Abstract
The Epstein-Barr virus (EBV) genome is present in a variety of tumor types, including virtually all undifferentiated nasopharyngeal carcinomas (NPC) and a portion of gastric carcinomas. The uniform presence of the EBV genome in certain tumors (versus only a very small number of normal B cells) suggests that novel therapies which specifically target EBV-positive cells for destruction might be effective for treating such tumors. Although the great majority of EBV-positive tumor cells are infected with one of the latent forms of EBV infection, expression of either viral immediate-early protein (BZLF1 or BRLF1) is sufficient to convert the virus to the lytic form of infection. Induction of the lytic form of EBV infection could potentially result in death of the tumor cell. Here we have examined the efficacy of adenovirus vectors expressing the BZLF1 or BRLF1 proteins for treatment of EBV-positive epithelial tumors. The BZLF1 and BRLF1 vectors induced preferential killing of EBV-positive, versus EBV-negative, gastric carcinoma cells in vitro. Infection of C18 NPC tumors (grown in nude mice) with either the BZLF1 or BRLF1 vector, but not a control adenovirus vector, induced expression of early lytic EBV genes in tumor cells. Injection of C18 tumors with the BZLF1 or BRLF1 adenovirus vector, but not the control vector, also significantly inhibited growth of the tumors in nude mice. The addition of ganciclovir did not significantly affect the antitumor effect of the BZLF1 and BRLF1 adenovirus vectors. These results suggest a potential cancer therapy against EBV-related tumors.
The Epstein-Barr virus (EBV) is a ubiquitous virus that infects more than 90% of the human population and has been linked to a growing number of malignant diseases (27, 41), including nasopharyngeal carcinoma (NPC) (57), Hodgkin's disease (52), Burkitt's lymphoma (34), gastric carcinoma (45), leiomyosarcoma (36), and possibly breast cancer (3). Regardless of whether EBV is required for the development and/or persistence of particular tumors, the near universal presence of the viral genome in certain tumor types suggests that it is a potential target for therapeutic antitumor strategies. Researchers have been exploring the concept that modulation of EBV gene transcription in tumor cells can be used to therapeutic advantage (25, 53, 54).
EBV can establish a latent or lytic infection in host cells (27, 41). However, EBV-infected tumor cells are almost always latently infected. In the latent forms of infection, the virus is replicated once per cell cycle as an episome using the viral oriP replication origin and host cell DNA polymerase (27, 41). EBV proteins known to contribute to cellular transformation are expressed in the latent types of infection (27, 41).
Expression of either one of the EBV immediate-early (IE) proteins, BZLF1 and BRLF1, is sufficient to induce a switch from latent to lytic viral replication in the infected cell (6, 26, 40, 42, 43, 49, 54, 56). BZLF1 and BRLF1 are both transcriptional activators (2, 5, 16, 20, 29), and each IE protein initially activates transcription of the other IE gene (2, 10, 29, 56). Both IE proteins are required together for induction of the full complement of early viral genes necessary for lytic viral replication (10). During lytic infection, the virus produces a linear genome and is replicated through the viral oriLyt replication origin (18, 27, 41). Lytic replication is mediated through the virally encoded DNA polymerase and requires a number of additional lytic EBV proteins (14). Induction of lytic EBV infection results in host cell killing in vitro (12, 24), although this point has not been clearly shown in vivo.
It has previously been shown that induction of lytic EBV infection allows the infected host cell to phosphorylate the prodrug ganciclovir (GCV) into its cytotoxic form (37, 54). It is already well established that expression of the herpes simplex virus thymidine kinase gene allows tumor cells to be killed by GCV (8, 15, 55). In the case of EBV, while it is not entirely certain which virally encoded protein phosphorylates GCV, this event is likely mediated by the EBV homologue of the herpes simplex virus-thymidine kinase protein (31, 32, 37) and/or the EBV homologue of the cytomegalovirus UL97 protein (BGLF4) (37, 47). Both of these EBV proteins are expressed only during the lytic form of viral infection, consistent with previous findings that lytic, but not latent, EBV infection results in GCV phosphorylation (37, 54). Westphal et al. have previously demonstrated that while treatment with GCV of lytically infected Burkitt lymphoma cells prevents the lytic form of EBV DNA replication, GCV nevertheless induces host cell death, presumably due to the toxic effect of phosphorylated GCV on the host cell DNA polymerase (54). Thus, induction of lytic EBV infection in EBV-positive tumor cells, with or without the addition of GCV, may be an effective method to treat EBV-associated tumors.
Using adenovirus vectors expressing the BZLF1 or BRLF1 proteins, Westphal et al. have previously shown that inoculation of Jijoye Burkitt lymphoma tumors grown in SCID mice induces the lytic form of EBV infection (54). However, it is not known whether expression of the EBV IE proteins induces cell death in EBV-positive tumor cells in vivo. In addition, it has not been previously demonstrated that delivery of IE proteins to EBV-positive tumors results in inhibition of tumor growth. The majority of EBV-positive B-cell lymphomas are not susceptible to adenovirus-mediated gene delivery, since most B cells lack the adenovirus receptor (28, 51). In contrast, epithelial tumors, such as NPC and gastric carcinoma, are usually highly susceptible to adenovirus infection.
In this study, we have examined the efficacy of adenovirus vectors expressing BZLF1 or BRLF1, with or without concomitant GCV treatment, for the treatment of EBV-positive epithelial tumors. We showed that both the BZLF1 and BRLF1 adenovirus vectors induce preferential killing of EBV-positive, versus EBV-negative, gastric carcinoma cells in vitro, although the BRLF1 vector is more toxic than the BZLF1 vector in the EBV-negative cells. We also showed that both the BZLF1 and the BRLF1 adenovirus vectors induce an abortively lytic form of EBV infection in C18 NPC tumors grown in nude mice. Furthermore, we demonstrated that injection of C18 NPC tumors with either the BZLF1 or the BRLF1 adenovirus vector, but not a control vector, significantly reduces tumor growth in nude mice. GCV did not significantly enhance the antitumor effect of the BZLF1 and BRLF1 adenovirus vectors at the doses used in our experiments. These results suggest that induction of lytic viral transcription using gene delivery techniques is an effective and novel method for treating EBV-positive malignancies.
MATERIALS AND METHODS
Cell lines.
NPC-KT is an EBV genome-positive NPC epithelial hybrid cell line, which was established by fusion of primary EBV genome-positive NPC epithelial cells with an epithelial cell line derived from human adenoid tissue (50). NPC-KT cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin-streptomycin. AGS is a gastric carcinoma cell line. The AGS-EBV cell line (a gift from Lindsey Hutt-Fletcher) was obtained by G418 selection of AGS cells that were infected with a recombinant virus (from Akata cells) in which a neomycin resistance cassette had been inserted into the nonessential BDLF3 open reading frame. AGS cells were maintained in Ham's F-12 medium with 10% fetal bovine serum; AGS-EBV cells also received 400 μg of G418/ml.
Generation of adenovirus vectors.
The construction and generation of the adenoviral vectors were described previously (54). Briefly, the BZLF1 and BRLF1 genes were cloned under the transcriptional control of the human cytomegalovirus IE promoter into a shuttle vector which contains a Lox P site, the left adenovirus terminal repeat, and a packaging signal. Then, via Cre-Lox-mediated recombination, these vectors were inserted into the Lox P site of a replication-deficient type 5 adenovirus lacking the E1 and E3 genes to create adenovirus BZLF1 (Ad-Z) and adenovirus BRLF1 (Ad-R). Control vectors (Ad-GFP and Ad-LacZ) containing the green fluorescent protein and beta-galactosidase genes, respectively, were made in the same manner.
Adenovirus infection in vitro.
NPC-KT cells were plated onto flasks 1 day prior to adenovirus infection. Cells were infected with no adenovirus (mock infection), Ad-GFP, Ad-Z, or Ad-R at a multiplicity of infection (MOI) of 20 (calculated from PFU).
In vitro adenovirus vector killing experiments.
AGS and AGS-EBV cells were grown to 70 to 80% confluence and then infected with Ad-LacZ, Ad-Z, or Ad-R at a MOI of 3, 10, or 20. The number of viable cells was determined by trypan blue exclusion 5 days postinfection. Cells were also harvested on day 5 for immunoblot analysis of BZLF1, BRLF1, and β-actin. All experiments were performed in triplicate.
Animal experiments.
The C18 NPC tumor is a patient-derived NPC that can be passaged in nude mice (4). Small minced pieces of C18 tumors were transplanted into the flanks of 5- to 6-week-old female nude mice, using matrigel as previously described (4). When tumors had become palpable (approximately 9 days after transplantation), they were inoculated with phosphate-buffered saline (PBS) or 4 × 109 PFU of Ad-GFP, Ad-Z, or Ad-R. The adenovirus was dialyzed in PBS prior to inoculation, suspended in 100 μl of PBS, and injected directly into the tumor, using a 30-gauge needle.
In preliminary experiments, mice were euthanized 2 days after injection of tumors and tumors were surgically removed. Tumor tissue from each treatment was then subjected to immunoblot analysis or a EBV DNA terminus assay or fixed in 4% paraformaldehyde (later replaced with 70% ethanol) (72 h) for the histochemical and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays.
For treatment studies, C18 tumors were treated as described above, with intratumoral injection of 4 × 109 PFU of Ad-GFP, Ad-Z, or Ad-R. A number of the animals also received intraperitoneal GCV (Warner-Lambert Company) (100 mg/kg of body weight), which was given twice daily for 5 consecutive days beginning 1 day after adenovirus inoculation. The treatment groups were as follows: Ad-GFP alone (7 tumors), Ad-GFP plus GCV (7 tumors), Ad-Z alone (6 tumors), Ad-Z plus GCV (7 tumors), Ad-R alone (7 tumors), and Ad-R plus GCV (8 tumors). Three times per week, the mice were examined and tumor measurements were obtained. Mice were euthanized when tumor size exceeded 1 cm3. Statistics were performed using the Excel t test.
Immunoblot analysis.
NPC-KT cells (either mock infected or infected with various adenovirus constructs) were harvested 24 h postinfection, and immunoblot analysis was subsequently performed, as described previously (1), using anti-BMRF1 (1:100; Capricorn), anti-BZLF1 (1:100; Argene), anti-BRLF1 (1:100; Argene), and anti-β-actin (1:5,000; Sigma) antibodies. Results were visualized using a chemiluminescence kit (Amersham).
For immunoblot analysis of C18 NPC tumors, tumors were removed after euthanasia of mice and tumor pieces were sonicated in lysis buffer containing 10 mM Tris-HCl (pH 8.0), 1% sodium dodecyl sulfate, 5% β-mercaptoethanol, and 1× proteinase inhibitors. The sonicated material was boiled for 10 min and centrifuged before being subjected to immunoblot analysis as described above.
TUNEL staining.
Tumor tissue sections were examined using a fluorescein isothiocyanate in situ cell death detection kit (Boehringer Mannheim). Briefly, tumor tissue sections were dewaxed and rehydrated. After washing in PBS, tissue sections were permeabilized with proteinase K (20 μg/ml; Sigma) for 30 min at room temperature. Cells were then incubated at 37°C for 1 h in a ratio of enzyme to fluorescein isothiocyanate label solution as specified by the manufacturer before a final wash in PBS. Tissue sections were covered with anti-fade (Biomeda) and analyzed under a fluorescence microscope.
Histological analysis.
Tumors were fixed in 4% paraformaldehyde and embedded in paraffin, and deparaffinized sections were subsequently stained with hematoxylin and eosin and examined microscopically.
EBV DNA terminus assay.
To evaluate episomal and linear structures of the EBV genome, DNA was isolated from C18 NPC tumor tissues 2 days after infection with Ad-GFP, Ad-Z, or Ad-R. DNA was cut with BamHI, run on a 0.8% agarose gel, blotted onto a Hybond nylon membrane, and hybridized with a 32P-labeled riboprobe targeting the EBV termini as previously described (39).
RESULTS
The BZLF1 and BRLF1 adenovirus vectors induce expression of lytic EBV genes in NPC-KT cells in vitro.
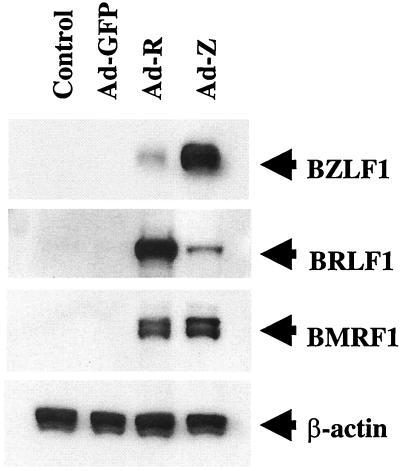
Transfection of either EBV IE gene, BZLF1 or BRLF1, has been previously shown to induce early lytic viral gene expression in NPC-KT cells (56). To examine the effects of the BZLF1 and BRLF1 proteins expressed in adenovirus vectors, NPC-KT cells were mock infected, infected with the BZLF1 or BRLF1 adenovirus vector, or infected with a control adenovirus vector expressing the GFP protein, using an MOI of 20 (PFU). The expression of BZLF1, BRLF1, and the early lytic cycle EBV protein, BMRF1, was examined by immunoblot analysis 2 days later. As shown in Fig. 1, infection with the control GFP adenovirus vector did not activate IE or early lytic gene expression in NPC-KT cells. In contrast, infection with either the BZLF1 or BRLF1 adenovirus vector induced BMRF1 expression in NPC-KT cells and each IE protein induced expression of the other IE protein. Thus, infection of this NPC-derived cell line in vitro with an adenovirus vector expressing either the BZLF1 or BRLF1 protein induced lytic EBV gene expression at similar levels of efficiency.
FIG. 1.
The BZLF1 and BRLF1 adenovirus vectors induce early lytic viral gene expression in NPC-KT cells. NPC-KT cells were infected with GFP, BZLF1, and BRLF1 adenoviruses (MOI of 20). The expression of the IE EBV proteins, BZLF1 and BRLF1, as well as the early lytic protein, BMRF1, was analyzed by immunoblot 24 h after infection.
The BZLF1 and BRLF1 adenovirus vectors preferentially kill EBV-positive gastric carcinoma cells.
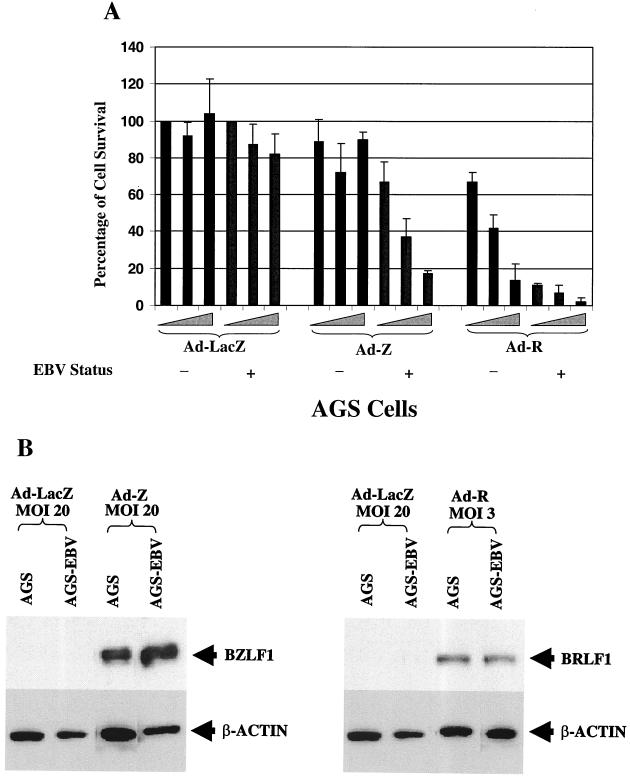
To determine whether the BZLF1 or the BRLF1 adenovirus vector preferentially kills EBV-positive gastric carcinoma cells, we infected EBV-negative versus EBV-positive AGS gastric carcinoma cells with increasing titers of each vector (MOI of 3, 10, or 20) or a control adenovirus vector expressing beta-galactosidase. The number of viable cells in each condition was quantitated 5 days after infection (Fig. 2A). As expected, both of the BZLF1 and BRLF1 vectors induced early lytic gene expression in AGS-EBV cells (data not shown). At the highest MOI (MOI of 20), the BZLF1 adenovirus vector killed over 80% of the EBV-positive cells versus fewer than 10% of the EBV-negative cells. In contrast, at the highest MOI (MOI of 20), the BRLF1 vector was toxic to both EBV-negative and EBV-positive cells. However, at the lowest MOI (MOI of 3), the BRLF1 vector induced preferential killing of EBV-positive cells. The EBV-positive and EBV-negative AGS extracts had equal levels of the BZLF1 and BRLF1 proteins following adenovirus infection (Fig. 2B), indicating that the two lines were infected at similar levels of efficiency. These data indicate that both the BZLF1 and BRLF1 proteins preferentially kill EBV-positive cells but that BRLF1 is considerably more toxic than BZLF1 in EBV-negative cells.
FIG. 2.
The BZLF1 and BRLF1 adenovirus vectors preferentially kill EBV-positive cells. (A) EBV-positive versus EBV-negative AGS gastric carcinoma cells were infected with increasing amounts (MOIs of 3, 10, and 20) of a control adenovirus vector (Ad-LacZ) or the BZLF1 or BRLF1 vector. The percentage of survival of cells in each infection condition (normalized to 100% viability for AGS cells infected with the lowest titer of Ad-LacZ) is shown, quantitated 5 days after infection. (B) Immunoblot analysis was performed to document equal levels of expression of the BRLF1 and BZLF1 proteins after infection with the adenovirus vectors in EBV-positive versus EBV-negative AGS cells.
The BZLF1 and BRLF1 adenovirus vectors induce early lytic EBV gene expression in C18 NPC tumors grown in nude mice.
The effect of the BZLF1 and BRLF1 adenovirus vectors in EBV super-infected AGS cells in vitro does not necessarily predict their effect in NPC tumors in vivo. Therefore, to investigate the effects of the BZLF1 and BRLF1 adenovirus vectors in a model more biologically relevant for NPC, patient-derived EBV-positive C18 NPC tumors were grown in nude mice. Nine days after transplantation into the flanks of nude mice, C18 tumors were directly inoculated with 4 × 109 PFU of the GFP, BZLF1, or BRLF1 adenovirus construct. Mice were euthanized 2 days after injection, and the tumors were harvested for immunoblot and histochemical analysis.
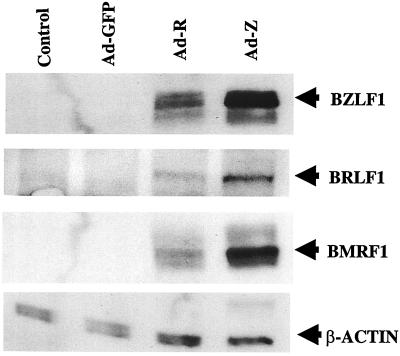
As shown in Fig. 3, injection of tumors with either the BZLF1 or BRLF1 adenovirus vector resulted in detectable BZLF1, BRLF1, and BMRF1 protein expression. In contrast, the control tumor and the Ad-GFP inoculated tumor had very little if any lytic EBV gene expression, consistent with previous observations indicating that the majority of NPC tumor cells contain only the latent form of EBV infection (30, 38). These results demonstrate that the C18 tumor is susceptible to adenovirus infection in vivo and that both the BZLF1- and the BRLF1-expressing vectors induce early lytic EBV gene expression. Adenovirus infection per se does not induce the expression of lytic EBV genes in C18 tumors. In contrast to the results in NPC-KT cells in vitro, infection of C18 tumors in vivo with the Ad-Z vector consistently induced somewhat more early lytic EBV gene expression than did infection with the Ad-R vector (Fig. 3 and data not shown).
FIG. 3.
The BZLF1 and BRLF1 adenovirus vectors induce transcription of early lytic EBV genes in C18 NPC tumors. C18 NPC tumors (grown in nude mice) were directly inoculated with the GFP, BZLF1, or BRLF1 adenovirus. Two days later, tumor extracts were analyzed by immunoblotting for the expression of BZLF1, BRLF1, BMRF1, or β-actin.
The BZLF1 and BRLF1 adenoviruses induce necrosis and apoptosis in C18 NPC tumor cells.
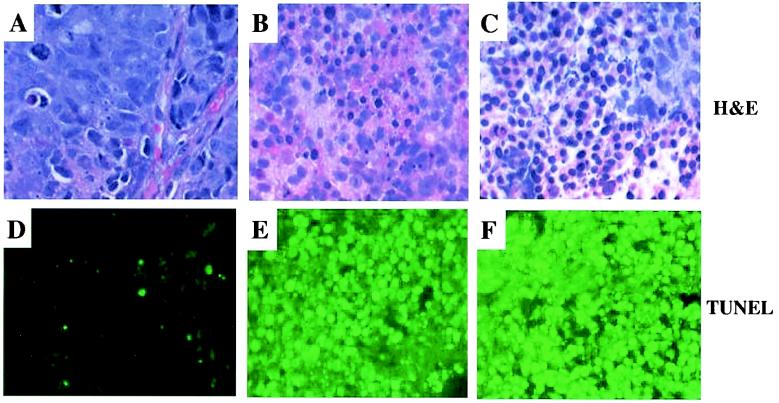
To further evaluate the effects of the BZLF1, BRLF1, and GFP adenovirus vectors in C18 tumors, we performed histochemical analysis of tumors harvested 2 days after injection with each vector (Fig. 4). Microscopic examination of the hematoxylin- and eosin-stained slides revealed that both BZLF1 and BRLF1 expression induced massive necrosis, as well as apoptosis, of C18 tumor cells. The necrotic zones were characterized by cells having smudged and pale nuclear chromatin, while the cytologically apoptotic cells had shrunken nuclei with condensed chromatin. In contrast, cells in the tumor inoculated with the control adenovirus vector appeared viable. Although most cells in the Ad-Z- and Ad-R-injected tumors appeared abnormal, small areas of viable cells could sometimes be found (for an example, see the upper-right-hand corner of Fig. 4C), likely reflecting cells which had not been infected. There was minimal inflammatory response to either the BZLF1 or the BRLF1 adenovirus vector. In situ TUNEL staining confirmed that injection of tumors with either the BZLF1 or BRLF1 vector, but not the control GFP vector, induced striking apoptosis (in addition to necrosis) of tumor cells. These results suggest that both of the BZLF1 and BRLF1 adenoviruses induce extensive cell death when inoculated directly into C18 NPC tumors at the doses used in these experiments.
FIG. 4.
The BZLF1 and BRLF1 adenoviruses induce cellular necrosis and apoptosis in C18 NPC tumors. GFP, BZLF1, or BRLF1 adenovirus was injected directly into C18 NPC tumors established in nude mice. Two days later, mice were euthanized and tumors were dissected and fixed. Sections of tumors, treated with GFP adenovirus (A and D), BZLF1 adenovirus (B and E), or BRLF1 adenovirus (C and F), were stained with hematoxylin and eosin (A, B, and C) (magnification, ×400) or TUNEL (D, E, and F) (magnification, ×200).
The BZLF1 and BRLF1 adenovirus vectors do not induce lytic EBV DNA replication in C18 NPC tumors.
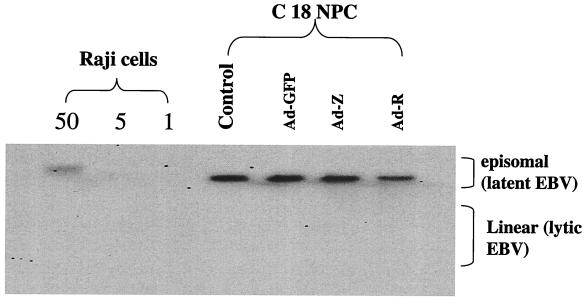
The immunoblot analysis of C18 tumors infected with the BRLF1 and BZLF1 adenoviruses indicated that these vectors induce the expression of at least some early lytic viral proteins. To determine if early lytic viral protein expression is accompanied by the lytic form of EBV DNA replication, tumor tissue was analyzed by Southern blot analysis using the terminus assay (39), which can distinguish between the latent (episomal) and lytic (linear) forms of EBV DNA. As shown in Fig. 5, only the episomal form of the EBV genome was detected in C18 tumor tissues infected with the BZLF1 and BRLF1 adenoviruses. The failure to detect the linear form of the EBV genome in Ad-Z- and Ad-R-infected C18 tumors is somewhat surprising and may indicate that the massive cell death associated with these vectors does not permit viral replication.
FIG. 5.
The BZLF1 and BRLF1 adenoviruses do not induce significant amounts of the lytic form of EBV replication in C18 NPC tumors. C18 NPC tumors were directly inoculated with the GFP, BZLF1, or BRLF1 adenovirus. Two days later, tumor DNA was harvested, cut with BamHI, and hybridized to a probe spanning the EBV termini for Southern blotting. The episomal form of EBV genome is seen in latent infection, whereas the linear form of the genome is produced by the lytic form of EBV DNA replication. Various amounts of Raji cell DNA were also loaded as a positive control for the episomal form of EBV DNA.
Treatment with either the BZLF1 or the BRLF1 adenovirus vector significantly inhibits growth of C18 NPC tumors.
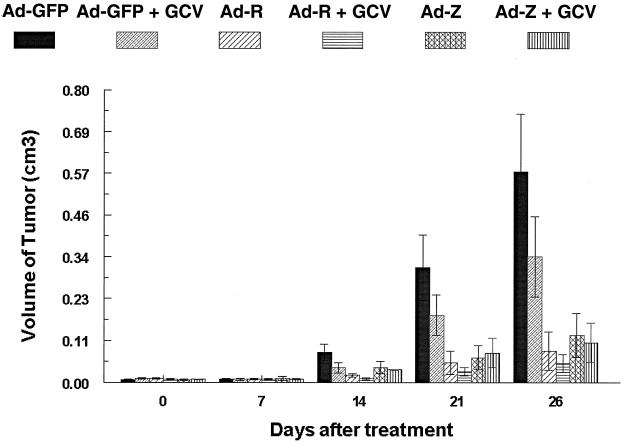
To determine whether injection of NPC tumors with either the BZLF1 or the BRLF1 adenovirus vector could be used therapeutically in vivo, C18 NPC tumors were transplanted into the flanks of nude mice. Nine days after transplantation, tumors were directly injected with 4 × 109 PFU of the GFP, BZLF1, or BRLF1 adenovirus. In addition, the mice in each treatment group were injected twice daily with either GCV (100 mg/kg body weight, intraperitoneally [i.p.]) or PBS for 5 days following injection with the adenovirus vectors. Experiments were terminated at day 26 after adenovirus injection.
The data from this experiment are summarized in Fig. 6. NPC tumors injected with the GFP adenovirus grow at a rate comparable to that of mock-infected tumors (data not shown). In comparison to that of the tumors injected with the GFP adenovirus, the growth of tumors injected with the BZLF1 or the BRLF1 adenovirus was significantly suppressed in the presence (Ad-Z + GCV [P = 0.01] or Ad-R + GCV [P = 0.003]) or absence (Ad-Z [P = 0.02] or Ad-R [P = 0.008]) of concomitant GCV therapy. We have found that approximately 1% of C18 tumor cells constitutively express the IE protein, BRLF1 (data not shown), and others have likewise reported that the lytic form of EBV infection is present within a small percentage of NPC tumor cells (7, 44). Consistent with this, GCV treatment of the Ad-GFP-injected tumors had a small inhibitory effect on C18 tumor growth. However, concomitant GCV treatment only slightly reduced the size of the tumors injected with the BZLF1 or BRLF1 vectors (Fig. 6). These data demonstrate that BZLF1 and BRLF1 adenovirus vectors, either alone or in combination with GCV, inhibit C18 NPC tumor growth.
FIG. 6.
The BZLF1 and BRLF1 adenoviruses, with or without GCV, significantly inhibit C18 NPC tumor growth in vivo. C18 NPC tumors were transplanted into the flanks of nude mice. Nine days later, tumors were directly inoculated with a GFP, BZLF1, or BRLF1 adenovirus vector, alone or in combination with GCV (100 mg per kg of body weight [i.p., twice a day for 5 days]). The average tumor volume for each treatment group is indicated at different time points.
DISCUSSION
The presence of the EBV genome within certain tumor types provides an attractive target for the development of new therapies. EBV is almost always present in undifferentiated NPC (30, 38), as well as in AIDS-associated central nervous system lymphomas (33), and is commonly present in lymphoproliferative disease (17) and leiomyosarcomas (36) occurring in immunocompromised patients. In addition, EBV is present in a subset of Hodgkin's disease cases (52), T-cell lymphomas (19), gastric carcinomas (45), liver cancers (46), and possibly breast cancers (3). In contrast, the EBV genome is present in only a very few normal B cells (27, 41). Thus, EBV-based cytotoxic therapies could potentially be applied to a wide variety of tumor types.
In this report, we have explored the therapeutic utility of inducing lytic EBV gene expression in EBV-positive tumor cells. Adenovirus vectors were constructed containing each of the EBV IE proteins, BZLF1 and BRLF1, and the ability of these vectors to induce lytic EBV gene expression was demonstrated both in vitro (in NPC-KT and AGS-EBV cells) and in vivo (in the C18 NPC tumor model). Both the BZLF1 and BRLF1 vectors induced preferential killing of EBV-positive, versus EBV-negative, gastric carcinoma cells in vitro. Most importantly, we demonstrated that adenovirus vectors expressing either of the EBV IE proteins produce tumor cell death when inoculated directly into C18 NPC tumors and inhibit C18 NPC tumor growth in nude mice. Our results suggest that therapeutic strategies that deliver the EBV IE proteins to tumor cells might be useful for treating both EBV-positive gastric carcinoma and NPC, irrespective of the role that EBV plays in their pathogenesis.
Our study demonstrates that both BZLF1 and BRLF1 adenovirus vectors can activate early lytic viral gene expression, and induce necrosis and apoptosis, in C18 NPC tumor cells. EBV encodes a variety of proteins that inhibit apoptosis, including the latent protein LMP-1 and the lytic bcl-2 homologs, BHRF1 and BALF1 (21, 27, 35, 41), and a recent in vitro study demonstrated that lytically infected EBV-positive Burkitt lymphoma cells do not undergo apoptosis when exposed to chemical inducing agents that normally cause apoptosis (22). The induction of apoptosis by the BZLF1 and BRLF1 vectors was therefore somewhat unexpected. Swenson et al. have previously shown that BRLF1 activates cell cycle progression and induces apoptosis in EBV-negative cells (48), and Westphal et al. and others have found that BZLF1 expression induces apoptosis in certain EBV-negative cell lines but not others (54; A. Mauser, A. Zanation, W. Yarborough, W. Kaufmann, and S. Kenney, submitted for publication). We speculate that the level of cellular BZLF1 and BRLF1 expression induced by the adenovirus vectors in C18 tumors is higher, and persists much longer, than that which occurs during normal EBV infection and thus that the antiapoptotic effects of other EBV encoded proteins are overwhelmed by the proapoptotic effects of the IE proteins.
Westphal et al. previously found that infection of Jijoye Burkitt lymphoma cells in vitro with either the BZLF1 or the BRLF1 adenovirus vector induces the linear form of EBV (54). The failure to observe the linear form of EBV following Ad-Z and Ad-R injection of C18 tumors in vivo suggests that at the doses used in this study, the vectors were primarily inducing an abortive form of lytic infection. It is possible that the host cell environment in C18 tumors suppresses fully lytic EBV infection. Alternatively, the early apoptotic death of the Ad-Z- and Ad-R-injected tumor cells may inhibit progression to the linear form of EBV DNA, even though these vectors induce transcriptional activation of at least some lytic viral replication proteins. Our in vitro studies in AGS-EBV cells indicate that lower doses (MOI of 3) of the Ad-Z and Ad-R vectors actually induce more lytic EBV DNA replication than higher doses (MOI of 20) (data not shown), suggesting that excessive and prolonged expression of the EBV IE proteins, while transcriptionally activating early viral genes, inhibits lytic EBV DNA replication. However, we cannot exclude the possibility that one or more adenovirus-encoded proteins inhibit lytic EBV replication.
Interestingly, cell death following lytic EBV infection is at least partially due to the expression of IE or early protein(s) (24), and thus, it was not entirely unexpected that abortive lytic EBV infection still results in host cell death. However, prior to these studies, it was unclear whether the BRLF1 and BZLF1 vectors would have similar killing effects in NPC tumors. We show here that the BZLF1 vector induces better expression than the BRLF1 vector of the lytic EBV gene, BMRF1, in C18 tumors. However, the two vectors inhibited NPC tumor growth to similar degrees. As BRLF1 expression is more cytotoxic than BZLF1 expression in EBV-negative cell lines, the intrinsic cytotoxicity of BRLF1, irrespective of the EBV genome, may contribute to tumor killing by the BRLF1 vector. Although we did not observe an effect on the level of β-actin, lytic EBV infection has been previously shown to inhibit cellular protein synthesis within 9 h of infection (11). It is possible that BRLF1, more than BZLF1, contributes to tumor cell killing through this mechanism. While we did not observe generalized toxicity to the mice when either the BZLF1 or BRLF1 adenovirus vector was injected directly into NPC tumors, it will be important to compare the nonspecific toxicity of the BZLF1 vector to that of the BRLF1 vector in EBV-negative cells in vivo before concluding that one vector is more useful than the other for treatment of NPC tumors in humans.
Here and elsewhere (13), it has been found that in the absence of the BZLF1 or BRLF1 adenovirus vector, GCV by itself induces a small but reproducible inhibition of NPC C18 tumor growth, in contrast to its lack of effect on an EBV-positive lymphoma (53). The ability of GCV to inhibit EBV-positive NPC tumor growth, but not lymphoma growth, likely reflects the increased number of tumor cells containing the lytic form of EBV infection in NPC tumors versus lymphomas (7, 44) and the known ability of GCV to induce a bystander killing effect (8, 15). Although GCV also slightly enhanced the inhibitory effects of the BZLF1 and BRLF1 adenovirus vectors, the effect was not nearly as dramatic as that which has been observed for combinations of GCV with agents that induce BZLF1 or BRLF1 transcription in tumors, such as gamma irradiation (53) and chemotherapy (13). We speculate that the relative lack of synergistic effect in this study reflects the potent killing effect of the BZLF1 and BRLF1 adenovirus vectors alone at the doses used, in contrast to the modest killing effect observed when agents which induce BZLF1 or BRLF1 transcription are given alone at relatively low doses (53). It remains possible that GCV synergy with the BZLF1 and BRLF1 adenovirus vectors would be more striking if the adenovirus vectors were delivered at lower doses. Alternatively, the abortively lytic form of EBV infection induced by Ad-Z and Ad-R in C18 tumors may not have resulted in significant GCV phosphorylation.
Unfortunately, although adenovirus can infect a wide range of cell types, including epithelial cells, most lymphocytes and lymphomas are not very susceptible to adenovirus infection, due to low expression of the major adenovirus receptor (28, 51). Thus, an adenovirus vector is not the optimal vehicle for delivering genes to EBV-positive lymphomas; other vectors would be preferable. Alternatively, approaches which increase adenovirus-mediated gene delivery to lymphocytes, including modification of the viral fiber protein to allow virus binding to alternative cell receptors (9) or the use of bispecific antibodies directed against the adenovirus fiber protein and lymphocyte cell surface antigens (23), may allow the efficient use of the BZLF1 and BRLF1 adenovirus vectors in EBV-positive lymphomas. Westphal et al. previously demonstrated that both the BZLF1 and BRLF1 adenovirus vectors induce lytic EBV infection when inoculated directly into an adenovirus-susceptible Burkitt lymphoma tumor (54), thus confirming that if efficient delivery of the BZLF1 or BRLF1 gene can be accomplished in EBV-positive lymphomas, it is likely to induce a similar therapeutic response to that observed for NPC tumors.
In summary, our present work demonstrates that gene transfer therapy using Ad-Z and Ad-R vectors, with or without concomitant GCV, effectively suppresses C18 NPC tumor growth, suggesting that this therapeutic strategy can be used for EBV-associated malignancies. It will be important to demonstrate that the therapeutic effect of BZLF1 and BRLF1 adenovirus vectors, as observed here using the C18 NPC tumor model, can also be observed in a variety of other patient-derived EBV-positive epithelial tumors. In addition, application of this therapy to patients with EBV-associated tumors will likely require enhancement of the efficacy and safety of presently available delivery vectors. Nevertheless, this is the first study to show that using gene delivery techniques, induction of the lytic form of EBV infection in tumors suppresses NPC tumor growth in vivo, and this work provides a basis for further development of novel EBV-targeted treatments for EBV-associated malignancies.
Acknowledgments
This work was supported by NIH grant R01 CA 66519.
We thank Natalie Edmund for help with animal experiments and the UNC Gene Therapy Center for preparation of the adenovirus vectors.
REFERENCES
- 1.Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. C. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson, A. L., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant, Z (S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet, M., J. M. Guinebretiere, E. Kremmer, V. Grunewald, E. Benhamou, G. Contesso, and I. Joab. 1999. Detection of Epstein-Barr virus in invasive breast cancers. J. Natl. Cancer Inst. 91:1376-1381. [DOI] [PubMed] [Google Scholar]
- 4.Busson, P., G. Ganem, P. Flores, F. Mugneret, B. Clausse, B. Caillou, K. Braham, H. Wakasugi, M. Lipinski, and T. Tursz. 1988. Establishment and characterization of three transplantable EBV-containing nasopharyngeal carcinomas. Int. J. Cancer 42:599-606. [DOI] [PubMed] [Google Scholar]
- 5.Chang, Y., D. Dong, G. Hayward, and S. D. Hayward. 1990. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J. Virol. 64:3358-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV) encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an early EBV promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochet, C., D. Martel-Renoir, V. Grunewald, J. Bosq, G. Cochet, G. Schwaab, J. F. Bernaudin, and I. Joab. 1993. Expression of the Epstein-Barr virus immediate-early gene, BZLF1, in nasopharyngeal carcinoma tumor cells. Virology 197:3358-3365. [DOI] [PubMed] [Google Scholar]
- 8.Conners, T. A. 1995. The choice of prodrugs for gene directed enzyme prodrug therapy of cancer. Gene Ther. 2:702-709. [PubMed] [Google Scholar]
- 9.Dmitriev, I., V. Krasnykh, C. R. Miller, M. Wang, E. Kashentseva, G. Mikheeva, N. Belousova, and D. T. Curiel. 1998. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 72:9706-9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the cooperative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feighny, R. J., M. P. Farrell, and J. S. Pagano. 1980. Polypeptide synthesis and phosphorylation in Epstein-Barr virus-infected cells. J. Virol. 34:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feighny, R. J., B. E. Henry II, and J. S. Pagano. 1981. Epstein-Barr virus-induced deoxynuclease and the reutilization of host-cell DNA degradation products in viral DNA replication. Virology 115:395-400. [DOI] [PubMed] [Google Scholar]
- 13.Feng, W.-H., B. Israel, N. Raab-Traub, P. Busson, and S. C. Kenney. 2002. Chemotherapy induces lytic Epstein-Barr virus (EBV) replication and confers ganciclovir susceptibility to EBV-positive tumors. Cancer Res. 62:1920-1926. [PubMed] [Google Scholar]
- 14.Fixman, E., G. Hayward, and S. D. Hayward. 1992. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J. Virol. 66:5030-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman, S. M., C. N. Abboud, K. A. Whartenby, C. H. Packman, D. S. Koeplin, D., F. L. Moolten, and G. N. Abraham. 1993. The “bystander” effect: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 53:5274-5283. [PubMed] [Google Scholar]
- 16.Gruffat, H., E. Manet, A. Rigolet, and A. Sergeant. 1990. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA binding protein. Nucleic Acids Res. 18:6835-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton-Dutoit, S. J., D. Rea, M. Raphael, K. Sandvej, J. Deleclues, C. Gisselbrecht, L. Marelle, J. van Krieken, and G. Pallesen. 1993. Epstein-Barr virus latent gene expression and tumor cell phenotype in acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma. Am. J. Pathol. 14:1072-1085. [PMC free article] [PubMed] [Google Scholar]
- 18.Hammerschmidt, W., and B. Sugden. 1988. Identification and characterization of oriLyt, a lytic origin of replication of Epstein-Barr virus. Cell 55:427-433. [DOI] [PubMed] [Google Scholar]
- 19.Harabuchi, Y., N. Yamanaka, A. Kataura, T. Kinoshita, F. Mizuno, and T. Osato. 1990. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal mid-line granuloma. Lancet 335:128-130. [DOI] [PubMed] [Google Scholar]
- 20.Hardwick, J. M., S. Lazarowits, and S. D. Hayward. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 62:2274-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson, S., D. Huen, M. Rowe, C. Dawson, G. Johnson, and A. B. Rickinson. 1993. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc. Natl. Acad. Sci. USA 90:8479-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inman, G. J., U. K. Binne, G. A. Parker, P. J. Farrell, and M. J. Allday. 2001. Activators of the Epstein-Barr virus lytic program concomitantly induce apoptosis, but lytic gene expression protects from cell death. J. Virol. 75:2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Israel, B. I., R. J. Pickles, D. M. Segal, R. D. Gerard, and S. C. Kenney. 2001. Enhancement of adenovirus vector entry into CD70-positive B-cell lines by using a bispecific CD70-adenovirus fiber antibody. J. Virol. 75:5215-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawanish, M. 1993. Epstein-Barr virus induces fragmentation of chromosomal DNA during lytic infection. J. Virol. 67:7654-7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenney, S., J.-Q. Ge, E. M. Westphal, and J. Olsen. 1998. Gene therapy strategies for treating Epstein-Barr virus-associated lymphomas: comparison of two different Epstein-Barr virus-based vectors. Hum. Gene Ther. 9:1131-1141. [DOI] [PubMed] [Google Scholar]
- 26.Kenney, S. C., J. Kamine, E. Holley-Guthrie, J.-C. Lin, E.-C. Mar, and J. Pagano. 1989. The Epstein-Barr virus (EBV) BZLF1 immediate-early gene product differentially affects latent versus productive EBV promoters. J. Virol. 63:1729-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2343-2397. In B. Fields, D. Knipe, and P. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 28.Leon, R. P., T. Hedlund, S. J. Meech, S. Li, J. Schaack, S. P. Hunger, R. C. Duke, and J. DeGregori. 1998. Adenoviral-mediated gene transfer in lymphocytes. Proc. Natl. Acad. Sci. USA 95:13159-13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Roux, F., A. Sergeant, and L. Corbo. 1996. Epstein-Barr virus (EBV) EB1/Zta protein provided in trans and competent for the activation of productive cycle genes does not activate the BZLF1 gene in the EBV genome. J. Gen. Virol. 77:501-509. [DOI] [PubMed] [Google Scholar]
- 30.Liebowitz, D. 1994. Nasopharyngeal carcinoma: the Epstein-Barr virus association. Semin. Oncol. 21:376-381. [PubMed] [Google Scholar]
- 31.Litter, E., and J. Arrand. 1998. Characterization of the Epstein-Barr virus-coded thymidine kinase expressed in heterologous eucaryotic and prokaryotic systems. J. Virol. 62:3892-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loubiere, L., M. Tiraby, C. Cazaux, E. Brisson, M. Grisoni, J. C. Zhao-Emonet, G. Tiraby, and D. Klatzmann. 1999. The equine herpes virus 4 thymidine kinase is a better suicide gene than the human herpes virus 1 thymidine kinase. Gene Ther. 6:1638-1642. [DOI] [PubMed] [Google Scholar]
- 33.MacMahon, E. M. E., J. D. Glass, S. D. Hayward, R. B. Mann, P. S. Becker, P. Charache, J. C. McArthur, and R. F. Ambinder. 1991. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet 338:969-973. [DOI] [PubMed] [Google Scholar]
- 34.Magrath, I., V. Jain, and K. Bhatia. 1992. Epstein-Barr virus and Burkitt's lymphoma. Semin. Cancer Biol. 3:285-295. [PubMed] [Google Scholar]
- 35.Marshall, W. L., C. Yim, E. Gustafson, T. Graf, D. R. Sage, K. Hanify, L. Williams, J. Fingeroth, and R. W. Finberg. 1999. Epstein-Barr virus encodes a novel homolog of the bcl-2 oncogene that inhibits apoptosis and associates with Bax and Bak. J. Virol. 73:5181-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClain, K., C. Leach, H. Jenson, V. Joshi, B. Pollock, R. Parmley, F. DiCarlo, F. Chadwick, and S. Murphy. 1995. Association of Epstein-Barr virus with leiomyosarcoma in children with AIDS. N. Engl. J. Med. 332:12-18. [DOI] [PubMed] [Google Scholar]
- 37.Moore, S. M., J. S. Cannon, Y. C. Tanhehco, F. M. Hamzeh, and R. F. Ambinder. 2001. Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues. Antimicrob. Agents Chemother. 45:2082-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raab-Traub, N. 1992. Epstein-Barr virus and nasopharyngeal carcinoma. Semin. Cancer Biol. 3:297-307. [PubMed] [Google Scholar]
- 39.Raab-Traub, N., and K. Flynn. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883-889. [DOI] [PubMed] [Google Scholar]
- 40.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein disrupts latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. Fields, D. Knipe, and P. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 42.Rooney, C., D. Rowe, T. Ragot, and P. Farrell. 1989. The spliced BZLF1 gene of Epstein-Barr virus transactivates an early EBV promoter and induces the lytic productive cycle. J. Virol. 63:3109-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rooney, C., N. Taylor, J. Countryman, H. Jenson, J. Kolman, and G. Miller. 1988. Genome rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc. Natl. Acad. Sci. USA 85:9801-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sbih-Lammali, F., F. Berger, P. Busson, and T. Ooka. 1996. Expression of the Dnase encoded by the BGLF5 gene of Epstein-Barr virus in nasopharyngeal carcinoma epithelial carcinoma cells. Virology 222:64-74. [DOI] [PubMed] [Google Scholar]
- 45.Shibata, D., and L. Weiss. 1992. Epstein-Barr virus-associated gastric carcinoma. Am. J. Pathol. 140:769-774. [PMC free article] [PubMed] [Google Scholar]
- 46.Sugawara, Y., M. Makuuchi, and K. Takada. 2000. Detection of Epstein-Barr virus DNA in hepatocellular carcinoma tissues from hepatitis C-positive patients. Scand. J. Gastroenterol. 35:981-984. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan, V., C. Talarico, S. Stanat, M. Davis, D. Coen, and K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358:162-164. [DOI] [PubMed] [Google Scholar]
- 48.Swenson, J., A. Mauser, W. Kaufmann, and S. Kenney. 1999. The Epstein-Barr virus protein BRLF1 activates S phase entry through E2F1 induction. J. Virol. 73:6540-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. trans activation of the latent Epstein-Barr virus genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takimoto, T., M. Kamide, and R. Umeda. 1984. Establishment of Epstein-Barr virus (EBV)-associated nuclear antigen (EBNA)-positive nasopharyngeal carcinoma hybrid cell line (NPC-KT). Arch. Oto-Rhino-Laryngol. 239:87-92. [DOI] [PubMed] [Google Scholar]
- 51.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss, L., L. Movahed, R. Warnke, and J. Sklar. 1989. Detection of Epstein-Bar viral genomes in Reed-Sternberg cells of Hodgkin's disease. N. Engl. J. Med. 320:502-506. [DOI] [PubMed] [Google Scholar]
- 53.Westphal, E. M., W. Blachstock, W.-H. Feng, B. Israel, and S. C. Kenney. 2000. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. 60:5781-5788. [PubMed] [Google Scholar]
- 54.Westphal, E. M., A. Mauser, J. Swenson, M. G. Davis, C. L. Talarico, and S. Kenney. 1999. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 59:1485-1491. [PubMed] [Google Scholar]
- 55.Wildner, O., R. M. Blaese, and F. Candotti. 1999. Enzyme prodrug gene therapy: synergistic use of the herpes simplex virus-cellular thymidine kinase/ganciclovir system and thymidylate synthase inhibitors for the treatment of colon cancer. Cancer Res. 59:5233-5238. [PubMed] [Google Scholar]
- 56.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.zur Hausen, H., H. Schulte-Holthausen, G. Klein, W. Henle, G. Henle, P. Clifford, and L. Santesson. 1970. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 228:1056-1058. [DOI] [PubMed] [Google Scholar]