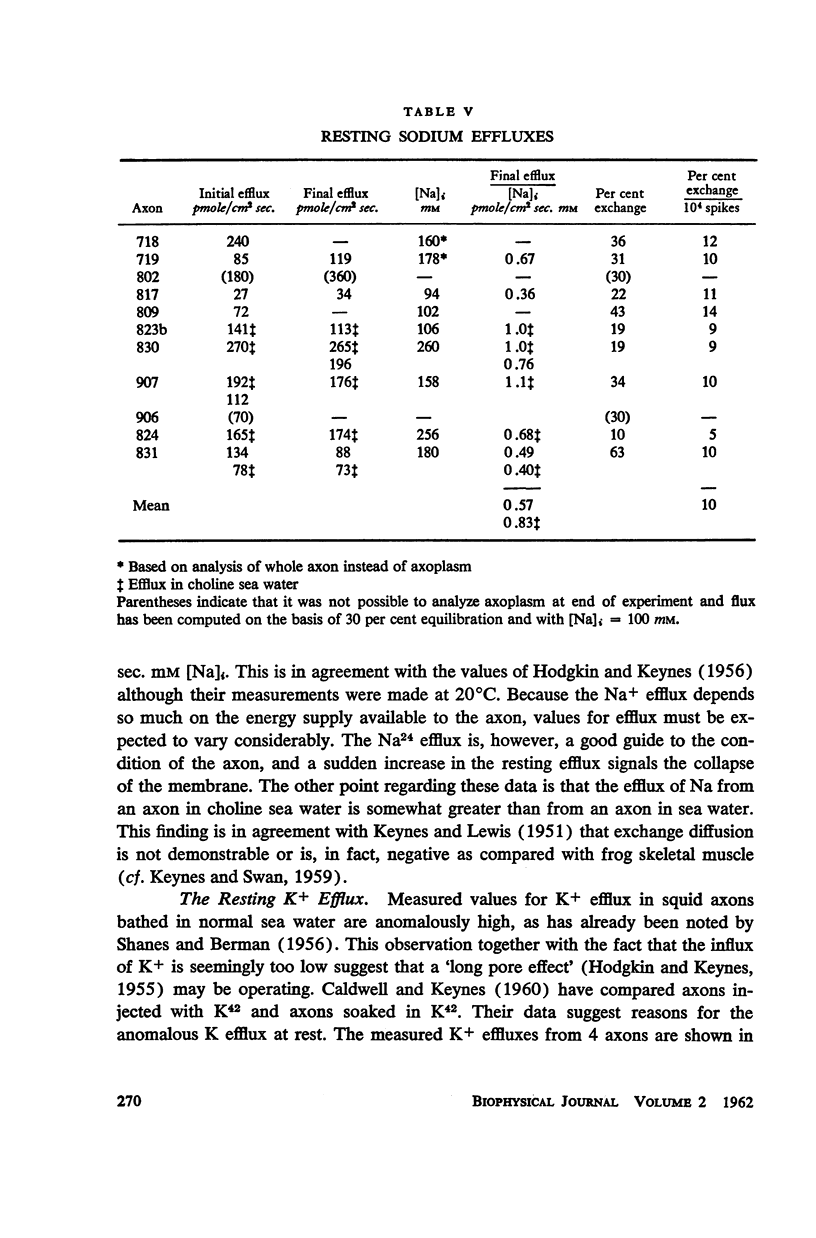
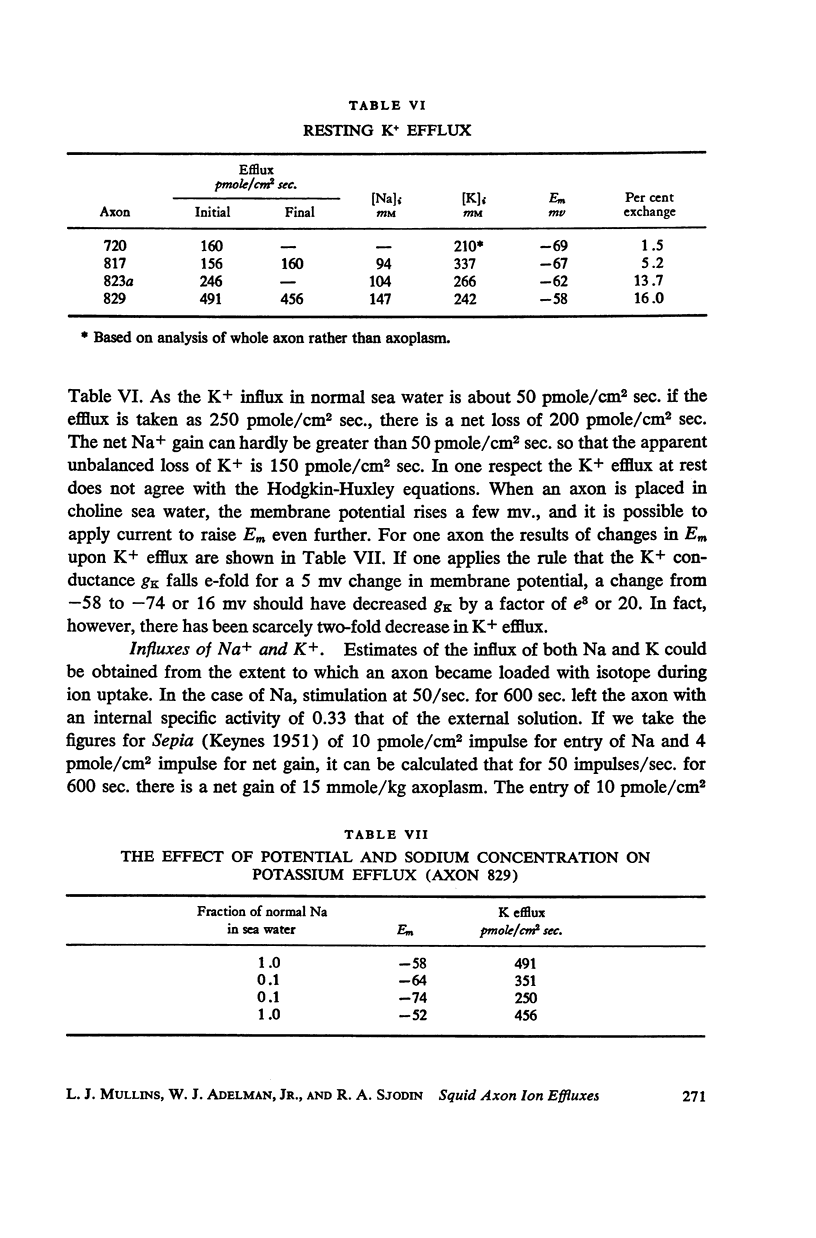
Squid giant axons loaded with Na24 were subjected to short duration (0.5 msec.) clamped depolarizations of about 100 mv at frequencies of 20/sec. and 60/sec. while in choline sea water. Under such conditions the early outward current was just about maximal at the time of termination of the clamping pulse. An integration of the early current versus time record gave 1.2 μcoulomb/cm2 pulse, while a measurement of the extra Na24 efflux resulting from repetitive pulsing gave a charge transfer of 1.4 μcoulomb/cm2 pulse. In sodium-containing sea water and with pulses 50-75 mv more positive than ENa the Na24 efflux is about 3 times the measured charge transfer. The efflux of K42 from a previously loaded axon into normal sea water is only 50 per cent of the measured charge transfer when the membrane is held for about 5 msec. at a potential such that there is no early current, and such pulses are at 10-20/sec. The experiments appear to confirm the suggestion that the early current during bioelectric activity is sodium but provide unsatisfactory support for the identification of the delayed but sustained current solely with potassium ions. Resting Na+ efflux is 0.6 pmole/cm2 sec. mmole [Na]1, while the apparent K+ efflux is about 250 pmole/cm2 sec. and is little affected by hyperpolarization.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELMAN W. J., TAYLOR R. E. Leakage current rectification in the squid giant axon. Nature. 1961 Jun 3;190:883–885. doi: 10.1038/190883a0. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C., KEYNES R. D. The permeability of the squid giant axon to radioactive potassium and chloride ions. J Physiol. 1960 Nov;154:177–189. doi: 10.1113/jphysiol.1960.sp006572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Ionic current measurements in the squid giant axon membrane. J Gen Physiol. 1960 Sep;44:123–167. doi: 10.1085/jgp.44.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Movement of radioactive potassium and membrane current in a giant axon. J Physiol. 1953 Aug;121(2):403–414. doi: 10.1113/jphysiol.1953.sp004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M., BERMAN M. D. Kinetics of ion movement in the squid giant axon. J Gen Physiol. 1955 Nov 20;39(2):279–300. doi: 10.1085/jgp.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]


